Body Mass Index Reduction and Selected Cardiometabolic Risk Factors in Obstructive Sleep Apnea: Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy, Inclusion and Exclusion Criteria
2.2. Data Extraction and Analysis
2.3. Statistical Approach
3. Results
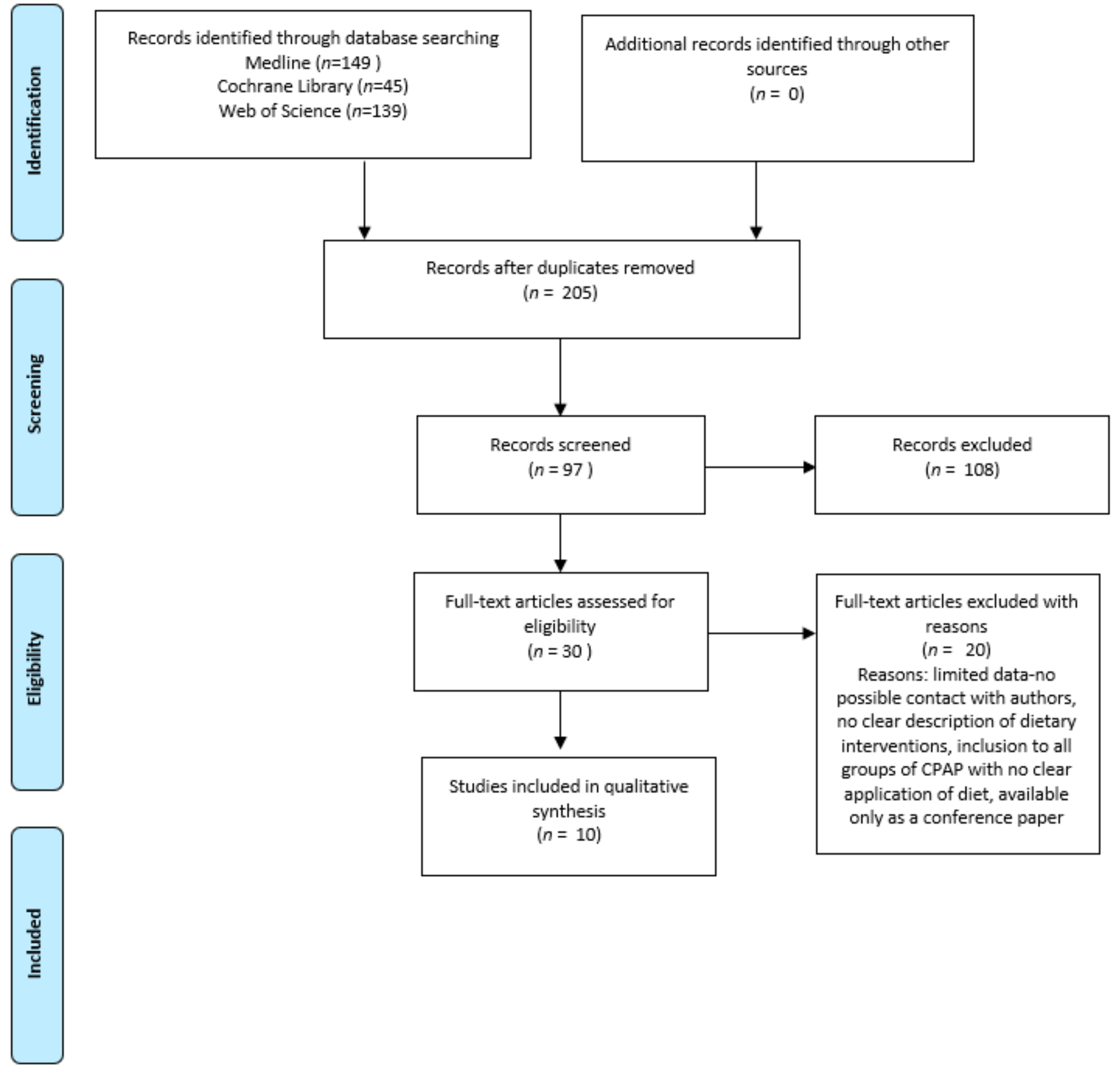
3.1. Search Results, Studies and Population Characteristics
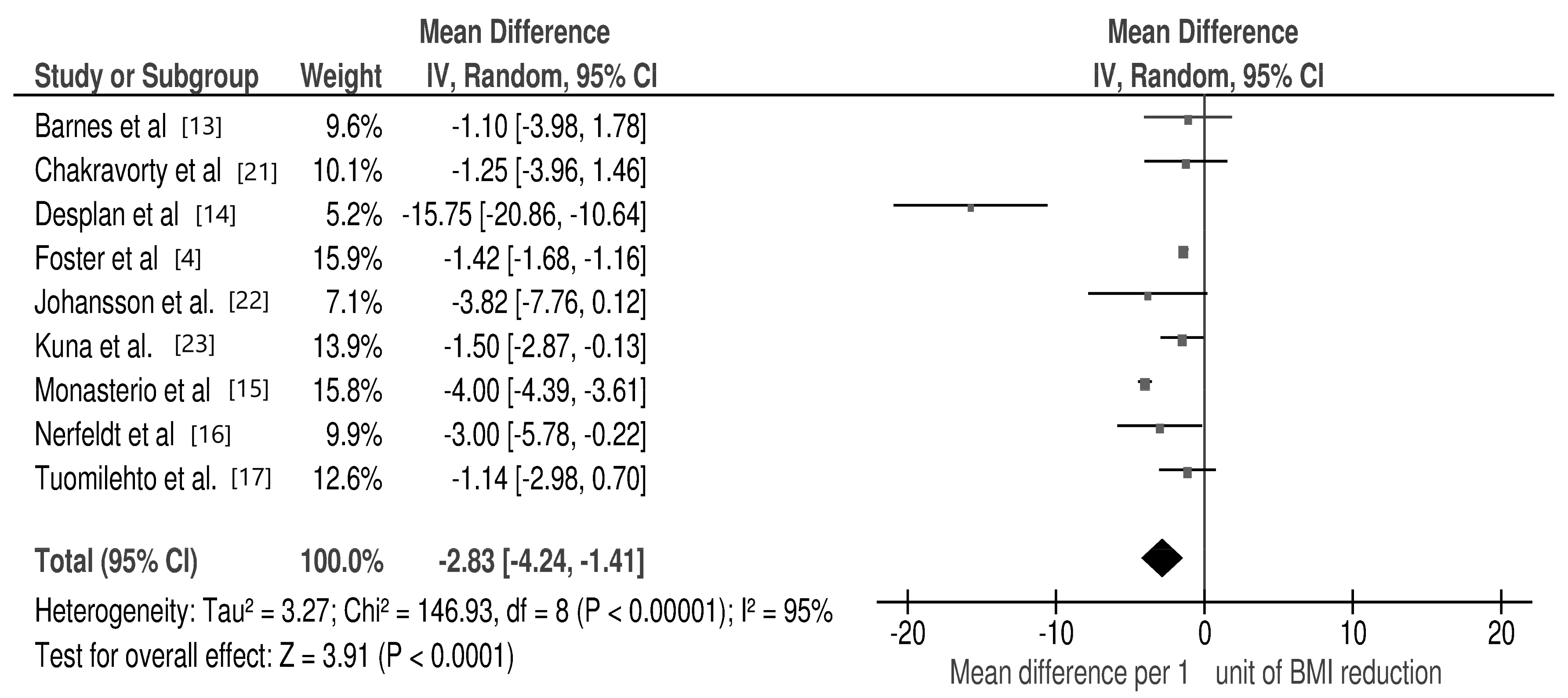
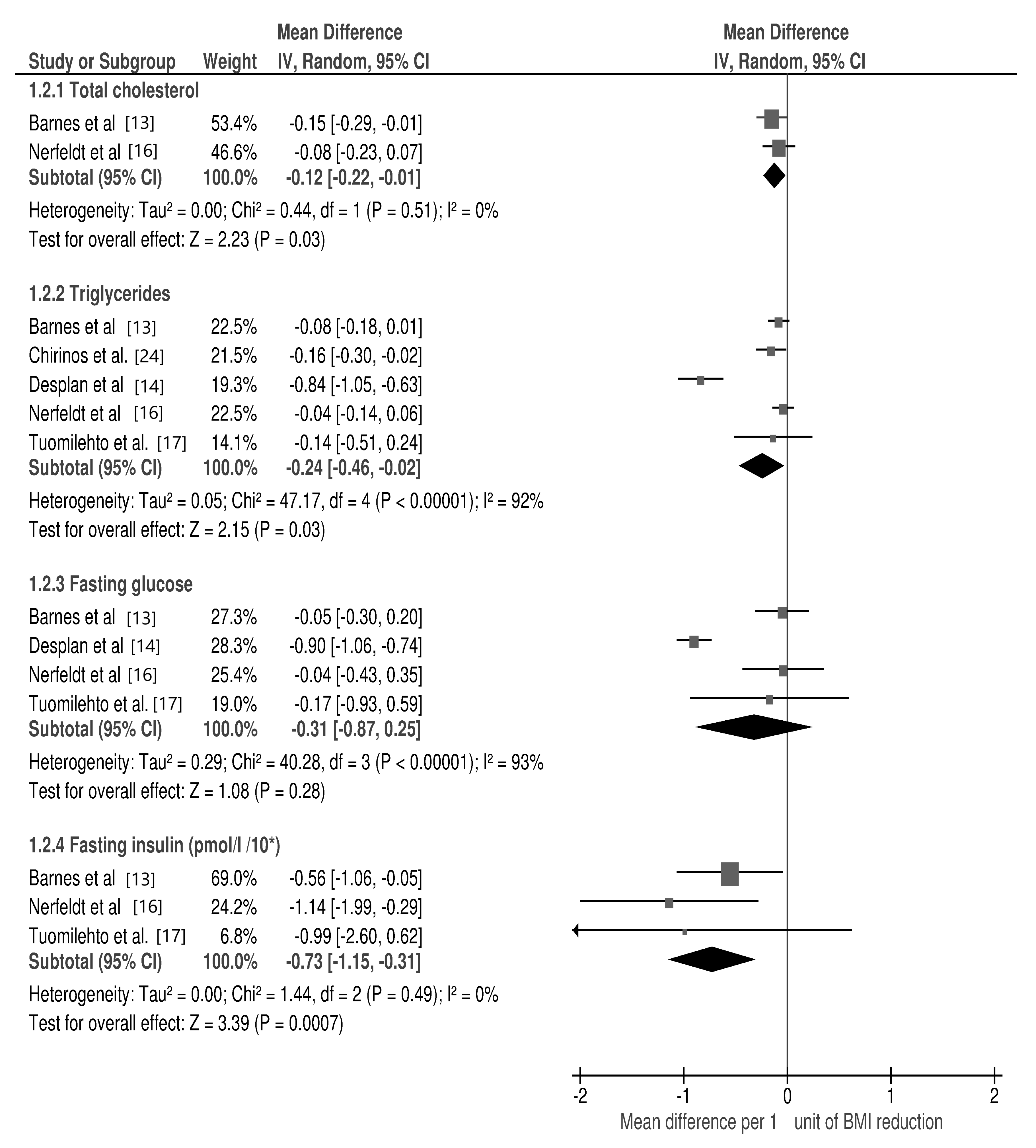
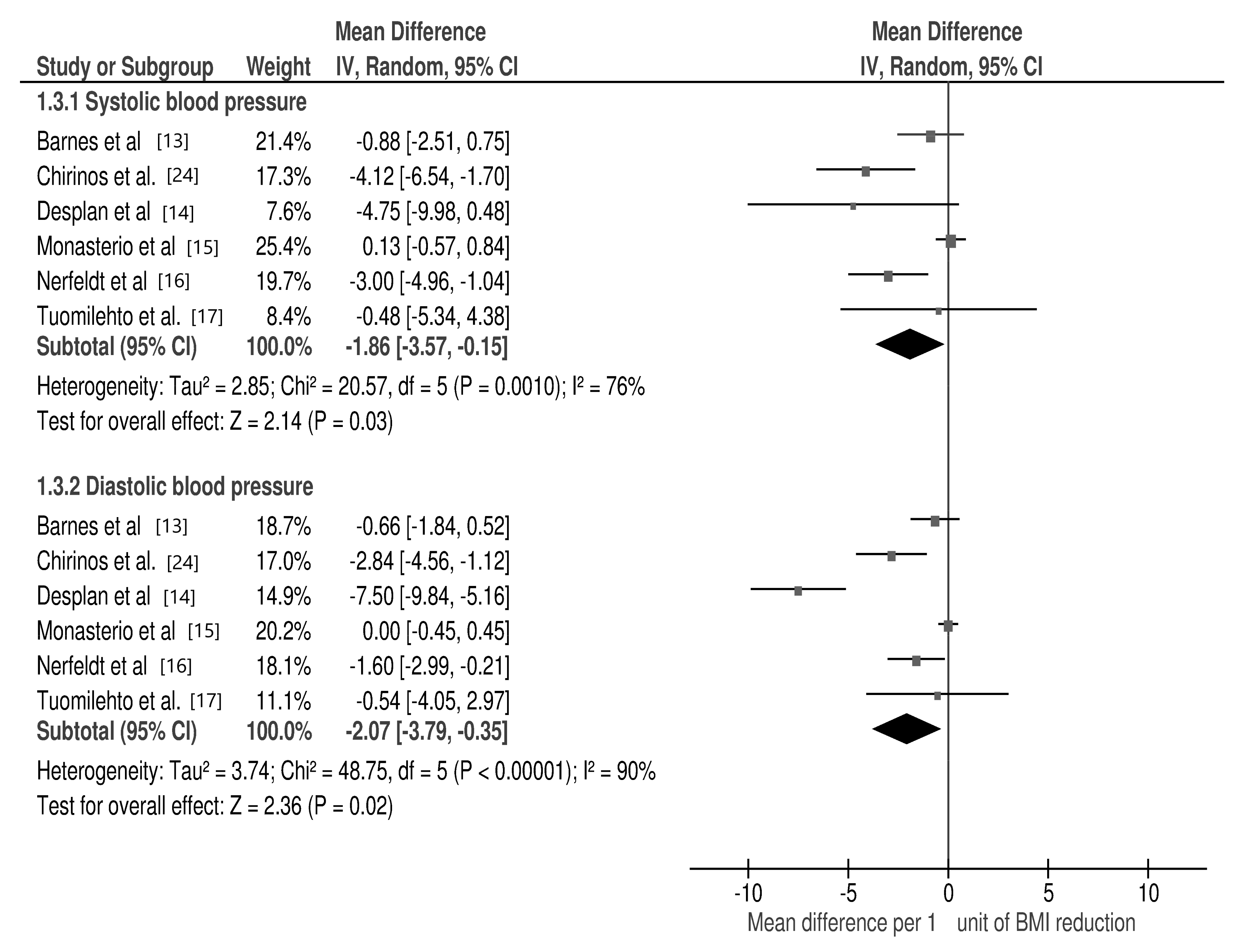
3.2. Changes in Selected Cardio-Metabolic Parameters during Lifestyle Modification in Relation to BMI Reduction
3.3. Subgroup Analyses
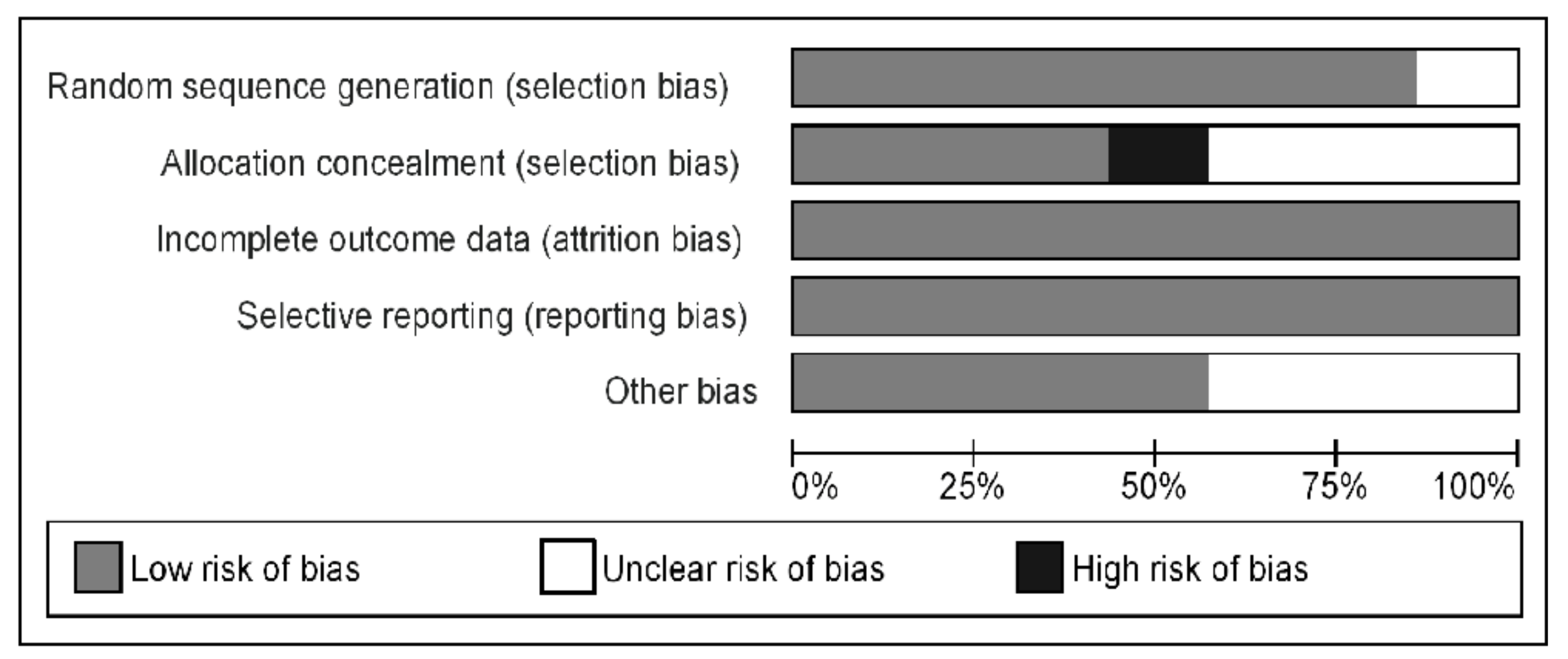
3.4. Risk of Bias and Publication Bias
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heinzer, R.; Petitpierre, N.J.; Marti-Soler, H.; Haba-Rubio, J. Prevalence and characteristics of positional sleep apnea in the HypnoLaus population-based cohort. Sleep Med. 2018, 48, 157–162. [Google Scholar] [CrossRef]
- Mirrakhimov, A.E. Obstructive sleep apnea and kidney disease: Is there any direct link? Sleep Breath. 2011, 16, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Dorasamy, P. Obstructive sleep apnea and cardiovascular risk. Ther. Clin. Risk Manag. 2007, 3, 1105–1111. [Google Scholar]
- Foster, G.D.; Borradaile, K.E.; Sanders, M.H.; Millman, R.; Zammit, G.; Newman, A.B.; Wadden, T.A.; Kelley, D.; Wing, R.R.; Kuna, S.T.; et al. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: The Sleep AHEAD study. Arch. Intern. Med. 2009, 169, 1619–1626. [Google Scholar] [CrossRef] [Green Version]
- Thomasouli, M.-A.; Brady, E.M.; Davies, M.J.; Hall, A.P.; Khunti, K.; Morris, D.H.; Gray, L.J. The impact of diet and lifestyle management strategies for obstructive sleep apnoea in adults: A systematic review and meta-analysis of randomised controlled trials. Sleep Breath. 2013, 17, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.L.; Gold, A.R.; Meyers, D.A.; Haponik, E.F.; Bleecker, E.R. Weight Loss in Mildly to Moderately Obese Patients with Obstructive Sleep Apnea. Ann. Intern. Med. 1985, 103, 850–855. [Google Scholar] [CrossRef]
