Effect of Upper Airway Stimulation in Patients with Obstructive Sleep Apnea (EFFECT): A Randomized Controlled Crossover Trial
Abstract
:1. Introduction
2. Materials and Methods
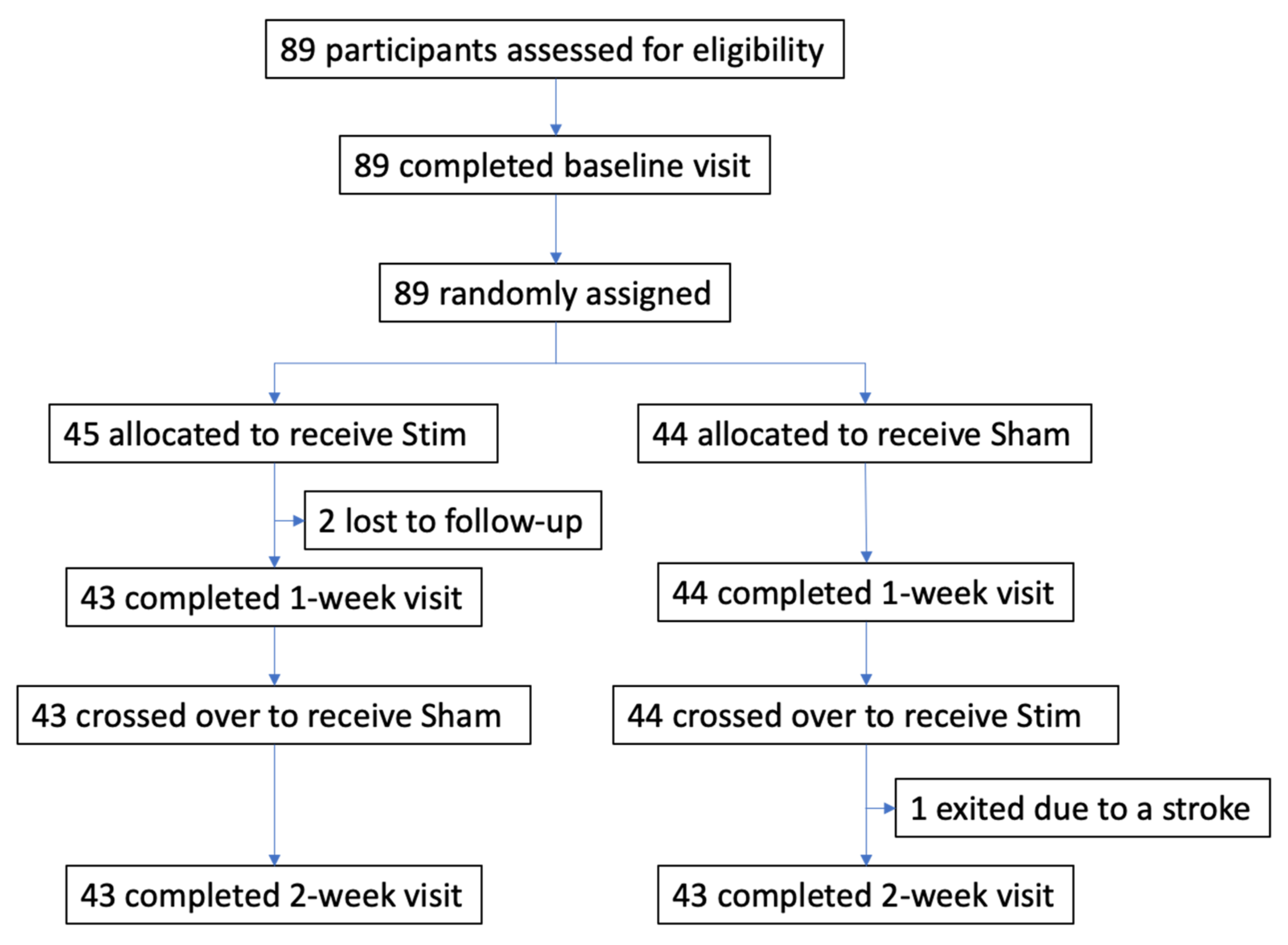
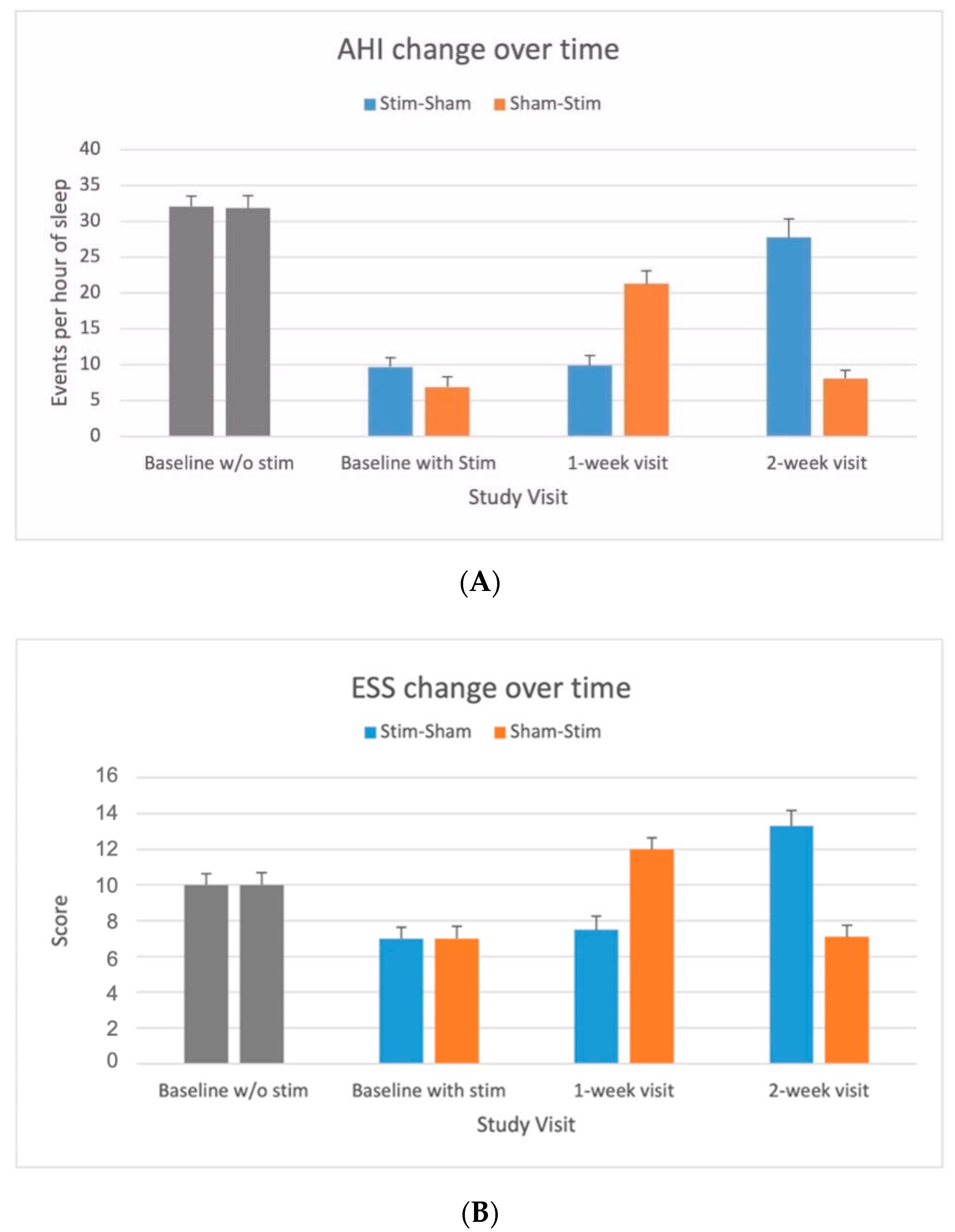
3. Results
4. Discussion
4.1. Strengths of the Study
4.2. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinzer, R.; Vat, S.; Marques-Vidal, P.; Marti-Soler, H.; Andries, D.; Tobback, N.; Mooser, V.; Preisig, M.; Malhotra, A.; Waeber, G.; et al. Prevalence of sleep-disordered breathing in the general population: The HypnoLaus study. Lancet Respir. Med. 2015, 3, 310–318. [Google Scholar] [CrossRef] [Green Version]
- Garvey, J.F.; Pengo, M.F.; Drakatos, P.; Kent, B.D. Epidemiological aspects of obstructive sleep apnea. J. Thorac. Dis. 2015, 7, 920–929. [Google Scholar] [PubMed]
- Verse, T.; Dreher, A.; Heiser, C.; Herzog, M.; Maurer, J.T.; Pirsig, W.; Rohde, K.; Rothmeier, N.; Sauter, A.; Steffen, A.; et al. ENT-specific therapy of obstructive sleep apnoea in adults: A revised version of the previously published German S2e guideline. Sleep Breath 2016, 20, 1301–1311. [Google Scholar] [CrossRef]
- Randerath, W.J.; Verbraecken, J.; Andreas, S.; Bettega, G.; Boudewyns, A.; Hamans, E.; Jalbert, F.; Paoli, J.R.; Sanner, B.; Smith, I.; et al. European Respiratory Society task force on non Ctisa. Non-CPAP therapies in obstructive sleep apnoea. Eur. Respir. J. 2011, 37, 1000–1028. [Google Scholar] [CrossRef] [Green Version]
- Heiser, C.; Hofauer, B. Hypoglossal nerve stimulation in patients with CPAP failure: Evolution of an alternative treatment for patients with obstructive sleep apnea. HNO 2017, 65, 99–106. [Google Scholar] [CrossRef]
- Schwartz, A.R.; Bennett, M.L.; Smith, P.L.; De Backer, W.; Hedner, J.; Boudewyns, A.; Van de Heyning, P.; Ejnell, H.; Hochban, W.; Knaack, L.; et al. Therapeutic Electrical Stimulation of the Hypoglossal Nerve in Obstructive Sleep Apnea. Arch. Otolaryngol. Head Neck Surg. 2001, 127, 1216–1223. [Google Scholar] [CrossRef] [Green Version]
