Abstract
This study aimed to investigate the association of add-on dipeptidyl peptidase-4 inhibitor (DPP4i) therapy and the progression of diabetic retinopathy (DR). In this retrospective population-based cohort study, we examined Taiwanese patients with type 2 diabetes, preexisting DR, and aged ≥40 years from 2009 to 2013. Prescription of DPP4i was defined as a medication possession ratio of ≥80% during the first 6 months. The outcomes included vitreous hemorrhage (VH), tractional retinal detachment, macular edema, and interventions including retinal laser therapy, intravitreal injection (IVI), and vitrectomy. Of 1,767,640 patients, 62,824 were eligible for analysis. After matching, the DPP4i and non-DPP4i groups each contained 20,444 patients. The risks of VH (p = 0.013) and macular edema (p = 0.035) were higher in the DPP4i group. The DPP4i group also had higher risks of receiving surgical interventions (retinal laser therapy (p < 0.001), IVI (p = 0.049), vitrectomy (p < 0.001), and any surgical intervention (p < 0.001)). More patients in the DPP4i group received retinal laser therapy (p < 0.001) and IVI (p = 0.001) than in the non-DPP4i group. No between-group differences in cardiovascular outcomes were noted. In the real-world database study, add-on DPP4i therapy may be associated with the progression of DR in patients with type 2 diabetes. No additional cardiovascular risks were found. The early progression of DR in rapid glycemic control was inconclusive in our study. The possible effect of add-on DPP4i therapy in the progression of DR in patients with type 2 diabetes requires further research.
1. Introduction
Diabetic retinopathy (DR), a common microvascular complication in patients with diabetes, is also a major cause of blindness in working-age adults [1]. The global number of patients with diabetes is estimated to reach 600 million by 2040, one-third of whom are expected to have DR [2]. Severe DR can lead to complications such as vitreous hemorrhage (VH), tractional retinal detachment (RD), and macular edema [3,4]. DR and its complications may require surgical intervention such as retinal laser therapy, intravitreal injection (IVI) of anti-vascular endothelial growth factor, and in some cases vitrectomy [3,4]. This imposes a substantial economic burden on patients with such conditions and their families [5].
Numerous studies have been conducted on preventing or slowing the progression of diabetic complications. A randomized controlled trial reported that appropriate glucose-lowering reduced the risk of cardiovascular diseases, microvascular complications, and all-cause mortality in patients with diabetes [6]. Another randomized controlled trial indicated that intensive glucose control effectively slowed DR progression in patients with type 2 diabetes [7]. Treatment for systemic conditions, such as hypertension and dyslipidemia, has been demonstrated to be associated with a low risk of DR development or progression [7,8].
Dipeptidyl peptidase-4 (DPP4) inhibitors (DPP4i) are a class of oral hypoglycemics, of which the first agent sitagliptin was approved in 2006 by the US Food and Drug Administration [9]. DPP4i suppress the function of DPP4 and indirectly prolong the serum level of glucagon-like peptide-1 (GLP-1), increasing insulin secretion and reducing glucagon secretion from the pancreas [10]. Although a meta-analysis reported that DPP4i exerted a better hypoglycemic effect than α-glucosidase inhibitors [11], other studies have observed associations between its use and an increased risk of heart failure [12,13]. Moreover, another meta-analysis indicated no beneficial association between DPP4i use and all-cause mortality [14]. Regarding DPP4i use in DR, sitagliptin prevented the effect of diabetes on the blood-retinal barrier in male Zucker diabetic fatty rats. Specifically, it improved endothelial function and prevented inflammation, nitrative stress, and apoptosis in animals [15]. However, the association between DPP4i and DR has not been fully characterized [16,17]. The first clinical study of the possible protective effects of DPP4i on DR progression, published in 2016, included 28 patients with type 2 diabetes [18]. A 2018 population-based study by Kim et al. that used data from the South Korean National Health Insurance Service reported a possible association of DPP4i use with an increased risk of DR events early in the treatment phase [19]. Using the same database, Chung et al. found a neutral association between DPP4i use and sulfonylurea added to metformin therapy and the risk of DR progression. The aggravation of DR by DPP4i remains a concern and requires more clinical investigation [20]. In this study, we investigated the association between add-on DPP4i therapy and DR progression in patients with type 2 diabetes and preexisting DR in a real-world setting.
2. Materials and Methods
2.1. Data Source
This retrospective population-based cohort study was conducted using the Taiwan National Health Insurance (NHI) Research Database (NHIRD) (Center for Biomedical Resources of National Health Research Institutes, Miaoli, Taiwan). More than 99.8% of the population in Taiwan (approximately 23.7 million people as of 2020) is covered by the NHI program, a single-payer system established in March 1995. The NHIRD contains de-identified information including medical claims data. Information on the NHI program and its databases has been described in detail in previous publications [21,22]. The present study was approved by the Chang Gung Memorial Hospital Ethics Institutional Review Board (IRB No. 201800199B1) and adheres to the principles of the Declaration of Helsinki.