- Georgoulis, M.; Yiannakouris, N.; Kechribari, I.; Lamprou, K.; Perraki, E.; Vagiakis, E.; Kontogianni, M.D. The effectiveness of a weight-loss Mediterranean diet/lifestyle intervention in the management of obstructive sleep apnea: Results of the “MIMOSA” randomized clinical trial. Clin. Nutr. 2021, 40, 850–859. [Google Scholar] [CrossRef]
- Kuna, S.T.; Reboussin, D.M.; Strotmeyer, E.S.; Millman, R.P.; Zammit, G.; Walkup, M.P.; Wadden, T.A.; Wing, R.R.; Pi-Sunyer, F.X.; Spira, A.P.; et al. Effects of Weight Loss on Obstructive Sleep Apnea Severity. Ten-Year Results of the Sleep AHEAD Study. Am. J. Respir. Crit. Care Med. 2021, 15, 221–229. [Google Scholar] [CrossRef]
- Shahar, E.; Whitney, C.W.; Redline, S.; Lee, E.T.; Newman, A.B.; Javier Nieto, F.; Boland, L.L.; Samet, J.M. Sleep-disordered breathing and cardiovascular disease: Cross-sectional results of the Sleep Heart Health Study. Am. J. Respir. Crit. Care Med. 2001, 163, 19–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, K.C.; Chow, W.-S.; Lam, J.C.; Lam, B.; Wong, W.-K.; Tam, S.; Ip, M.S. HDL dysfunction in obstructive sleep apnea. Atherosclerosis 2006, 184, 377–382. [Google Scholar] [CrossRef]
- Al-Delaimy, W.K.; Manson, J.E.; Willett, W.C.; Stampfer, M.J.; Hu, F.B. Snoring as a risk factor for type II diabetes mellitus: A prospective study. Am. J. Epidemiol. 2002, 155, 387–393. [Google Scholar] [CrossRef] [Green Version]
- Jean-Louis, G.; Zizi, F.; Clark, L.T.; Brown, C.D.; McFarlane, S.I. Obstructive Sleep Apnea and Cardiovascular Disease: Role of the Metabolic Syndrome and Its Components. J. Clin. Sleep Med. 2008, 4, 261–272. [Google Scholar] [CrossRef] [Green Version]
- Barnes, M.; Goldsworthy, U.R.; Cary, B.A.; Hill, C.J. A Diet and Exercise Program to Improve Clinical Outcomes in Patients with Obstructive Sleep Apnea—A Feasibility Study. J. Clin. Sleep Med. 2009, 5, 409–415. [Google Scholar] [CrossRef] [Green Version]
- Desplan, M.; Mercier, J.; Sabaté, M.; Ninot, G.; Prefaut, C.; Dauvilliers, Y. A comprehensive rehabilitation program improves disease severity in patients with obstructive sleep apnea syndrome: A pilot randomized controlled study. Sleep Med. 2014, 15, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Monasterio, C.; Vidal, S.; Duran, J.; Ferrer, M.; Carmona, C.; Barbé, F.; Mayos, M.; Gonzalez-Mangado, N.; Juncadella, M.; Navarro, A.; et