- Heiser, C.; Steffen, A.; Randerath, W.; Penzel, T. Hypoglossal nerve stimulation in obstructive sleep apnea. Somnologie 2017, 21, 140–148. [Google Scholar] [CrossRef]
- Heiser, C.; Hofauer, B. Addressing the Tone and Synchrony Issue during Sleep: Pacing the Hypoglossal Nerve. Sleep Med. Clin. 2019, 14, 91–97. [Google Scholar] [CrossRef]
- Strollo, P.J.; Soose, R.J.; Maurer, J.T.; de Vries, N.; Cornelius, J.; Froymovich, O.; Hanson, R.D.; Padhya, T.A.; Steward, D.L.; Gillespie, M.B.; et al. Upper-Airway Stimulation for Obstructive Sleep Apnea. N. Engl. J. Med. 2014, 370, 139–149. [Google Scholar] [CrossRef] [Green Version]
- Stimulation Therapy for Apnea Reduction (STAR) Trial Group; Strollo, P.J.; Gillespie, M.B.; Soose, R.J.; Maurer, J.T.; de Vries, N.; Cornelius, J.; Hanson, R.D.; Padhya, T.A.; Steward, D.L.; et al. Upper Airway Stimulation for Obstructive Sleep Apnea: Durability of the Treatment Effect at 18 Months. Sleep 2015, 38, 1593–1598. [Google Scholar]
- Soose, R.J.; Woodson, B.T.; Gillespie, M.B.; Maurer, J.T.; de Vries, N.; Steward, D.L.; Strohl, K.P.; Baskin, J.Z.; Padhya, T.A.; Badr, M.S.; et al. Upper Airway Stimulation for Obstructive Sleep Apnea: Self-Reported Outcomes at 24 Months. J. Clin. Sleep Med. 2016, 12, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Woodson, B.T.; Soose, R.J.; Gillespie, M.B.; Strohl, K.P.; Maurer, J.T.; de Vries, N.; Steward, D.L.; Baskin, J.Z.; Badr, M.S.; Lin, H.S.; et al. Three-Year Outcomes of Cranial Nerve Stimulation for Obstructive Sleep Apnea: The STAR Trial. Otolaryngol. Head Neck Surg. 2016, 154, 181–188. [Google Scholar] [CrossRef]
- Gillespie, M.B.; Soose, R.J.; Woodson, B.T.; Strohl, K.P.; Maurer, J.T.; de Vries, N.; Steward, D.L.; Baskin, J.Z.; Badr, M.S.; Lin, H.-S.; et al. Upper Airway Stimulation for Obstructive Sleep Apnea: Patient-Reported Outcomes after 48 Months of Follow-up. Otolaryngol. Neck Surg. 2017, 156, 765–771. [Google Scholar] [CrossRef]