2.2. Inclusion and Exclusion Criteria
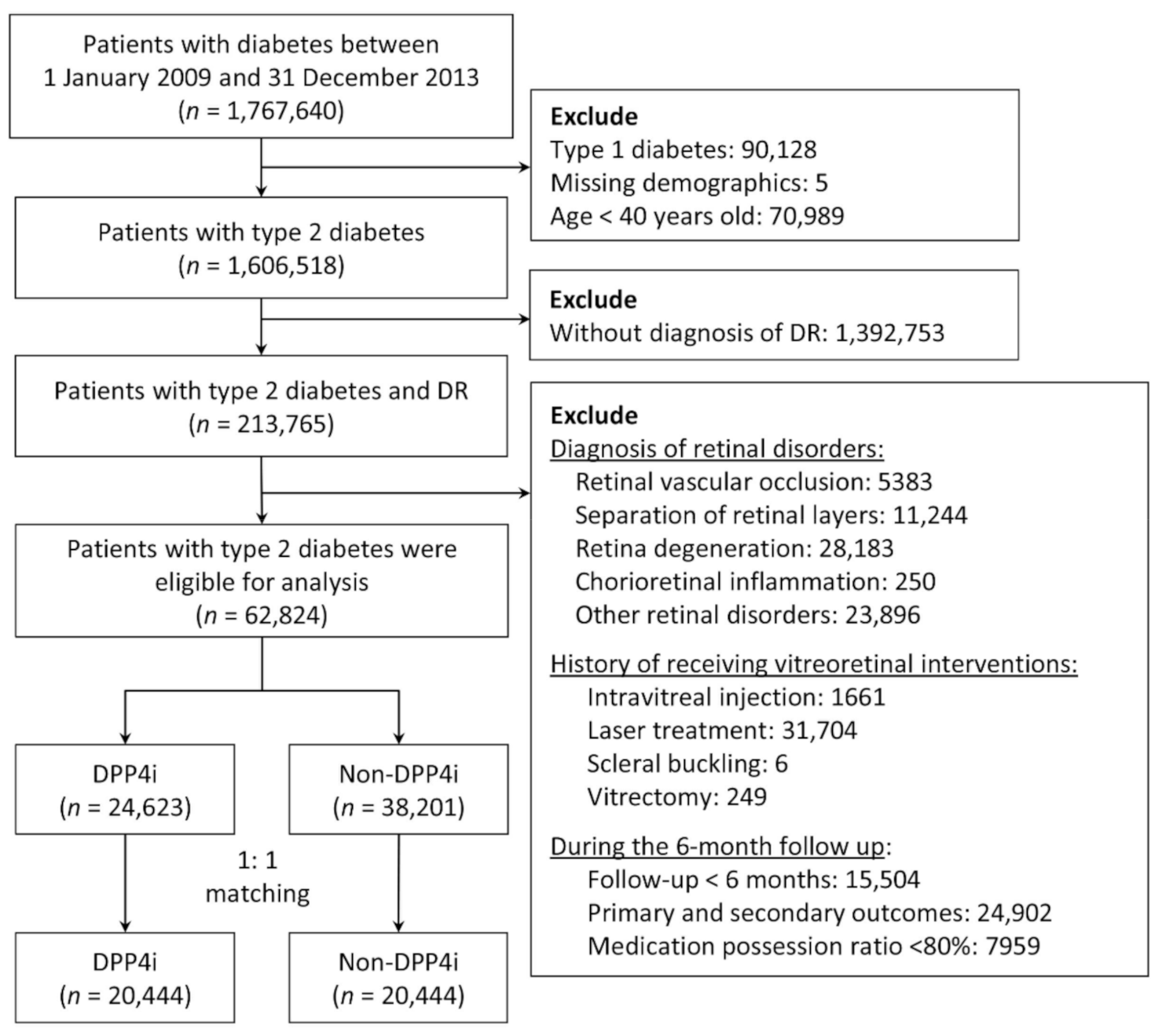
From 2009 to 2013, we identified patients with diabetes in the NHIRD by using the diagnostic codes of the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). These codes were validated in a study on the accuracy of diabetes diagnosis in NHI claims data. Specifically, at least four outpatient visits for diabetes corresponded to a 95.7% accuracy [23]. Another study observed that a prescription of any oral hypoglycemic agent corresponded to an accuracy of 99% [24]. Therefore, in the present study, we included patients with at least five outpatient diagnoses of type 2 diabetes who were also taking any oral hypoglycemics. Patients with type 2 diabetes and preexisting DR were included in the analysis. We excluded patients who were aged under 40 years as well as those with missing demographic data, type 1 diabetes, retinal disorders (including retinal vascular occlusion, separation of retinal layers, retina degeneration, and chorioretinal inflammation), a history of receiving vitreoretinal interventions (including IVI, retinal laser therapy, scleral buckling, and vitrectomy), or were followed up for less than 6 months (Figure 1).
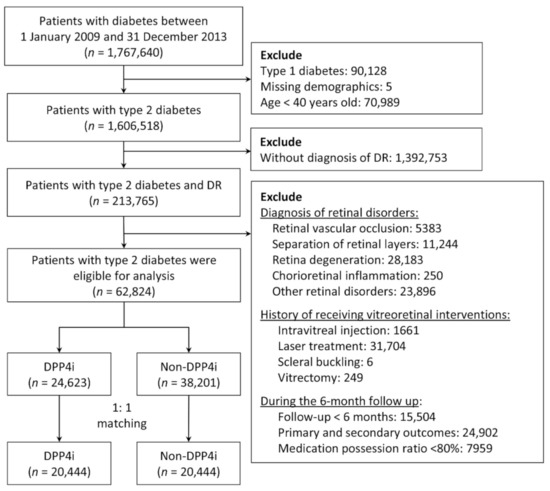
Figure 1.
Flowchart of the inclusion and exclusion criteria of the patients. DR, diabetes retinopathy; DPP4i, dipeptidyl peptidase 4 inhibitors.
2.3. Group Definition
The index date of the DPP4i group was defined as the date of the first DPP4i prescription between 2009 and 2013. To prevent the immortal time bias, the index date of the non-DPP4i group was assigned as the index date of the DPP4i group through an approach known as prescription time-distribution matching [25]. To ascertain the compliance of DPP4i use, patients in the DPP4i group with a medication possession ratio (MPR) of less than 80% during the first 6 months of follow-up [26], specifically 144 days (180 days × 0.8), were excluded from further analysis (Figure 1).
2.4. Outcomes
In this study, the primary ocular outcome was the composite DR outcome, which consisted of any one of the following: VH, tractional RD, and macular edema. The secondary ocular outcome was the composite outcome of any surgical intervention, namely retinal laser therapy, IVI, and vitrectomy. The cardiovascular outcomes, including myocardial infarction, hospitalization for heart failure, ischemic stroke, and hemorrhagic stroke, were defined as safety outcomes. The primary DR outcome and its components were defined as diagnosis after at least three outpatient diagnoses or one inpatient diagnosis. The surgical interventions and other ocular outcomes were examined using the Taiwan NHI reimbursement codes from the claims data for outpatient and inpatient visits. The occurrence of safety outcomes was determined using the principal discharge diagnosis. Mortality and cardiovascular events selected for analysis have been validated previously [27,28].
2.5. Covariates
Covariates were sex, age, proxy variables for compliance (i.e., the number of outpatient visits for diabetes management), proxy variables for DR severity (previous proliferative DR and previous DR duration), comorbidities as well as scores on the Charlson Comorbidity Index, indicators for diabetic severity (diabetes duration, diabetic neuropathy, and diabetic foot ulcer), and concomitant medications. Comorbidities, namely dyslipidemia, hypertension, ischemic heart disease, chronic kidney disease, peripheral arterial disease, ischemic stroke, heart failure, and atrial fibrillation, were confirmed after at least three outpatient diagnoses or one inpatient diagnosis in the previous year. Medications during the first 6 months of follow-up were classified into three categories: antidiabetics, antihypertensives, and other medications. Details of the ICD-9-CM diagnostic codes used in this study are provided in Supplementary Materials (Table S1). The Charlson Comorbidity Index scores were calculated as described previously [29].