al. Effectiveness of Continuous Positive Airway Pressure in Mild Sleep Apnea–Hypopnea Syndrome. Am. J. Respir. Crit. Care Med. 2001, 164, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Nerfeldt, P.; Nilsson, B.Y.; Mayor, L.; Uddén, J.; Friberg, D. A Two-Year Weight Reduction Program in Obese Sleep Apnea Patients. J. Clin. Sleep Med. 2010, 6, 479–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuomilehto, H.P.; Seppä, J.M.; Partinen, M.M.; Peltonen, M.; Gylling, H.; Tuomilehto, J.O.; Vanninen, E.J.; Randell, J.; Uusitupa, M.; Martikainen, T.; et al. Lifestyle intervention with weight reduction: First-line treatment in mild obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2009, 179, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and me-ta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tigwell, P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta–Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed on 7 August 2015).
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Chakravorty, I.; Cayton, R.; Szczepura, A. Health utilities in evaluating intervention in the sleep apnoea/hypopnoea syndrome. Eur. Respir. J. 2002, 20, 1233–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, K.; Hemmingsson, E.; Harlid, R.; Lagerros, Y.T.; Granath, F.; Rössner, S.; Neovius, M. Longer term effects of very low energy diet on obstructive sleep apnoea in cohort derived from randomised controlled trial: Prospective observational follow-up study. BMJ 2011, 342, d3017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuna, S.T.; Reboussin, D.M.; Borradaile, K.E.; Sanders, M.H.; Millman, R.P.; Zammit, G.; Newman, A.B.; Wadden, T.A.; Wing, R.R.; Foster, G.D.; et al. Long-term effect of weight loss on obstructive sleep apnea severity in obese patients with type 2 diabetes. Sleep 2013, 36, 641–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chirinos, J.A.; Gurubhagavatula, I.; Teff, K.; Rader, D.J.; Wadden, T.A.; Townsend, R.; Foster, G.D.; Maislin, G.; Saif, H.; Broderick, P.; et al. CPAP, Weight Loss, or Both for Obstructive Sleep Apnea. N. Engl. J. Med. 2014, 370, 2265–2275. [Google Scholar] [CrossRef] [Green Version]