- Woodson, B.T.; Strohl, K.P.; Soose, R.J.; Gillespie, M.B.; Maurer, J.T.; de Vries, N.; Padhya, T.A.; Badr, M.S.; Lin, H.-S.; Vanderveken, O.M.; et al. Upper Airway Stimulation for Obstructive Sleep Apnea: 5-Year Outcomes. Otolaryngol. Neck Surg. 2018, 159, 194–202. [Google Scholar] [CrossRef] [Green Version]
- Heiser, C.; Maurer, J.T.; Hofauer, B.; Sommer, J.U.; Seitz, A.; Steffen, A. Outcomes of Upper Airway Stimulation for Obstructive Sleep Apnea in a Multicenter German Postmarket Study. Otolaryngol. Neck Surg. 2016, 156, 378–384. [Google Scholar] [CrossRef]
- Steffen, A.; Sommer, J.U.; Hofauer, B.; Maurer, J.T.; Hasselbacher, K.; Heiser, C. Outcome after one year of upper airway stimulation for obstructive sleep apnea in a multicenter German post-market study. Laryngoscope 2018, 128, 509–515. [Google Scholar] [CrossRef]
- Heiser, C.; Steffen, A.; Boon, M.; Hofauer, B.; Doghramji, K.; Maurer, J.T.; Sommer, J.U.; Soose, R.; Strollo, P.J.; Schwab, R.; et al. Post-approval upper airway stimulation predictors of treatment effectiveness in the ADHERE registry. Eur. Respir. J. 2019, 53, 1801405. [Google Scholar] [CrossRef]
- Thaler, E.; Schwab, R.; Maurer, J.; Soose, R.; Larsen, C.; Stevens, S.; Stevens, D.; Boon, M.; Huntley, C.; Doghramji, K.; et al. Results of the ADHERE upper airway stimulation registry and predictors of therapy efficacy. Laryngoscope 2020, 130, 1333–1338. [Google Scholar] [CrossRef]
- Woodson, B.T.; Gillespie, M.B.; Soose, R.J.; Maurer, J.T.; de Vries, N.; Steward, D.L.; Baskin, J.Z.; Padhya, T.A.; Lin, H.S.; Mickelson, S.; et al. Randomized controlled withdrawal study of upper airway stimulation on OSA: Short- and long-term effect. Otolaryngol. Head Neck Surg. 2014, 151, 880–887. [Google Scholar] [CrossRef]
- Sher, A.E.; Schechtman, K.B.; Piccirillo, J.F. The Efficacy of Surgical Modifications of the Upper Airway in Adults with Obstructive Sleep Apnea Syndrome. Sleep 1996, 19, 156–177. [Google Scholar] [CrossRef] [Green Version]
- Berry, R.B.; Brooks, R.; Gamaldo, C.; Harding, S.M.; Lloyd, R.M.; Quan, S.F.; Troester, M.T.; Vaughn, B.V. AASM Scoring Manual Updates for 2017 (Version 2.4). J. Clin. Sleep Med. 2017, 13, 665–666. [Google Scholar] [CrossRef]