2.6. Statistics
To reduce confounding effects, the analysis of differences in outcomes between the DPP4i and non-DPP4i groups was performed after propensity score matching (PSM). The propensity score was the predicted probability given the value of the covariates, which was calculated using a multivariable logistic regression model in which the study groups (1: DPP4i and 0: non-DPP4i) were regressed on the selected covariates. The matching was processed using a greedy nearest-neighbor algorithm with a caliper of 0.2 times the standard deviation of the logit of the propensity score. The matching order was random, and replacement was not allowed. Each patient in the DPP4i group was matched with a non-DPP4i control. The matching quality was assessed after PSM by using the absolute value of the standardized difference between the groups, where a value of less than 0.1 was considered negligible.
The Fine–Gray subdistribution hazard model, which considers all-cause mortality a competing risk, was used to compare the occurrence of time-to-event outcomes between the groups. The average number of surgical interventions per decade was also analyzed and compared using the Poisson model, in which the natural logarithm of the follow-up duration was an offset variable. The study groups (DPP4i vs. non-DPP4i) were the only explanatory variable in the regression analysis. The within-pair clustering of outcomes after PSM was accounted for by using robust standard errors through the generalized estimating equation approach [30]. Further subgroup analyses were conducted to evaluate the consistency of the observed treatment effect on the specified outcomes across different levels of subgroup variables. The outcomes of interest comprised the primary and secondary endpoints, namely the composite DR outcome and the composite outcome of any surgical intervention, respectively. The selected subgroups were sex, age (dichotomized at 65 years), previous proliferative DR, hypertension, dyslipidemia, ischemic heart disease, ischemic stroke, chronic kidney disease, peripheral arterial disease, diabetes duration (dichotomized at 10 years), diabetic neuropathy, diabetic foot ulcer, and the use of concomitant antidiabetics (e.g., metformin, sulfonylurea, thiazolidinediones, alpha-glucosidase inhibitors, meglitinides, and insulin). A two-sided p-value of <0.05 was considered to be significant. All analyses were performed using SAS software, Version 9.4 of the SAS System (SAS Institute Inc., Cary, NC, USA), including the % cif macro for generating cumulative incidence functions under the Fine–Gray sub-distribution hazard method.
3. Results
3.1. Participants
Between 2009 and 2013, a total of 1,767,640 patients with diabetes were identified. After the exclusion of patients aged under 40 years as well as those with type 1 diabetes, missing demographic data, and no DR diagnosis, 213,765 patients remained. We further excluded patients who were followed up for less than 6 months or developed any of the primary or secondary ocular outcomes within 6 months after the index date, as well as those with retinal disorders, a history of receiving vitreoretinal interventions or who had an MPR of less than 80%. After these procedures, 62,824 patients remained. After 1:1 PSM, the non-DPP4i and DPP4i groups comprised 20,444 patients each (Figure 1).
3.2. Demographic Characteristics
Table 1 presents the demographic characteristics of the study groups before and after matching. Before matching, the patients in the DPP4i group were younger; had more outpatient visits for diabetes management in the previous year; were more likely to have undergone a dilated fundus examination in the previous year; had a higher prevalence of dyslipidemia; had a longer diabetes duration; had more prescriptions of sulfonylurea, alpha-glucosidase inhibitors, meglitinides, beta-blockers, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, antiplatelets, statins, and fenofibrates, and fewer prescriptions of insulin. After matching, the two groups were well balanced in terms of sex, age, comorbidities, indicators for diabetic severity, underlying ocular diseases, medications, and follow-up duration.

Table 1.
Characteristics of patients with type 2 diabetes and diabetic retinopathy before and after matching. Balance achieved between the DPP4i and non-DPP4i groups after matching.
3.3. Primary Ocular Outcomes
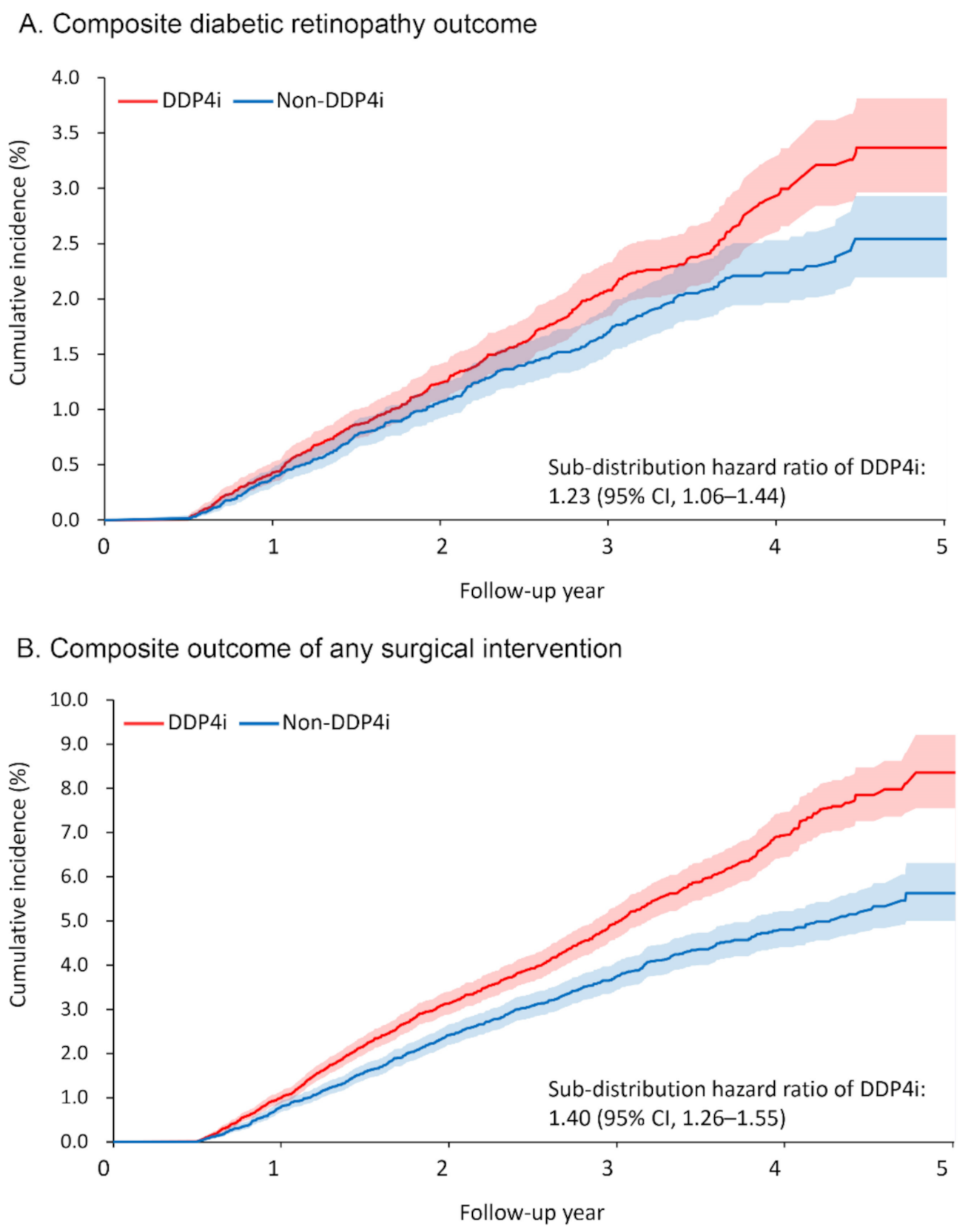
Table 2 presents the primary ocular outcomes of the patients, including any surgical intervention taken. Over a mean follow-up duration of 2.5 years, 366 and 294 patients (1.8% and 1.4%, respectively) in the DPP4i and non-DPP4i groups developed the primary ocular outcome, namely the composite DR outcome. The risk of developing the composite DR outcome was significantly higher in the DPP4i group (sub-distribution hazard ratio [SHR] 1.23, 95% confidence interval [CI] 1.06–1.44; Figure 2A). Among the individual components of the composite DR outcome, the risks of VH (SHR 1.24, 95% CI 1.05–1.48) and macular edema (SHR 1.48, 95% CI 1.03–2.13) were significantly higher in the DPP4i group.