- Fogelholm, M.; Kronholm, E.; Kukkonen-Harjula, K.; Partonen, T.; Partinen, M.; Härmä, M. Sleep-related disturbances and physical inactivity are independently associated with obesity in adults. Int. J. Obes. 2007, 31, 1713–1721. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.S.; Waight, C.; Doyle, G.; Rossa, K.R.; Sullivan, K.A. Liking for high fat foods in patients with Obstructive Sleep Apneea. Appetite 2014, 78, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Peppard, P.E.; Young, T.; Palta, M.; Dempsey, J.; Skatrud, J. Longitudinal Study of Moderate Weight Change and Sleep-Disordered Breathing. JAMA 2000, 284, 3015–3021. [Google Scholar] [CrossRef] [Green Version]
- Tuomilehto, H.; Gylling, H.; Peltonen, M.; Martikainen, T.; Sahlman, J.; Kokkarinen, J.; Randel, J.; Tukiainen, H.; Vannien, E.; Partinen, M.; et al. Sustained improvement in mild obstructive sleep apnea after a diet- and physical activity-based lifestyle intervention: Postinterventional follow-up. Am. J. Clin. Nutr. 2010, 92, 688–696. [Google Scholar] [PubMed]
- Stelmach-Mardas, M.; Mardas, M.; Warchoł, W.; Jamka, M.; Walkowiak, J. Successful maintenance of body weight reduction after individualized dietary counseling in obese subjects. Sci. Rep. 2014, 4, 6620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stelmach-Mardas, M.; Mardas, M.; Walkowiak, J.; Boeing, H. Long-term weight status in regainers after weight loss by lifestyle intervention: Status and challenges. Proc. Nutr. Soc. 2014, 73, 509–518. [Google Scholar] [CrossRef] [Green Version]
- Stelmach-Mardas, M.; Rodacki, T.; Dobrowolska-Iwanek, J.; Brzozowska, A.; Walkowiak, J.; Wojtanowska-Krosniak, A.; Zagrodzki, P.; Bechthold, A.; Mardas, M.; Boeing, H. Link between Food Energy Density and Body Weight Changes in Obese Adults. Nutrients 2016, 8, 229. [Google Scholar] [CrossRef]
- De Melo, C.M.; Dos Santos Quaresma, M.V.L.; Del Re, M.P.; Ribeiro, S.M.L.; Moreira Antunes, H.K.; Togeiro, S.M.; Tufik, S.; de Mello, M.T. One-month of a low-energy diet, with no additional effect of high-protein, reduces Obstructive Sleep Apnea severity and improve metabolic parameters in obese males. Clin. Nutr. ESPEN 2021, 42, 82–89. [Google Scholar] [CrossRef]