- Weaver, T.E.; Laizner, A.M.; Evans, L.K.; Maislin, G.; Chugh, D.K.; Lyon, K.; Smith, P.L.; Schwartz, A.R.; Redline, S.; Pack, A.; et al. An Instrument to Measure Functional Status Outcomes for Disorders of Excessive Sleepiness. Sleep 1997, 20, 835–843. [Google Scholar]
- Johns, M.; Hocking, B. Daytime Sleepiness and Sleep Habits of Australian Workers. Sleep 1997, 20, 844–847. [Google Scholar] [CrossRef]
- Heiser, C.; Knopf, A.; Bas, M.; Gahleitner, C.; Hofauer, B. Selective upper airway stimulation for obstructive sleep apnea: A single center clinical experience. Eur. Arch. Oto Rhino Laryngol. 2016, 274, 1727–1734. [Google Scholar] [CrossRef]
- Steffen, A.; Sommer, U.J.; Maurer, J.T.; Abrams, N.; Hofauer, B.; Heiser, C. Long-term follow-up of the German post-market study for upper airway stimulation for obstructive sleep apnea. Sleep Breath. 2019, 24, 979–984. [Google Scholar] [CrossRef]
- Bradley, T.D.; Floras, J.S. Obstructive sleep apnoea and its cardiovascular consequences. Lancet 2009, 373, 82–93. [Google Scholar] [CrossRef]
- Zinchuk, A.; Yaggi, H.K. Sleep Apnea Heterogeneity, Phenotypes, and Cardiovascular Risk. Implications for Trial Design and Precision Sleep Medicine. Am. J. Respir. Crit. Care Med. 2019, 200, 412–413. [Google Scholar] [CrossRef]
- Penzel, T.; Kantelhardt, J.W.; Bartsch, R.; Riedl, M.; Kraemer, J.F.; Wessel, N.; Garcia, C.; Glos, M.; Fietze, I.; Schöbel, C. Modulations of Heart Rate, ECG, and Cardio-Respiratory Coupling Observed in Polysomnography. Front. Physiol. 2016, 7, 460. [Google Scholar] [CrossRef] [Green Version]
- Aurora, R.N.; Crainiceanu, C.; Gottlieb, D.J.; Kim, J.S.; Punjabi, N.M. Obstructive Sleep Apnea during REM Sleep and Cardiovascular Disease. Am. J. Respir. Crit. Care Med. 2018, 197, 653–660. [Google Scholar] [CrossRef]