Table 2.
Primary ocular outcomes, including any surgical intervention taken, of patients with type 2 diabetes and diabetic retinopathy demonstrating significantly higher risks of composite diabetic retinopathy and surgical interventions in the DPP4i group.
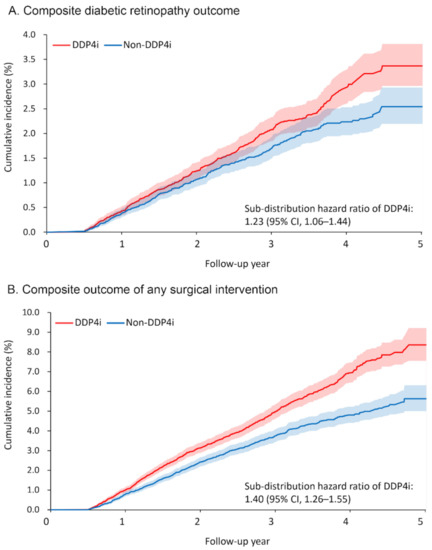
Figure 2.
Cumulative incidence function of (A) composite diabetic retinopathy outcome and (B) composite outcome of any surgical intervention between the DPP4i and non-DPP4i group after propensity score matching. DPP4i, dipeptidyl peptidase 4 inhibitor; CI, confidence interval.
The DPP4i group also had a higher risk of receiving surgical intervention for severe DR or its complications (retinal laser therapy: SHR 1.75, 95% CI 1.33–2.30; IVI: SHR 1.32, 95% CI 1.001–1.74; vitrectomy: SHR 1.32, 95% CI 1.24–1.40; any surgical intervention: SHR 1.40, 95% CI 1.26–1.55; Figure 2B). As for the number of interventions, more patients in the DPP4i group received retinal laser therapy (rate ratio (RR) 1.39, 95% CI 1.23–1.58) and IVI (RR 1.84, 95% CI 1.28–2.63) than in the non-DPP4i group.
3.4. Safety Outcomes
