Peripheral Nervous System Involvement in Non-Primary Pediatric Cancer: From Neurotoxicity to Possible Etiologies
Abstract
:1. Introduction
Methods
2. Chemotherapy-Induced Peripheral Neuropathy (CIPN)
2.1. Risk Factors
2.1.1. Treatment Factors
2.1.2. Disease Factors
2.1.3. Patient Factors
2.1.4. Genetic Risk Factors
2.2. CIPN of Platinum Compounds
2.3. CIPN of Anti-Microtubule Agents
2.3.1. Vinca Alkaloids CIPN
2.3.2. Taxane-Based CIPN
2.4. CIPN of Proteasome Inhibitors
2.5. Nelarabine CIPN
2.6. CIPN Clinical Assessment
2.7. CIPN Clinical Neurophysiology
2.8. Therapeutic Options and Prevention Approach
2.9. Long-Term Outcomes
3. Autoimmune Peripheral Neuropathy (APN)
3.1. Immune Checkpoint Inhibitor(ICI)-Induced APN
3.2. Vinca Alkaloid-Induced APN
3.3. Proteasome Inhibitor Induced APN
4. Radiation-Induced Peripheral Neuropathy (RIPN)
4.1. Pathophysiology of RIPN
4.2. Clinical Features
4.3. Treatment of RIPN
5. Enteric Nervous System and Chemotherapy-Induced Enteric Neurotoxicity
6. Critical Illness Polyneuropathy in Pediatric Cancer
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pike, C.T.; Birnbaum, H.G.; Muehlenbein, C.E.; Pohl, G.M.; Natale, R.B. Healthcare costs and workloss burden of patients with chemotherapy-associated peripheral neuropathy in breast, ovarian, head and neck, and nonsmall cell lung cancer. Chemother. Res. Pract. 2012, 913848. [Google Scholar] [CrossRef] [PubMed]
- Starobova, H.; Vetter, I. Pathophysiology of chemotherapy-induced peripheral neuropathy. Front. Mol. Neurosci. 2017, 31, 174. [Google Scholar] [CrossRef]
- Kandula, T.; Park, S.B.; Cohn, R.J.; Krishnan, A.V.; Farrar, M.A. Pediatric chemotherapy induced peripheral neuropathy: A systematic review of current knowledge. Cancer Treat. Rev. 2016, 50, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Bjornard, K.L.; Gilchrist, L.S.; Inaba, H.; Diouf, B.; Hockenberry, M.J.; Kadan-Lottick, N.S.; Bowers, D.C.; Dolan, M.E.; Ullrich, N.J.; Evans, W.E.; et al. Peripheral neuropathy in children and adolescents treated for cancer. Lancet Child. Adolesc. Health. 2018, 10, 744–754. [Google Scholar] [CrossRef]
- Park, S.B.; Goldstein, D.; Krishnan, A.V.; Lin, C.S.; Friedlander, M.L.; Cassidy, J.; Koltzenburg, M.; Kiernan, M.C. Chemotherapy-induced peripheral neurotoxicity: A critical analysis. CA Cancer J. Clin. 2013, 63, 419–437. [Google Scholar] [CrossRef] [PubMed]
- Kandula, T.; Farrar, M.A.; Cohn, R.J.; Mizrahi, D.; Carey, K.; Johnston, K.; Kiernan, M.C.; Krishnan, A.V.; Park, S.B. Chemotherapy-induced peripheral neuropathy in long-term survivors of childhood cancer: Clinical, neurophysiological, functional, and patient-reported outcomes. JAMA Neurol. 2018, 75, 980–988. [Google Scholar] [CrossRef] [PubMed]
- McHaney, V.A.; Thibadoux, G.; Hayes, F.A.; Green, A.A. Hearing loss in children receiving cisplatin chemotherapy. J. Pediatr. 1983, 102, 314–317. [Google Scholar] [CrossRef]
- Mollman, J.E. Cisplatin neurotoxicity. N. Engl. J. Med. 1990, 322, 126–127. [Google Scholar] [CrossRef]
- Tuxen, M.K.; Hansen, S.W. Neurotoxicity secondary to antineoplastic drugs. Cancer Treat. Rev. 1994, 20, 191–214. [Google Scholar] [CrossRef]
- Cvitkovic, E. Cumulative toxicities from cisplatin therapy and current cytoprotective measures. Cancer Treat. Rev. 1998, 24, 265–281. [Google Scholar] [CrossRef]