- Ng, S.S.; Chan, R.S.; Woo, J.; Chan, T.-O.; Cheung, B.H.; Sea, M.M.; To, K.-W.; Chan, K.K.; Ngai, J.; Yip, W.-H.; et al. A Randomized Controlled Study to Examine the Effect of a Lifestyle Modification Program in OSA. Chest 2015, 148, 1193–1203. [Google Scholar] [CrossRef]
- Liu, A.; Cardell, J.; Ariel, D.; Lamendola, C.; Abbasi, F.; Kim, S.H.; Holmes, T.H.; Tomasso, V.; Mojaddidi, H.; Grove, K.; et al. Abnormalities of Lipoprotein Concentrations in Obstructive Sleep Apnea Are Related to Insulin Resistance. Sleep 2015, 38, 793–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, J.-Y.; Zhang, Y.-H.; Qin, L.-Q. Obstructive sleep apnea and cardiovascular risk: Meta-analysis of prospective cohort studies. Atherosclerosis 2013, 229, 489–495. [Google Scholar] [CrossRef]
- García-Unciti, M.; Martinez, J.A.; Izquierdo, M.; Gorostiaga, E.M.; Grijalba, A.; Ibañez, J. Effect of resistance training and hypocaloric diets with different protein content on body composition and lipid profile in hypercholesterolemic obese women. Nutr. Hosp. 2013, 27, 1511–1520. [Google Scholar]
- Snel, M.; Jonker, J.T.; Hammer, S.; Kerpershoek, G.; Lamb, H.J.; Meinders, A.E.; Pijl, H.; De Roos, A.; Romijn, J.A.; Smit, J.W.; et al. Long-Term Beneficial Effect of a 16-Week Very Low Calorie Diet on Pericardial Fat in Obese Type 2 Diabetes Mellitus Patients. Obesity 2012, 20, 1572–1576. [Google Scholar] [CrossRef]
- Petersen, K.F.; Dufour, S.; Befroy, D.; Lehrke, M.; Hendler, R.E.; Shulman, G.I. Reversal of Nonalcoholic Hepatic Steatosis, Hepatic Insulin Resistance, and Hyperglycemia by Moderate Weight Reduction in Patients with Type 2 Diabetes. Diabetes 2005, 54, 603–608. [Google Scholar] [CrossRef] [Green Version]
- Moss, J.; Tew, G.A.; Copeland, R.J.; Stout, M.; Billings, C.G.; Saxton, J.M.; Winter, E.M.; Bianchi, S.M. Effects of a Pragmatic Lifestyle Intervention for Reducing Body Mass in Obese Adults with Obstructive Sleep Apnoea: A Randomised Controlled Trial. BioMed Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gabryelska, A.; Białasiewicz, P. Association between excessive daytime sleepiness, REM phenotype and severity of obstructive sleep apnea. Sci. Rep. 2020, 10, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mokros, Ł.; Kuczyński, W.; Gabryelska, A.; Franczak, Ł.; Spałka, J.; Białasiewicz, P. High