The results of the safety outcomes are shown in Table 3. No between-group differences were observed in any of the safety outcomes, namely myocardial infarction, hospitalization for heart failure, ischemic stroke, hemorrhagic stroke, and the composite outcome of major adverse cardiovascular events.

Table 3.
Safety outcomes of patients with type 2 diabetes and diabetic retinopathy showing no significant risk in both groups.
3.5. Subgroup Analysis
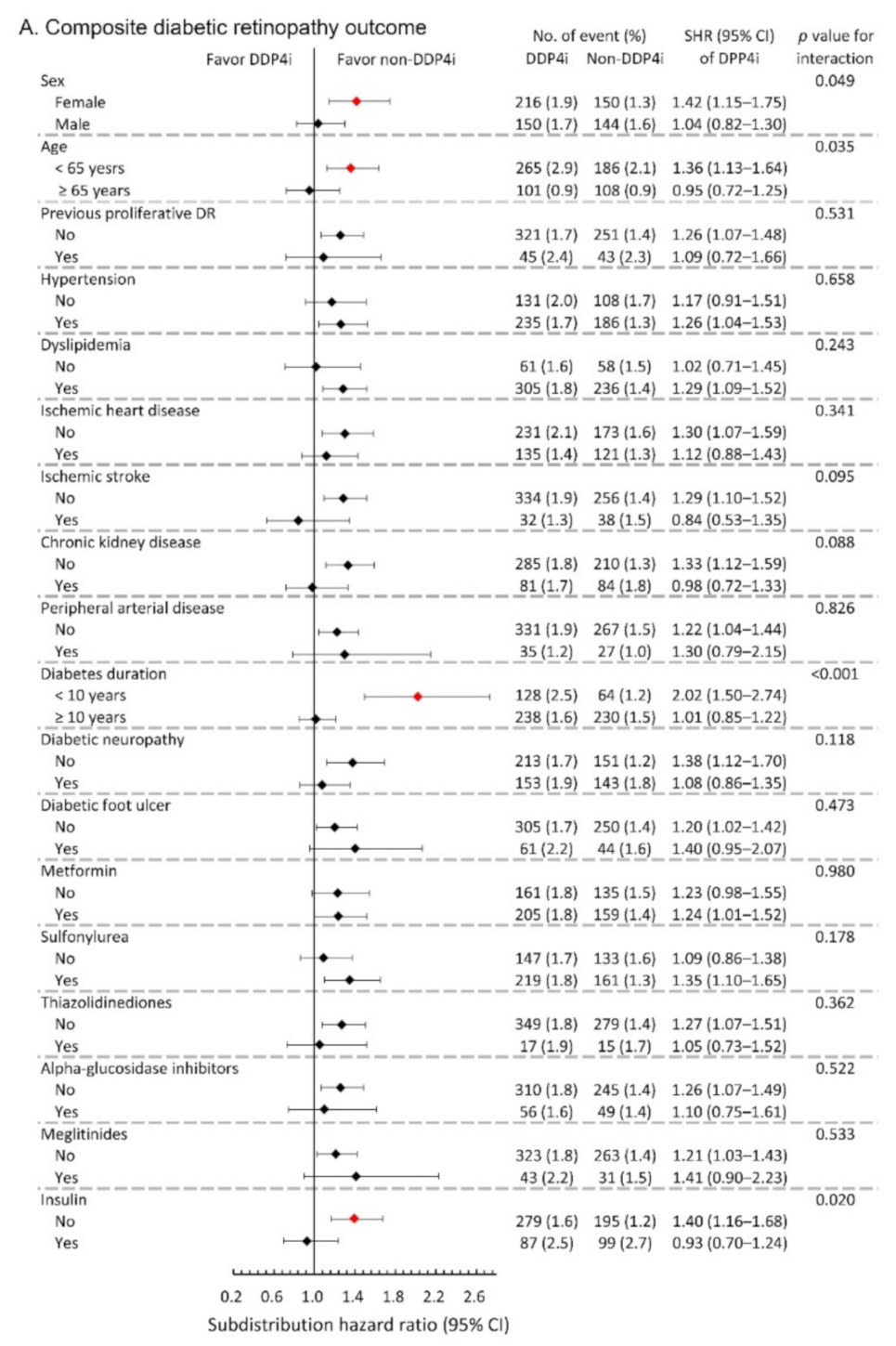
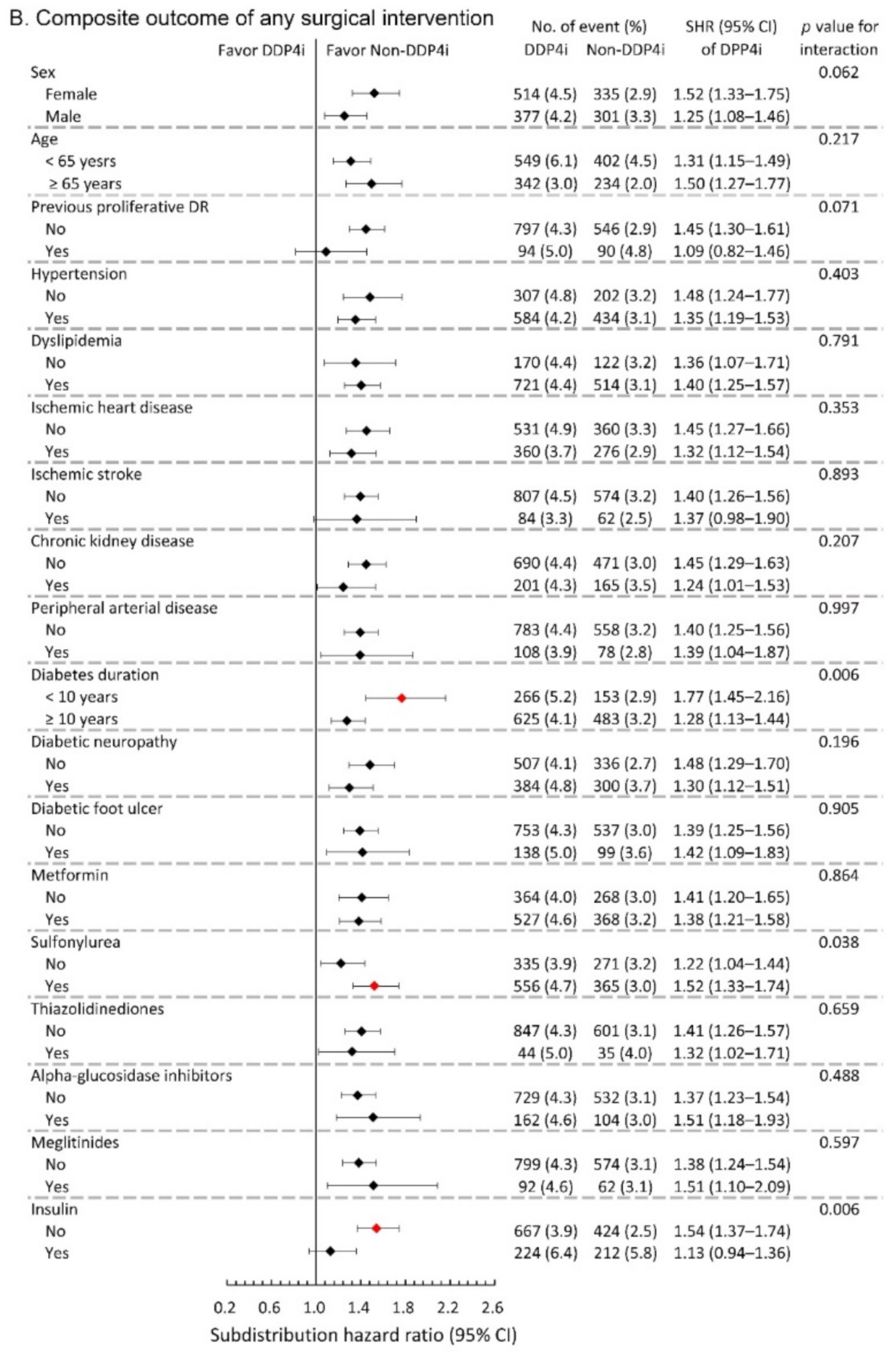
We further conducted subgroup analysis on the primary composite DR outcome and the composite outcome of any surgical interventions. The results showed that the observed hazardous effect of DPP4i on the risk of primary composite DR outcome was particularly obvious in the following population: females, younger patients, patients with relatively shorter diabetes duration, and those without taking insulin (All p-values for interaction <0.05; Figure 3A). Similarly, the observed increased risk of the composite outcome of any surgical interventions due to DPP4i was more apparent in patients with relatively shorter diabetes duration, and those who took sulfonylurea, and those without insulin therapy (All p-values for interaction <0.05; Figure 3B).
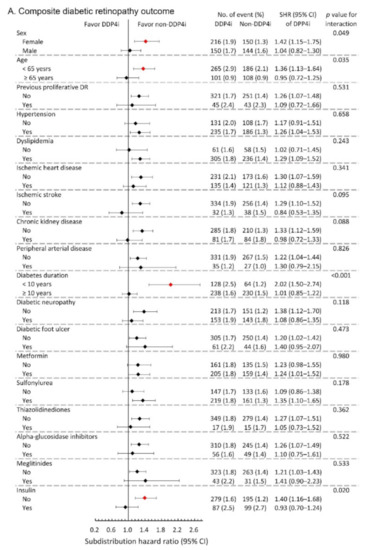
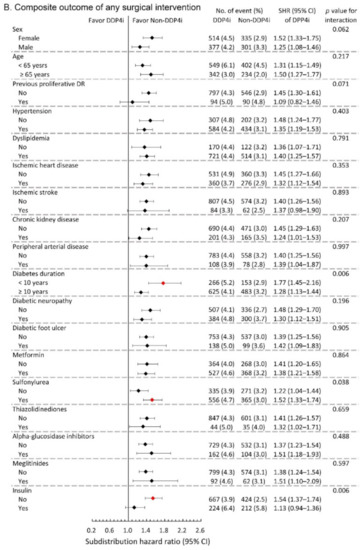
Figure 3.
