Ultrasound Evaluation of the Rectus Femoris for Sarcopenia in Patients with Early Subacute Stroke
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Measurements
2.3. Assessment of Lean Mass and Definition of Sarcopenia
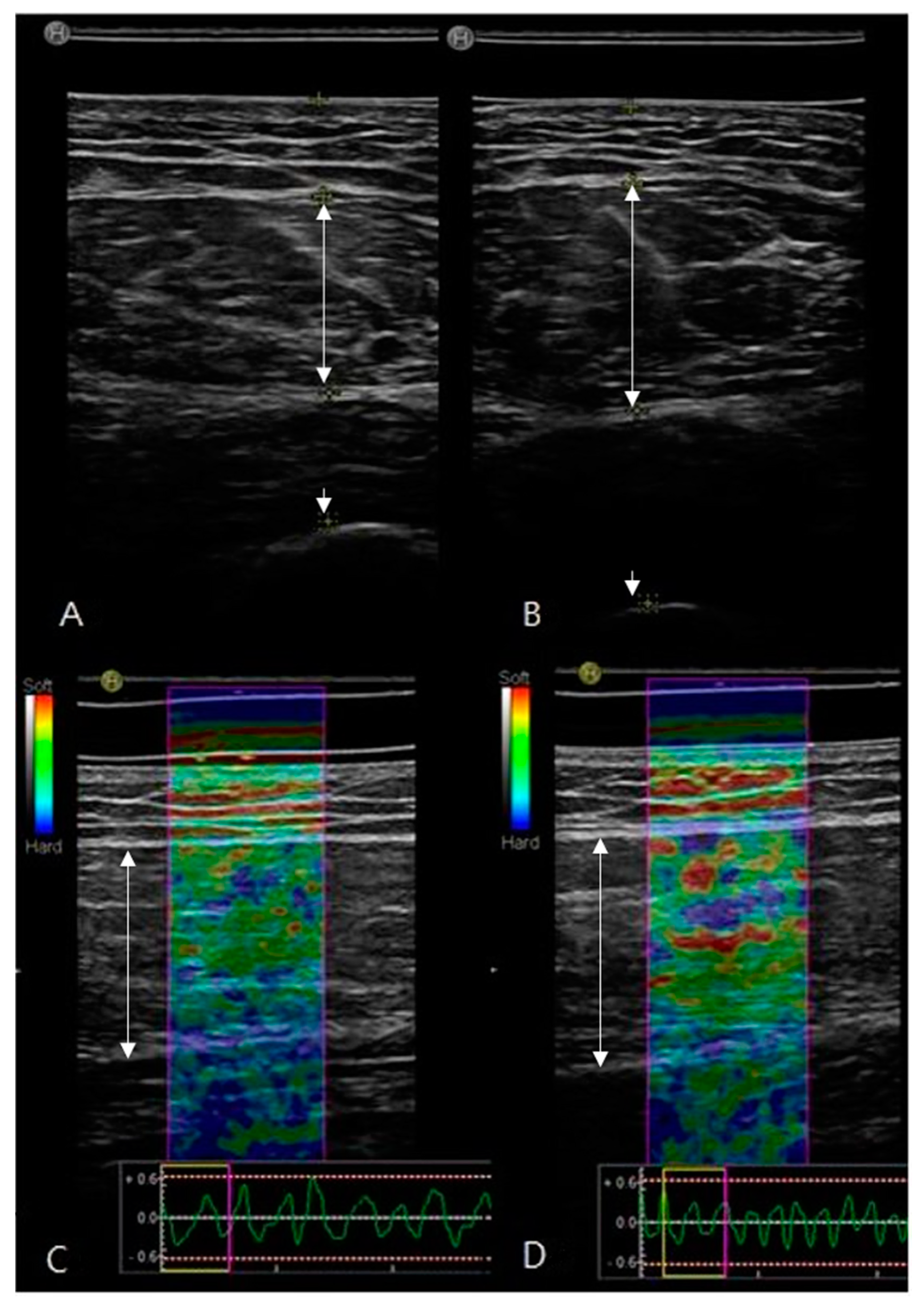
2.4. Ultrasound Image Recording and Estimation
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Arasaki, K.; Igarashi, O.; Ichikawa, Y.; Machida, T.; Shirozu, I.; Hyodo, A.; Ushijima, R. Reduction in the motor unit number estimate (MUNE) after cerebral infarction. J. Neurol. Sci. 2006, 250, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Scherbakov, N.; von Haehling, S.; Anker, S.D.; Dirnagl, U.; Doehner, W. Stroke induced Sarcopenia: Muscle wasting and disability after stroke. Int. J. Cardiol. 2013, 170, 89–94. [Google Scholar] [CrossRef]
- Shiraishi, A.; Yoshimura, Y.; Wakabayashi, H.; Tsuji, Y. Prevalence of stroke-related sarcopenia and its association with poor oral status in post-acute stroke patients: Implications for oral sarcopenia. Clin. Nutr. 2018, 37, 204–207. [Google Scholar] [CrossRef]
- Harris, M.L.; Polkey, I.M.; Bath, P.; Moxham, J. Quadriceps muscle weakness following acute hemiplegic stroke. Clin. Rehabil. 2001, 15, 274–281. [Google Scholar] [CrossRef]
- Puthucheary, Z.A.; Rawal, J.; McPhail, M.; Connolly, B.; Ratnayake, G.; Chan, P.; Hopkinson, N.; Padhke, R.; Dew, T.; Sidhu, P.; et al. Acute Skeletal Muscle Wasting in Critical Illness. JAMA 2013, 310, 1591–1600. [Google Scholar] [CrossRef] [Green Version]
- Scherbakov, N.; Doehner, W. Sarcopenia in stroke-facts and numbers on muscle loss accounting for disability after stroke. J. Cachex Sarcopenia Muscle 2011, 2, 5–8. [Google Scholar] [CrossRef] [Green Version]
- Hafer-Macko, C.E. Skeletal muscle changes after hemiparetic stroke and potential beneficial effects of exercise intervention strategies. J. Rehabil. Res. Dev. 2008, 45, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.S.; Buscemi, A.; Forrester, L.; Hafer-Macko, C.E.; Ivey, F.M. Atrophy and Intramuscular Fat in Specific Muscles of the Thigh. Neurorehabilit. Neural Repair 2011, 25, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Carlson, C.L.; Visser, M.; Kelley, D.E.; Scherzinger, A.; Harris, T.B.; Stamm, E.; Newman, A.B. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J. Appl. Physiol. 2001, 90, 2157–2165. [Google Scholar] [CrossRef] [PubMed]
- Prado-Medeiros, C.L.; Silva, M.P.; Lessi, G.C.; Alves, M.Z.; Tannus, A.; Lindquist, A.R.; Salvini, T. Muscle Atrophy and Functional Deficits of Knee Extensors and Flexors in People with Chronic Stroke. Phys. Ther. 2012, 92, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Yamaga, M.; Koga, H. Systemic inflammation and sarcopenia in recovery stage of stroke: The negative impact on functional rehabilitation outcomes. Ann. Phys. Rehabil. Med. 2018, 61, e63. [Google Scholar] [CrossRef]