Negative Predictive Value of Normal Body Mass Index for Obstructive Sleep Apnea in the Lateral Sleeping Position. J. Clin. Sleep Med. 2018, 14, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Drager, L.F.; Peker, Y.; Vgontzas, A.N.; Phillips, C.L.; Hoyos, C.M.; Salles, G.F.; Guo, M.; Li, Y. Effect of CPAP on Weight and Local Adiposity in Adults with Obstructive Sleep Apnea: A Meta-Analysis. Ann. Am. Thorac. Soc. 2021. [Google Scholar] [CrossRef] [PubMed]

| Study | Study Design | Subjects (n) § | Age (Years) Mean ± SD | % of Women | Nationality | Analyzed Groups | Intervention | Time of Intervention | BMI (kg/m2) Mean ± SD | AHI (A + H/h) Mean ± SD | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Intervention | Baseline | Intervention | |||||||||
| Barnes et al. 2009 [13] | NRS | 12 | All: 42.3 ± 10.4 | 100 | Australia | SG | VLED * | 16 wk | 36.1 ± 4.3 | 30.1 ± 4.2 | 24.6 ± 12.0 | 18.3 ± 11.9 |
| Chakravorty et al. 2002 [21] | RCT | 53 | All: 49.0 ± 11.0 | - | Great Britain | SG CG | CPAP Diet | 12 wk | 40.0 ± 14.5 32.5 ± 5.5 | 40.0 ± 12.5 31.7 ± 5.6 | 55.0 ± 28.7 35.0 ± 19.1 | 8.0 ± 28.0 34.0 ± 21.0 |
| Chirinos et al. 2014 [24] | RCT | 146 | 48.3 49.8 49.0 | 41.0 59.7 49.0 | American | SG 1 SG 2 CG | Diet CPAP Diet + CPAP | 24 wk | 38.3 ±5.5 38.2 ±7.2 37.7 ±5.5 | - | 39.7 ± 20.3 41.2 ± 20.96 47.1 ± 26.86 | - |
| Desplan et al. 2014 [14] | RCT | 22 | All: 35–70 | - | France | SG CG | Diet + EAS * EAS | 4 wk | 29.9 ± 3.4 31.3 ± 2.5 | 29.1 ± 3.1 31.3 ± 2.2 | 40.6 ± 19.439.8 ± 19.2 | 28.0 ± 19.3 45.4 ± 22.5 |
| Foster et al. 2009 [4] | RCT | 264 | All: 61.2 ± 6.5 | - | American | SG CG | Diet * Education support | 16 wk | −3.8 ± 0.3 −0.2 ± 0.3 | −5.4 ± 1.5 4.2 ± 1.4 | ||
| Johansson et al. 2011 [22] | NRS | 62 | All: 48.7± 7.3 | - | Sweden | SG | VLCD | 9 wk | −5.5 ± 2.0 | −21 ± 16 | ||
| Kuna et al. 2013 [23] | RCT | 264 | All: 61.3 ± 6.5 | 59 | American | SG CG | Diet * Education support | 16 wk | −3.8 ± 0.74 *** −0.2 ± 0.66 *** | −5.7 ± 1.5 4.0 ± 1.4 | ||
| Monasterio et al. 2001 [15] | RCT | 142 | 54.0 ±9.0 53.0 ± 9.0 | Spain | SG CG | Sleep hyg. + diet Sleep hyg. + diet + CPAP | 12 wk | 29.5 ±3.3 29.4 ±3.7 | 28.5 ±3.5 ** 29.5 ±2.8 ** | 21.0± 6.0 20.0 ± 6.0 | 17.0 ± 10.0 * 6.0 ± 8.0 * | |