Subgroup analysis of (A) composite diabetic retinopathy outcomes and (B) composite outcome of any surgical interventions of the diabetic patients with DR between the DPP4i users and non-DPP4i controls in the propensity score-matched cohort. The red color indicates a statistical significance.
4. Discussion
The use of DPP4i in glucose-lowering for diabetes has increased considerably over the past decade after being introduced in 2006 [31]. To reduce mortality and morbidity, measuring drugs’ protective effects and related diabetes complications are essential. As mentioned, DR, a major microvascular complication in diabetes, can cause severe visual impairment. Thus, in this population-based study, we evaluated the association between the add-on DPP4i therapy and the progression of preexisting DR in patients with type 2 diabetes aged ≥ 40 years. During the 2.5-year follow-up, the add-on DPP4i therapy was associated with increased risks of composite DR outcome and needs of surgical interventions. However, it did not increase the risk of cardiovascular events.
The association between DPP4i and DR remains a matter of contention in the literature. A study including 82 patients with type 2 diabetes reported that DPP4i use had protective effects on DR progression [18]. A study using a cohort representative of individuals in the US population aged ≥ 65 years observed that DPP4i use had a neutral effect on DR [32]. Some other studies have found that DPP4i cause adverse retinal outcomes. In the Trial Evaluating Cardiovascular Outcomes With Sitagliptin (TECOS), DR occurred more frequently in patients under add-on sitagliptin therapy than in those who were not (2.8% vs. 2.2%) [33]. Another study, using a sample representative of the South Korean population, also indicated an increased risk of DR in early DPP4i treatment (<12 months) [19]. These findings indicate that the pharmacodynamic or effects of DPP4i may vary with population or patient characteristics.
The non-DPP4i and DPP4i groups in the present study comprised 20,444 patients (after matching) with type 2 diabetes (mean duration of 11 years since onset) and preexisting DR, respectively. VH and macular edema occurred significantly more frequently in the DPP4i group than in the non-DPP4i group. Furthermore, patients in the DPP4i group under add-on DPP4i therapy for diabetes control were more likely to receive surgical intervention for advanced DR. In short, add-on DPP4i therapy increased the risk of DR progression. However, no significant between-group differences in safety outcomes were noted. In addition, DPP4i was not associated with an increased risk of cardiovascular events.
Although the exact mechanism remains uncertain, biochemical changes in retinal cells after DPP4i administration in experimental studies have been inconsistent. Numerous laboratory studies have reported the protective effects of DPP4i on retinal health. For example, Gonçalves et al. found that sitagliptin had an antioxidative effect on rat retinas [34]. In another of their studies, sitagliptin ameliorated bovine retinal endothelial dysfunction caused by inflammation [15]. Another study noted that linagliptin had anti-angiogenic effects on mice with oxygen-induced retinopathy [35]. However, Lee et al. indicated that DPP4i caused disruptions in endothelial cell-to-cell junctions by accumulating stromal cell-derived factor 1α and phosphorylating vascular endothelial cadherin, as well as further increasing retinal vascular permeability [36]. In a 2020 experimental study, the results revealed that prolonged DPP4 inhibition destabilized the blood-retina barrier, potentially inducing retinal edema [37]. Early deterioration of DR was also reported in a GLP-1 analog, semaglutide, although the pharmacodynamic may be different with the DPP4i [38]. Retinal changes under DPP4i therapy may depend on the duration of DPP4i treatment and the severity of diabetes and its complications. Long-term administration of DPP4i in patients with preexisting DR might induce the development of excess vasculature as well as vascular permeability, potentially contributing to exudate production and further exacerbating DR. Thus, more awareness of DR progression may be necessary for patients under long-term DPP4i treatment.
Cardiovascular complications of DPP4i remain the topic of an ongoing debate. Some studies have reported a decreased risk of cardiovascular events after DPP4i therapy [39,40]. By contrast, other studies have indicated that DPP4i use increased the risk of cardiovascular disorders [17,41]. In our study, the safety outcomes (including myocardial infarction, heart failure, ischemic stroke, hemorrhagic stroke, and composite cardiovascular outcomes) did not differ significantly between the groups. This is consistent with the assessment from the TECOS [33], the Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care, and the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus (SAVOR)—Thrombolysis in Myocardial Infarction (TIMI) [12]. Thus, our additional finding also supported a neutral association between DPP4i use and the occurrence of major adverse cardiovascular events.
A limited number of large-scale clinical studies have evaluated the association of DPP4i and the progression of retinopathy in patients with diabetes. The strength of the present study is that, to the best of our knowledge, it is the first observational investigation of the association of an add-on DPP4i in DR progression in a population-based cohort. By systemically assessing the possible confounding factors and making adjustments through PSM, we minimized detection bias and balanced the clinical characteristics between the groups. The approximately 2.5-year follow-up also means that the present findings demonstrate the long-term impacts—as opposed to the short-term effects—of DPP4i use. The potential harm that may accompany DPP4i use indicated in the present study raises substantial concerns regarding its safe use as an antidiabetic.
This study has some limitations. First, because only patients older than 40 years were included, the present findings cannot be extrapolated to other age groups. Moreover, because the patients were all Taiwanese, it remains unclear whether our findings are generalizable to other populations. Second, we could not completely prevent confounding effects. Nevertheless, we performed matching by systematically considering various variables, minimizing any imbalance between the groups. Third, we could not obtain information on the patients’ diabetes control, as well as the hypoglycemic events, which are important factors of diabetes management. Nevertheless, we have matched the patients in the two groups based on their hypoglycemic agent use. Fourth, data on laboratory tests, such as the serum glucose level or hemoglobin A1c, are not available in the NHIRD. Rapid reduction of hemoglobin A1c may affect early worsening of DR [8]. However, this phenomenon should be counterbalanced in a long-term observation in the patients with better glucose control, which has been reported in the Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes (SUSTAIN) study [38]. In our study, the follow-up period of 2.5 years is comparable with the previous study, and our case number (n = 20,444 in both study and control groups) is higher than the SUSTAIN study (n = 8105 across the SUSTAIN 1 to 6 studies) [38]. Whether the DR progression in the add-on DPP4i use is related to rapid hypoglycemic response needs further study. Fifth, the between-patient variation in diabetes severity (with some patients in severe condition) means that the alleviation of systemic disorders with medications remains challenging. The blood pressure change in our study was also not available. Nevertheless, we have matched the groups according to their disease duration, complications, medications, and underlying conditions. Therefore, the clinical characteristics of patients in the two groups were comparable at least in theory. Last, the database did not contain the results of ocular exams including optical coherence tomography, which is essential to differentiate the involvement of diabetic macular edema. The association of DPP4i and the involvement of diabetic macular edema may need further investigations. A prospective randomized trial may be required for understanding the possible effect of add-on DPP4i therapy in the progression of DR in patients with type 2 diabetes.