- Abe, T.; Loenneke, J.P.; Thiebaud, R.S. Ultrasound assessment of hamstring muscle size using posterior thigh muscle thickness. Clin. Physiol. Funct. Imaging 2014, 36, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Alley, D.E.; Shardell, M.D.; Peters, K.W.; McLean, R.R.; Dam, T.-T.L.; Kenny, A.M.; Fragala, M.S.; Harris, T.B.; Kiel, D.; Guralnik, J.M.; et al. Grip Strength Cutpoints for the Identification of Clinically Relevant Weakness. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2014, 69, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Loenneke, J.P.; Young, K.C.; Thiebaud, R.S.; Nahar, V.K.; Hollaway, K.M.; Stover, C.D.; Ford, M.A.; Bass, M.A.; Loftin, M. Validity of Ultrasound Prediction Equations for Total and Regional Muscularity in Middle-aged and Older Men and Women. Ultrasound Med. Biol. 2015, 41, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Eng, J.J.; Chu, K.S.; Dawson, A.S.; Kim, C.; Hepburn, K.E. Functional Walk Tests in Individuals with Stroke. Stroke 2002, 33, 756–761. [Google Scholar] [CrossRef]
- Sousa-Santos, A.R.; Amaral, T.F. Differences in handgrip strength protocols to identify sarcopenia and frailty—A systematic review. BMC Geriatr. 2017, 17, 1–21. [Google Scholar] [CrossRef] [Green Version]
- De Souza, V.A.; Oliveira, D.; Cupolilo, E.N.; Miranda, C.S.; Colugnati, F.A.B.; Mansur, H.N.; Fernandes, N.M.D.S.; Bastos, M.G. Rectus femoris muscle mass evaluation by ultrasound: Facilitating sarcopenia diagnosis in pre-dialysis chronic kidney disease stages. Clinics 2018, 73, e392. [Google Scholar] [CrossRef]
- Boutin, R.D.; Yao, L.; Canter, R.; Lenchik, L. Sarcopenia: Current Concepts and Imaging Implications. Am. J. Roentgenol. 2015, 205, W255–W266. [Google Scholar] [CrossRef]
- Gao, J.; He, W.; Du, L.-J.; Li, S.; Cheng, L.-G.; Shih, G.; Rubin, J. Ultrasound strain elastography in assessment of resting biceps brachii muscle stiffness in patients with Parkinson’s disease: A primary observation. Clin. Imaging 2016, 40, 440–444. [Google Scholar] [CrossRef]
- Sanada, K.; Kearns, C.F.; Midorikawa, T.; Abe, T. Prediction and validation of total and regional skeletal muscle mass by ultrasound in Japanese adults. Graefe’s Arch. Clin. Exp. Ophthalmol. 2005, 96, 24–31. [Google Scholar] [CrossRef]
- Berger, J.; Bunout, D.; Barrera, G.; de la Maza, M.P.; Henriquez, S.; Leiva, L.; Hirsch, S. Rectus femoris (RF) ultrasound for the assessment of muscle mass in older people. Arch. Gerontol. Geriatr. 2015, 61, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH Sarcopenia Project: Rationale, Study Description, Conference Recommendations, and Final Estimates. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Heckmatt, J.; Leeman, S.; Dubowitz, V. Ultrasound imaging in the diagnosis of muscle disease. J. Pediatr. 1982, 101, 656–660. [Google Scholar] [CrossRef]