| Nerfeldt et al. 2010 [16] | NRS | 33 | All: 52(31–68) | 27.2 | Sweden | SG | VLCD + behavioral support | 8-wk (observation: 24mo) | 40.0 ± 5.0 | 35.0 ± 3.0 | 43.0 ± 24.0 | 28.0 ± 19.0 |
| Tuomilehto et al. 2009 [17] | RCT | 72 | 51.8 (9.0) 50.9 (8.6) | 35 | Finland | SG CG | VLCD + lifestyle modification lifestyle modification | 12 wk (observation: 12mo) | −3.5 ± 2.1 −0.8 ± 2.0 | −4.0 ± 5.6 0.3 ± 8.0 | ||
| Study | Analyzed Groups | TC (mmol/L) Mean ± SD | TG (mmol/L) Mean ± SD | Fasting Glc (mmol/L) Mean ± SD | Fasting Insulin (pmol/L) Mean ± SD | SBP/DBP (mmHg) Mean ± SD | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Intervention | Baseline | Intervention | Baseline | Intervention | Baseline | Intervention | Baseline | Intervention | ||
| Barnes et al. 2009 [13] | SG | 5.3 ± 1.1 | 4.4 ± 1.0 | 1.5 ± 0.8 | 1.0 ± 0.6 | 6.1 ± 2.2 | 5.8 ± 1.5 | 107.6 ± 41.7 | 74.3 ± 32.6 | 125.8 ± 14.0/77.3 ± 10.6 | 120.5 ± 9.8/ 73.3 ± 6.3 |
| Chirinos et al. 2014 [24] | SG 1 SG 2 CG | - | - | −0.26 (−0.49 to −0.03) ** −0.08 (−0.27 to 0.11) ** −0.60 (−0.86 to −0.34) ** | - | - | - | −6.8 (−10.8 to −2.7)/ −4.7 (−7.7 to −1.7) ** −3 (−6.5 to 0.5)/ −3.5 (−6.1 to −0.9) ** −14.1 (−18.7 to −9.5)/ −10.6 (−14 to −7.2) ** | |||
| Desplan et al. 2014 [14] | SG CG | - | - | 1.87 ± 1.01 1.70 ± 0.53 | 1.20 ± 0.29 1.72 ± 0.81 | 5.61 (4.94–6.17) * 5.44 (5.17–5.67) * | 4.78 (4.5–5.44) * 5.17 (4.89–6.0) * | - | - | 141.8 ± 15.8/ 79.0 ± 7.1 128.1 ± 14.4/ 82.5 ± 7.1 | 145.6 ± 23.2/ 73.0 ± 10.3 128.0 ± 18.2/ 78.8 ± 9.7 |
| Monasterio et al. 2001 [15] | SG CG | - | - | - | - | - | - | - | - | 132 ± 17/84 ± 11 126 ± 17 81 ± 12 | 131 ± 16/84 ± 10 126 ± 15/81 ± 10 |
| Nerfeldt et al. 2010 [16] | SG | 5.3 ± 1.1 | 4.9 ± 1.2 | 1.8 ± 0.8 | 1.6 ± 0.7 | 7.2 ± 2.7 | 7.0 ± 3.3 | 147 ± 78 | 90 ± 52 | 144 ± 19/89 ± 14 | 129 ± 10/81 ± 6 |
| Tuomilehto et al. 2009 [17] | SG CG | - | - | −0.48 ± 1.13 −0.006 ± 0.65 | −0.6 ± 2.3 −0.4 ± 1.4 | −34.7 ± 48.6 8.33 ± 23.6 | −1.7 ± 14.7/−1.9 ± 10.6 −1.1 ± 19.6/−0.4 ± 12.6 | ||||
| Analyzed Parameter | Duration | Design | Intervention | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Short-Term | Long-Term | p | RCT | NRS | p | LC Diet | VLCD | p-Value | |