5. Conclusions
In conclusion, add-on DPP4i therapy may be associated with the progression of preexisting DR in patients with type 2 diabetes aged ≥40 years, but the cause and effect need further research DPP4i therapy did not increase the risk of cardiovascular events. Therefore, when choosing hypoglycemic treatments for patients with diabetes and preexisting DR, the possible promoting effect of DPP4i on DR progression should be considered. A close retinal evaluation may be necessary for long-term DPP4i administration.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jcm10132871/s1, Table S1: ICD-9 CM diagnostic codes used in the study.
Author Contributions
Y.-S.H. and T.-H.C. have full access to the data and takes overall responsibility. Conception and design: E.Y.-C.K., T.-H.C. and Y.-S.H.; Data collection and collation: C.K., W.-C.W. and C.-C.S.; Data analysis and interpretation: K.-J.C. and C.-C.L.; Writing: E.Y.-C.K. and C.K. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the Ministry of Science and Technology, Taiwan (MOST 106-2314-B-182A-045 -MY3), and Chang Gung Memorial Hospital, Taiwan (BMRPF29). The sponsor had no role in the design or conduct of this research.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Chang Gung Memorial Hospital, Taiwan (No. 201800199B1).
Informed Consent Statement
Patient consent was waived due to the de-identified database.
Data Availability Statement
The data used for the current study cannot be made publicly available according to the NHIRD regulations of personal data protection, allowing only the person responsible for the data management to approach the data after approval from Taiwan NHI bureau.
Acknowledgments
We thank two biostatisticians, Alfred Hsing-Fen Lin and Ben Yu-Lin Chou, for their valuable assistance with the statistical analysis in the present study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Klein, B.E. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 2007, 14, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.W.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef]
- Antonetti, D.A.; Klein, R.; Gardner, T.W. Diabetic retinopathy. N. Engl. J. Med. 2012, 366, 1227–1239. [Google Scholar] [CrossRef] [Green Version]
- International Diabetes Federation. IDF Diabetes Atlas, 8th ed.; International Diabetes Federation: Brussels, Belgium, 2019; Available online: http://www.diabetesatlas.org/ (accessed on 13 January 2021).
- Gerstein, H.C.; Miller, M.E.; Genuth, S.; Ismail-Beigi, F.; Buse, J.B.; Goff, D.C., Jr.; Probstfield, J.L.; Cushman, W.C.; Ginsberg, H.N.; Bigger, J.T.; et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N. Engl. J. Med. 2011, 364, 818–828. [Google Scholar] [CrossRef] [Green Version]
- Chew, E.Y.; Ambrosius, W.T.; Davis, M.D.; Danis, R.P.; Gangaputra, S.; Greven, C.M.; Hubbard, L.; Esser, B.A.; Lovato, J.F.; Perdue, L.H.; et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N. Engl. J. Med. 2010, 363, 233–244. [Google Scholar] [CrossRef] [Green Version]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998, 352, 837–853. [Google Scholar] [CrossRef]
- The, U.S. Food and Drug Administration. Drug Approval Package; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2006; Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/021995s000TOC.cfm (accessed on 13 January 2021).
- Baetta, R.; Corsini, A. Pharmacology of dipeptidyl peptidase-4 inhibitors: Similarities and differences. Drugs 2011, 71, 1441–1467. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, L.; Yu, L.; Yang, J. Head-to-Head Comparison of the Hypoglycemic Efficacy and Safety Between Dipeptidyl Peptidase-4 Inhibitors and alpha-Glucosidase Inhibitors in Patients With Type 2 Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 2019, 10, 777. [Google Scholar] [CrossRef]
- Scirica, B.M.; Bhatt, D.L.; Braunwald, E.; Steg, P.G.; Davidson, J.; Hirshberg, B.; Ohman, P.; Frederich, R.; Wiviott, S.D.; Hoffman, E.B.; et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N. Engl. J. Med. 2013, 369, 1317–1326. [Google Scholar] [CrossRef] [Green Version]
- Zannad, F.; Cannon, C.P.; Cushman, W.C.; Bakris, G.L.; Menon, V.; Perez, A.T.; Fleck, P.R.; Mehta, C.R.; Kupfer, S.; Wilson, C.; et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: A multicentre, randomised, double-blind trial. Lancet 2015, 385, 2067–2076. [Google Scholar] [CrossRef]
- Zheng, S.L.; Roddick, A.J.; Aghar-Jaffar, R.; Shun-Shin, M.J.; Francis, D.; Oliver, N.; Meeran, K. Association Between Use of Sodium-Glucose Cotransporter 2 Inhibitors, Glucagon-like Peptide 1 Agonists, and Dipeptidyl Peptidase 4 Inhibitors With All-Cause Mortality in Patients With Type 2 Diabetes: A Systematic Review and Meta-analysis. JAMA 2018, 319, 1580–1591. [Google Scholar] [CrossRef]
- Gonçalves, A.; Leal, E.; Paiva, A.; Teixeira Lemos, E.; Teixeira, F.; Ribeiro, C.F.; Reis, F.; Ambrosio, A.F.; Fernandes, R. Protective effects of the dipeptidyl peptidase IV inhibitor sitagliptin in the blood-retinal barrier in a type 2 diabetes animal model. Diabetes Obes. Metab. 2012, 14, 454–463. [Google Scholar] [CrossRef]
- Avogaro, A.; Fadini, G.P. The effects of dipeptidyl peptidase-4 inhibition on microvascular diabetes complications. Diabetes Care 2014, 37, 2884–2894. [Google Scholar] [CrossRef] [Green Version]
- Rehman, M.B.; Tudrej, B.V.; Soustre, J.; Buisson, M.; Archambault, P.; Pouchain, D.; Vaillant-Roussel, H.; Gueyffier, F.; Faillie, J.L.; Perault-Pochat, M.C.; et al. Efficacy and safety of DPP-4 inhibitors in patients with type 2 diabetes: Meta-analysis of placebo-controlled randomized clinical trials. Diabetes Obes. Metab. 2017, 43, 48–58. [Google Scholar] [CrossRef]