- Drakonaki, E.E.; Allen, G.M.; Wilson, D. Ultrasound elastography for musculoskeletal applications. Br. J. Radiol. 2012, 85, 1435–1445. [Google Scholar] [CrossRef] [Green Version]
- Bickerstaffe, A.; Beelen, A.; Zwarts, M.J.; Nollet, F.; van Dijk, J.P. Quantitative muscle ultrasound and quadriceps strength in patients with post-polio syndrome. Muscle Nerve 2015, 51, 24–29. [Google Scholar] [CrossRef]
- Ryan, A.S.; Dobrovolny, C.; Smith, G.V.; Silver, K.H.; Macko, R.F. Hemiparetic muscle atrophy and increased intramuscular fat in stroke patients. Arch. Phys. Med. Rehabil. 2002, 83, 1703–1707. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Yamaguchi, S.; Sasho, T.; Fukawa, T.; Akatsu, Y.; Nagashima, K.; Takahashi, K. Quantitative Ultrasound Elastography with an Acoustic Coupler for Achilles Tendon Elasticity. J. Ultrasound Med. 2016, 35, 159–166. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Fukumoto, Y.; Ikezoe, T.; Yamada, Y.; Tsukagoshi, R.; Nakamura, M.; Mori, N.; Kimura, M.; Ichihashi, N. Skeletal muscle quality assessed from echo intensity is associated with muscle strength of middle-aged and elderly persons. Graefe’s Arch. Clin. Exp. Ophthalmol. 2012, 112, 1519–1525. [Google Scholar] [CrossRef] [Green Version]
- Abe, T.; Kawakami, Y.; Bemben, M.G.; Fukunaga, T. Comparison of Age-Related, Site-Specific Muscle Loss Between Young and Old Active and Inactive Japanese Women. J. Geriatr. Phys. Ther. 2011, 34, 168–173. [Google Scholar] [CrossRef]
- Takeshima, N.; Shimada, K.; Islam, M.M.; Kanehisa, H.; Ishida, Y.; Brechue, W.F. Progressive, Site-Specific Loss of Muscle Mass in Older, Frail Nursing Home Residents. J. Aging Phys. Act. 2015, 23, 452–459. [Google Scholar] [CrossRef]
- Kortebein, P.; Ferrando, A.; Lombeida, J.; Wolfe, R.; Evans, W.J. Effect of 10 Days of Bed Rest on Skeletal Muscle in Healthy Older Adults. JAMA 2007, 297, 1769–1774. [Google Scholar] [CrossRef]
- Buford, T.W.; Anton, S.D.; Judge, A.R.; Marzetti, E.; Wohlgemuth, S.E.; Carter, C.S.; Leeuwenburgh, C.; Pahor, M.; Manini, T.M. Models of accelerated sarcopenia: Critical pieces for solving the puzzle of age-related muscle atrophy. Ageing Res. Rev. 2010, 9, 369–383. [Google Scholar] [CrossRef] [Green Version]
- Celik, B.; Ones, K.; Ince, N. Body composition after stroke. Int. J. Rehabil. Res. 2008, 31, 93–96. [Google Scholar] [CrossRef]
- Metoki, N.; Sato, Y.; Satoh, K.; Okumura, K.; Iwamoto, J. Muscular Atrophy in the Hemiplegic Thigh in Patients After Stroke. Am. J. Phys. Med. Rehabil. 2003, 82, 862–865. [Google Scholar] [CrossRef]