| AHI [A + H/h] | −7.24 (14.15, −0.34) | −1.87 (−3.37, −0.37) | 0.14 | −2.95 (−4.65, −1.26) | −2.44 (−4.22, −0.65) | 0.68 | −3.35 (−5.25, −1.46) | −1.81 (−3.09, −0.53) | 0.19 |
| TC (mmol/L) | - | - | - | - | - | - | - | - | - |
| TG (mmol/L) | −0.47 (−1.25, 0.31) | −0.11 (−0.19, −0.03) | 0.37 | −0.39 (−0.87, 0.10) | −0.08 (−0.18, 0.02) | 0.22 | −0.50 (−1.16, 0.17) | −0.06 (−0.13, 0.01) | 0.21 |
| fasting Glc (mmol/L) | −0.49 (−1.33, 0.35) | −0.06 (−0.30, 0.18) | 0.34 | −0.63 (−1.32, 0.06) | −0.05 (−0.26, 0.16) | 0.11 | - | - | - |
| fasting insulin (pmol/L) | - | - | - | - | - | - | - | - | - |
| SBP (mmHg) | −3.22 (−5.05, −1.38) | −1.22 (−3.05, 0.62) | 0.12 | −2.05 (−4.98, 0.89) | −1.87 (−3.94, 0.21) | 0.92 | −2.49 (−6.11, 1.14) | −1.70 (−3.29, −0.10) | 0.70 |
| DBP (mmHg) | −4.47 (−10.25, 1.31) | −0.68 (−1.41, 0.05) | 0.20 | −2.70 (−6.01, 0.60) | −0.87 (−1.63, −0.10) | 0.29 | −3.31 (−7.33, 0.71) | −1.02 (−1.89, −0.15) | 0.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stelmach-Mardas, M.; Brajer-Luftmann, B.; Kuśnierczak, M.; Batura-Gabryel, H.; Piorunek, T.; Mardas, M. Body Mass Index Reduction and Selected Cardiometabolic Risk Factors in Obstructive Sleep Apnea: Meta-Analysis. J. Clin. Med. 2021, 10, 1485. https://doi.org/10.3390/jcm10071485
Stelmach-Mardas M, Brajer-Luftmann B, Kuśnierczak M, Batura-Gabryel H, Piorunek T, Mardas M. Body Mass Index Reduction and Selected Cardiometabolic Risk Factors in Obstructive Sleep Apnea: Meta-Analysis. Journal of Clinical Medicine. 2021; 10(7):1485. https://doi.org/10.3390/jcm10071485
Chicago/Turabian StyleStelmach-Mardas, Marta, Beata Brajer-Luftmann, Marta Kuśnierczak, Halina Batura-Gabryel, Tomasz Piorunek, and Marcin Mardas. 2021. "Body Mass Index Reduction and Selected Cardiometabolic Risk Factors in Obstructive Sleep Apnea: Meta-Analysis" Journal of Clinical Medicine 10, no. 7: 1485. https://doi.org/10.3390/jcm10071485
APA StyleStelmach-Mardas, M., Brajer-Luftmann, B., Kuśnierczak, M., Batura-Gabryel, H., Piorunek, T., & Mardas, M. (2021). Body Mass Index Reduction and Selected Cardiometabolic Risk Factors in Obstructive Sleep Apnea: Meta-Analysis. Journal of Clinical Medicine, 10(7), 1485. https://doi.org/10.3390/jcm10071485