- Chung, Y.R.; Park, S.W.; Kim, J.W.; Kim, J.H.; Lee, K. Protective Effects of Dipeptidyl peptidase-4 inhibitors on Progression of Diabetic Retinopathy in Patient with Type 2 Diabetes. Retina 2016, 36, 2357–2363. [Google Scholar] [CrossRef]
- Kim, N.H.; Choi, J.; Kim, N.H.; Choi, K.M.; Baik, S.H.; Lee, J.; Kim, S.G. Dipeptidyl peptidase-4 inhibitor use and risk of diabetic retinopathy: A population-based study. Diabetes Obes. Metab. 2018, 44, 361–367. [Google Scholar] [CrossRef]
- Chung, Y.R.; Ha, K.H.; Kim, H.C.; Park, S.J.; Lee, K.; Kim, D.J. Dipeptidyl Peptidase-4 Inhibitors versus Other Antidiabetic Drugs Added to Metformin Monotherapy in Diabetic Retinopathy Progression: A Real World-Based Cohort Study. Diabetes Metab. J. 2019. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Su, C.C.; Shao, S.C.; Sung, S.F.; Lin, S.J.; Kao Yang, Y.H.; Lai, E.C. Taiwan’s National Health Insurance Research Database: Past and future. Clin. Epidemiol. 2019, 11, 349–358. [Google Scholar] [CrossRef] [Green Version]
- Hsing, A.W.; Ioannidis, J.P. Nationwide Population Science: Lessons From the Taiwan National Health Insurance Research Database. JAMA Intern. Med. 2015, 175, 1527–1529. [Google Scholar] [CrossRef]
- Lin, C.C.; Lai, M.S.; Syu, C.Y.; Chang, S.C.; Tseng, F.Y. Accuracy of diabetes diagnosis in health insurance claims data in Taiwan. J. Med. Assoc. 2005, 104, 157–163. [Google Scholar]
- Wu, C.S.; Lai, M.S.; Gau, S.S.; Wang, S.C.; Tsai, H.J. Concordance between patient self-reports and claims data on clinical diagnoses, medication use, and health system utilization in Taiwan. PLoS ONE 2014, 9, e112257. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Rahme, E.; Abrahamowicz, M.; Pilote, L. Survival bias associated with time-to-treatment initiation in drug effectiveness evaluation: A comparison of methods. Am. J. Epidemiol. 2005, 162, 1016–1023. [Google Scholar] [CrossRef] [Green Version]
- Kang, E.Y.; Chen, T.H.; Garg, S.J.; Sun, C.C.; Kang, J.H.; Wu, W.C.; Hung, M.J.; Lai, C.C.; Cherng, W.J.; Hwang, Y.S. Association of Statin Therapy With Prevention of Vision-Threatening Diabetic Retinopathy. JAMA Ophthal. 2019, 137, 363–371. [Google Scholar] [CrossRef]
- Cheng, C.L.; Chien, H.C.; Lee, C.H.; Lin, S.J.; Yang, Y.H. Validity of in-hospital mortality data among patients with acute myocardial infarction or stroke in National Health Insurance Research Database in Taiwan. Int. J. Cardiol. 2015, 201, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-L.; Lee, C.-H.; Chen, P.-S.; Li, Y.-H.; Lin, S.-J.; Yang, Y.-H.K. Validation of Acute Myocardial Infarction Cases in the National Health Insurance Research Database in Taiwan. J. Epidemiol. 2014, 24, 500–507. [Google Scholar] [CrossRef] [Green Version]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Austin, P.C.; Fine, J.P. Propensity-score matching with competing risks in survival analysis. Stat. Med. 2019, 38, 751–777. [Google Scholar] [CrossRef] [Green Version]
- Gallwitz, B. Clinical Use of DPP-4 Inhibitors. Front. Endocrinol. 2019, 10, 389. [Google Scholar] [CrossRef]
- Wang, T.; Hong, J.L.; Gower, E.W.; Pate, V.; Garg, S.; Buse, J.B.; Stürmer, T. Incretin-Based Therapies and Diabetic Retinopathy: Real-World Evidence in Older U.S. Adults. Diabetes Care 2018, 41, 1998–2009. [Google Scholar] [CrossRef] [Green Version]
- Green, J.B.; Bethel, M.A.; Armstrong, P.W.; Buse, J.B.; Engel, S.S.; Garg, J.; Josse, R.; Kaufman, K.D.; Koglin, J.; Korn, S.; et al. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2015, 373 (Suppl. 235S), 232–242. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, A.; Almeida, L.; Silva, A.P.; Fontes-Ribeiro, C.; Ambrósio, A.F.; Cristóvão, A.; Fernandes, R. The dipeptidyl peptidase-4 (DPP-4) inhibitor sitagliptin ameliorates retinal endothelial cell dysfunction triggered by inflammation. Biomed. Pharmacother. 2018, 102, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Kolibabka, M.; Dietrich, N.; Klein, T.; Hammes, H.P. Anti-angiogenic effects of the DPP-4 inhibitor linagliptin via inhibition of VEGFR signalling in the mouse model of oxygen-induced retinopathy. Diabetologia 2018, 61, 2412–2421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.S.; Kim, Y.G.; Cho, H.J.; Park, J.; Jeong, H.; Lee, S.E.; Lee, S.P.; Kang, H.J.; Kim, H.S. Dipeptidyl Peptidase-4 Inhibitor Increases Vascular Leakage in Retina through VE-cadherin Phosphorylation. Sci. Rep. 2016, 6, 29393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jäckle, A.; Ziemssen, F.; Kuhn, E.M.; Kampmeier, J.; Lang, G.K.; Lang, G.E.; Deissler, H.; Deissler, H.L. Sitagliptin and the Blood-Retina Barrier: Effects on Retinal Endothelial Cells Manifested Only after Prolonged Exposure. J. Diabetes Res. 2020, 2020, 2450781. [Google Scholar] [CrossRef] [PubMed]
- Vilsbøll, T.; Bain, S.C.; Leiter, L.A.; Lingvay, I.; Matthews, D.; Simó, R.; Helmark, I.C.; Wijayasinghe, N.; Larsen, M. Semaglutide, reduction in glycated haemoglobin and the risk of diabetic retinopathy. Diabetes Obes. Metab. 2018, 20, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Ussher, J.R.; Drucker, D.J. Cardiovascular actions of incretin-based therapies. Circ. Res. 2014, 114, 1788–1803. [Google Scholar] [CrossRef] [Green Version]
- Bae, E.J. DPP-4 inhibitors in diabetic complications: Role of DPP-4 beyond glucose control. Arch. Pharmacal. Res. 2016, 39, 1114–1128. [Google Scholar] [CrossRef]
- Li, L.; Li, S.; Deng, K.; Liu, J.; Vandvik, P.O.; Zhao, P.; Zhang, L.; Shen, J.; Bala, M.M.; Sohani, Z.N.; et al. Dipeptidyl peptidase-4 inhibitors and risk of heart failure in type 2 diabetes: Systematic review and meta-analysis of randomised and observational studies. BMJ 2016, 352, i610. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).