- Reimers, K.; Reimers, C.D.; Wagner, S.; Paetzke, I.; E Pongratz, D. Skeletal muscle sonography: A correlative study of echogenicity and morphology. J. Ultrasound Med. 1993, 12, 73–77. [Google Scholar] [CrossRef]
- Harris-Love, M.O.; Avila, N.A.; Adams, B.; Zhou, J.; Seamon, B.; Ismail, C.; Zaidi, S.H.; Kassner, C.A.; Liu, F.; Blackman, M.R. The Comparative Associations of Ultrasound and Computed Tomography Estimates of Muscle Quality with Physical Performance and Metabolic Parameters in Older Men. J. Clin. Med. 2018, 7, 340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Booth, F.W. Effect of limb immobilization on skeletal muscle. J. Appl. Physiol. 1982, 52, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Gracies, J.-M. Pathophysiology of spastic paresis. I: Paresis and soft tissue changes. Muscle Nerve 2005, 31, 535–551. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, A.A.; Lane, H.W.; Stuart, C.A.; Davis-Street, J.; Wolfe, R.R. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am. J. Physiol. Metab. 1996, 270, E627–E633. [Google Scholar] [CrossRef] [PubMed]
- Rahemi, H.; Nigam, N.; Wakeling, J.M. The effect of intramuscular fat on skeletal muscle mechanics: Implications for the elderly and obese. J. R. Soc. Interface 2015, 12, 20150365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Sarcopenia (n = 14) | Non-Sarcopenia (n = 16) | p-Value | |
|---|---|---|---|
| Age (year) | 69.79 ± 9.82 | 65.38 ± 12.29 | 0.291 |
| Male | 9 (64.3%) | 11 (68.8%) | 0.804 |
| Post onset (days) | 19.77 ± 7.07 | 26.85 ± 13.35 | 0.108 |
| Body Mass Index (kg/m2) | 22.52 ± 3.23 | 24.38 ± 3.02 | 0.126 |
| pCircumference of Thigh (cm) | 36 ± 2.12 | 36.5 ± 3.32 | 0.643 |
| npCircumference of Thigh (cm) | 37 ± 5.57 | 36.63 ± 3.48 | 0.826 |
| Modified Bathel index in Korean | 51.21 ± 25.03 | 60.19 ± 27.35 | 0.359 |
| Functional ambulation categories | 2.57 ± 1.09 | 2.81 ± 2.81 | 0.499 |
| MMSE-K | 22.69 ± 5.06 | 22.81 ± 6.48 | 0.957 |
| Sarcopenia (n = 14) | Non-Sarcopenia (n = 16) | p-Value | |
|---|---|---|---|
| pLean Mass of Arm (kg) | 2.08 ± 0.39 | 2.75 ± 0.41 | 0.000 |
| npLean Mass of Arm (kg) | 2.15 ± 0.41 | 2.92 ± 0.48 | 0.000 |
| pLean Mass of Leg (kg) | 5.70 ± 1.01 | 7.65 ± 1.36 | 0.000 |
| npLean Mass of Leg (kg) | 5.85 ± 0.90 | 7.85 ± 1.43 | 0.000 |
| Appendicular Lean Mass (kg) | 15.81 ± 2.68 | 20.86 ± 3.70 | 0.000 |
| pFat Mass of Arm (kg) | 0.72 ± 0.46 | 0.77 ± 0.48 | 0.762 |
| npFat Mass of Arm (kg) | 0.74 ± 0.47 | 0.81 ± 0.47 | 0.709 |
| pFat Mass of Leg (kg) | 2.21 ± 1.04 | 2.17 ± 1.26 | 0.933 |
| npFat Mass of Leg (kg) | 2.25 ± 1.12 | 2.22 ± 1.22 | 0.942 |
| Total Fat Mass (kg) | 16.56 ± 8.16 | 17.59 ± 8.18 | 0.742 |
| Sarcopenia (n = 14) | Non-Sarcopenia (n = 16) | p-Value | |
|---|---|---|---|