- Mehra, R.; Steffen, A.; Heiser, C.; Hofauer, B.; Withrow, K.; Doghramji, K.; Boon, M.; Huntley, C.; Soose, R.J.; Stevens, S.; et al. Upper Airway Stimulation versus Untreated Comparators in Positive Airway Pressure Treatment–Refractory Obstructive Sleep Apnea. Ann. Am. Thorac. Soc. 2020, 17, 1610–1619. [Google Scholar] [CrossRef] [PubMed]
| All n = 89 | Stim–Sham n = 45 | Sham–Stim n = 44 | |
|---|---|---|---|
| Age, years | 57.5 ± 9.8 | 58.3 ± 9.4 | 56.6 ± 10.4 |
| BMI, kg/m2 | 29.2 ± 4.4 | 28.6 ± 3.7 | 29.5 ± 3.9 |
| Male sex, % | 81.0 | 82.2 | 79.5 |
| Race, % Caucasian | 100 | 100 | 100 |
| Baseline ESS | 7.0 ± 4.4 | 7.0 ± 4.2 | 7.0 ± 4.6 |
| Baseline ESS before implantation | 10.6 ± 3.8 | 10.0 ± 4.7 | 10.0 ± 4.7 |
| Baseline AHI | 8.3 ± 8.9 | 9.7 ± 8.5 | 6.9 ± 9.2 |
| Baseline AHI before implantation | 32.3 ± 11.4 | 32.1 ± 9.8 | 31.9 ± 11.4 |
| Endpoint | Treatment 1 | Treatment 2 | Difference (95% CI) p-Value |
|---|---|---|---|
| AHI ≤ 15 (ITT) | 73.3% (33/45) | 29.5% (13/44) | 43.8% (25.1, 62.5) < 0.001 |
| Endpoint | Treatment 1 | Treatment 2 |
|---|---|---|
| AHI ≤ 10 (ITT) | 51.1% (23/45) | 15.9% (7/44) |
| AHI ≤ 5 (ITT) | 35.6% (16/45) | 0.0% (0/44) |
| Parameter | Stim (n = 86) | Sham (n = 86) | Treatment Difference | p-Value |
|---|---|---|---|---|
| PSG Parameters | ||||
| AHI (events/h) | 0.6 (−1.8, 2.9) | 16.1 (13.7, 18.4) | −15.5 (−18.3, −12.8) | <0.001 |
| ODI (events/h) | 0.6 (−1.9, 3.0) | 12.7 (10.3, 15.2) | −12.2 (−14.8, −9.6) | <0.001 |
| Apnea index (events/h) | 0.5 (−1.2, 2.3) | 8.9 (7.2, 10.7) | −8.4 (−10.6, −6.2) | <0.001 |
| AHI in supine position (events/h) | 2.2 (−2.3, 6.6) | 23.8 (19.4, 28.2) | −21.6 (−27.2, −16.0) | <0.001 |
| AHI in non-supine position (events/h) | −0.1 (−3.2, 2.9) | 3.1 (0.1, 6.1) | −3.3 (−6.4, −0.1) | 0.044 |
| AHI in REM sleep (events/h) | 2.0 (−1.6, 5.6) | 17.1 (13.5, 20.6) | −15.1 (−19.7, −10.5) | <0.001 |
| AHI in non-REM sleep (events/h) | 0.0 (−2.4, 2.5) | 15.7 (13.3, 18.2) | −15.7 (−18.5, −12.8) | <0.001 |
| Central Apnea Index (events/h) | 0.1 (−0.1, 0.4) | 0.3 (0.0, 0.5) | −0.1 (−0.4, 0.1) | 0.285 |
| Mixed Apnea Index (events/h) | 0.1 (−0.3, 0.4) | 0.3 (−0.1, 0.6) | −0.2 (−0.6, 0.2) | 0.355 |