| pThickness of Fat Layer (cm) | 10.44 ± 5.65 | 8.39 ± 4.23 | 0.304 |
| npThickness of Fat Layer (cm) | 9.98 ± 5.27 | 8.60 ± 4.11 | 0.465 |
| pTRF (cm) | 14.51 ± 2.47 | 17.32 ± 3.83 | 0.026 |
| npTRF (cm) | 15.31 ± 2.49 | 18.50 ± 3.66 | 0.010 |
| pEI | 43.94 ± 15.58 | 35.75 ± 11.59 | 0.111 |
| npEI | 40.35 ± 14.45 | 34.51 ± 15.52 | 0.297 |
| pSRE | 1.86 ± 1.06 | 1.41 ± 0.63 | 0.166 |
| npSRE | 1.68 ± 0.77 | 1.36 ± 0.53 | 0.200 |
| pEI to TRF ratio | 3.21 ± 1.58 | 2.23 ± 1.00 | 0.049 |
| npEI to TRF ratio | 2.81 ± 1.40 | 2.03 ± 1.27 | 0.124 |
| Univariate Analysis | Multivariate Analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | OR | p-Value | 95% CI | B | OR | p-Value | 95% CI | |||
| Sarcopenia M < 19.75 F < 15.02 (kg) | Age | 0.37 | 1.038 | 0.283 | 0.970–1.111 | Age | −0.60 | 0.971 | 0.316 | 0.837–1.392 |
| Male | −0.201 | 0.818 | 0.796 | 0.179–3.744 | Male | 0.664 | 1.943 | 0.542 | 0.230–16.394 | |
| Body Mass Index | −0.208 | 0.812 | 0.135 | 0.618–1.067 | Body Mass Index | −0.29 | 0.971 | 0.873 | 0.667–1.392 | |
| pThickness of Fat Layer | 0.09 | 1.094 | 0.298 | 0.924–1.296 | npTRF | −0.485 | 0.616 | 0.048 | 0.381–0.996 | |
| pTRF | −0.3 | 0.741 | 0.044 | 0.553–0.992 | ||||||
| pEI | 0.047 | 1.048 | 0.118 | 0.988–1.111 | ||||||
| pSRE | 0.719 | 2.052 | 0.192 | 0.697–6.044 | Age | −0.14 | 0.696 | 0.92 | 0.456–1.060 | |
| pEI to TRF ratio | 0.65 | 1.916 | 0.071 | 0.947–3.877 | Male | 0.262 | 1.300 | 0.807 | 0.158–10.682 | |
| npThickness of Fat Layer | 0.067 | 1.069 | 0.45 | 0.899–1.271 | Body Mass Index | −0.62 | 0.940 | 0.772 | 0.670–1.320 | |
| npTRF | −0.357 | 0.699 | 0.021 | 0.516–0.948 | pTRF | −0.363 | 0.696 | 0.92 | 0.456–1.060 | |
| npEI | 0.027 | 1.028 | 0.289 | 0.977–1.081 | ||||||
| npSRE | 0.783 | 2.189 | 0.203 | 0.656–7.307 | ||||||
| npEI to TRF ratio | 0.466 | 1.593 | 0.134 | 0.866–2.932 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.; Im, S.; Park, G.-Y. Ultrasound Evaluation of the Rectus Femoris for Sarcopenia in Patients with Early Subacute Stroke. J. Clin. Med. 2021, 10, 3010. https://doi.org/10.3390/jcm10143010
Choi Y, Im S, Park G-Y. Ultrasound Evaluation of the Rectus Femoris for Sarcopenia in Patients with Early Subacute Stroke. Journal of Clinical Medicine. 2021; 10(14):3010. https://doi.org/10.3390/jcm10143010
Chicago/Turabian StyleChoi, Yongmin, Sun Im, and Geun-Young Park. 2021. "Ultrasound Evaluation of the Rectus Femoris for Sarcopenia in Patients with Early Subacute Stroke" Journal of Clinical Medicine 10, no. 14: 3010. https://doi.org/10.3390/jcm10143010
APA StyleChoi, Y., Im, S., & Park, G. -Y. (2021). Ultrasound Evaluation of the Rectus Femoris for Sarcopenia in Patients with Early Subacute Stroke. Journal of Clinical Medicine, 10(14), 3010. https://doi.org/10.3390/jcm10143010