| Central Mixed Apnea Index (events/h) | −0.0 (−0.8, 0.7) | 0.4 (−0.3, 1.1) | −0.4 (−1.2, 0.4) | 0.283 |
| Hypopnea Index (events/h) | 0.0 (−1.6, 1.6) | 7.0 (5.4, 8.6) | −7.0 (−8.9, −5.1) | <0.001 |
| Minimal measured SaO2 (%) | −0.9 (−1.9, 0.2) | −4.0 (−5.0, −3.0) | 3.1 (2.1, 4.2) | <0.001 |
| Mean SaO2 (%) | −0.2 (−0.9, 0.4) | −0.5 (−1.2, 0.1) | 0.3 (−0.5, 1.1) | 0.493 |
| Total time SaO2 <90% | 2.4 (−1.7, 6.4) | 9.0 (4.9, 13.0) | −6.6 (−11.2, −2.0) | 0.005 |
| Quality of life measures | ||||
| ESS (points) | 0.2 (−0.7, 1.1) | 3.5 (2.6, 4.4) | −3.3 (−4.4, −2.2) | <0.001 |
| FOSQ (points) | 0.2 (−0.5, 0.9) | −1.9 (−2.6, −1.2) | 2.1 (1.4, 2.8) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heiser, C.; Steffen, A.; Hofauer, B.; Mehra, R.; Strollo, P.J., Jr.; Vanderveken, O.M.; Maurer, J.T. Effect of Upper Airway Stimulation in Patients with Obstructive Sleep Apnea (EFFECT): A Randomized Controlled Crossover Trial. J. Clin. Med. 2021, 10, 2880. https://doi.org/10.3390/jcm10132880
Heiser C, Steffen A, Hofauer B, Mehra R, Strollo PJ Jr., Vanderveken OM, Maurer JT. Effect of Upper Airway Stimulation in Patients with Obstructive Sleep Apnea (EFFECT): A Randomized Controlled Crossover Trial. Journal of Clinical Medicine. 2021; 10(13):2880. https://doi.org/10.3390/jcm10132880
Chicago/Turabian StyleHeiser, Clemens, Armin Steffen, Benedikt Hofauer, Reena Mehra, Patrick J. Strollo, Jr., Olivier M. Vanderveken, and Joachim T. Maurer. 2021. "Effect of Upper Airway Stimulation in Patients with Obstructive Sleep Apnea (EFFECT): A Randomized Controlled Crossover Trial" Journal of Clinical Medicine 10, no. 13: 2880. https://doi.org/10.3390/jcm10132880
APA StyleHeiser, C., Steffen, A., Hofauer, B., Mehra, R., Strollo, P. J., Jr., Vanderveken, O. M., & Maurer, J. T. (2021). Effect of Upper Airway Stimulation in Patients with Obstructive Sleep Apnea (EFFECT): A Randomized Controlled Crossover Trial. Journal of Clinical Medicine, 10(13), 2880. https://doi.org/10.3390/jcm10132880