- Geisler, S.; Doan, R.A.; Strickland, A.; Huang, X.; Milbrandt, J.; DiAntonio, A. Prevention of vincristine-induced peripheral neuropathy by genetic deletion of SARM1 in mice. Brain 2016, 139, 3092–3108. [Google Scholar] [CrossRef] [Green Version]
- Sittl, R.; Lampert, A.; Huth, T.; Schuy, E.T.; Link, A.S.; Fleckenstein, J.; Alzheimer, C.; Grafe, P.; Carr, R.W. Anticancer drug oxaliplatin induces acute cooling-aggravated neuropathy via sodium channel subtype Na(V)1.6-resurgent and persistent current. Proc. Natl. Acad. Sci. USA 2012, 109, 6704–6709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mora, E.; Smith, E.M.; Donohoe, C.; Hertz, D.L. Vincristine-induced peripheral neuropathy in pediatric cancer patients. Am. J. Cancer Res. 2016, 6, 2416–2430. [Google Scholar] [PubMed]
- Lavoie Smith, E.M.; Li, L.; Chiang, C.; Thomas, K.; Hutchinson, R.J.; Wells, E.M.; Ho, R.H.; Skiles, J.; Chakraborty, A.; Bridges, C.M.; et al. Patterns and severity of vincristine-induced peripheral neuropathy in children with acute lymphoblastic leukemia. J. Peripher. Nerv. Syst. 2015, 20, 37–46. [Google Scholar] [CrossRef]
- Egbelakin, A.; Ferguson, M.J.; MacGill, E.A.; Lehmann, A.S.; Topletz, A.R.; Quinney, S.K.; Li, L.; McCammack, K.C.; Hall, S.D.; Renbarger, J.L. Increased risk of vincristine neurotoxicity associated with low CYP3A5 expression genotype in children with acute lymphoblastic leukemia. Pediatr. Blood Cancer 2011, 56, 361–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purser, M.J.; Johnston, D.L.; McMillan, H.J. Chemotherapy-induced peripheral neuropathy among Paediatric Oncology Patients. Can. J. Neurol. Sci. 2014, 41, 442–447. [Google Scholar] [CrossRef] [Green Version]
- Courtemanche, H.; Magot, A.; Ollivier, Y.; Rialland, F.; Leclair-Visonneau, L.; Fayet, G.; Camdessanché, J.P.; Péréon, Y. Vincristine-induced neuropathy: Atypical electrophysiological patterns in children. Muscle Nerve 2015, 52, 981–985. [Google Scholar] [CrossRef]
- Bay, A.; Yilmaz, C.; Yilmaz, N.; Oner, A.F. Vincristine induced cranial polyneuropathy. Indian J. Pediatr. 2006, 73, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Lassaletta, A.; Scheinemann, K.; Zelcer, S.M.; Hukin, J.; Wilson, B.A.; Jabado, N.; Carret, A.S.; Lafay-Cousin, L.; Larouche, V.; Hawkins, C.E.; et al. Phase II weekly vinblastine for chemotherapy-naïve children with progressive low-grade glioma: A canadian pediatric brain tumor consortium study. J. Clin. Oncol. 2016, 34, 3537–3543. [Google Scholar] [CrossRef]
- Cavaletti, G.; Gilardini, A.; Canta, A.; Rigamonti, L.; Rodriguez-Menendez, V.; Ceresa, C.; Marmiroli, P.; Bossi, M.; Oggioni, N.; D’Incalci, M.; et al. Bortezomib-induced peripheral neurotoxicity: A neurophysiological and pathological study in the rat. Exp. Neurol. 2007, 204, 317–325. [Google Scholar] [CrossRef]
- Schiff, D.; Wen, P.Y.; van den Bent, M.J. Neurological adverse effects caused by cytotoxic and targeted therapies. Nat. Rev. Clin. Oncol. 2009, 6, 596–603. [Google Scholar] [CrossRef]
- Dawkins, J.L.; Hulme, D.J.; Brahmbhatt, S.B.; Auer-Grumbach, M.; Nicholson, G.A. Mutations in SPTLC1, encoding serine palmitoyltransferase, long chain base subunit-1, cause hereditary sensory neuropathy type I. Nat. Genet. 2001, 27, 309–312. [Google Scholar] [CrossRef]
- Dunsmore, K.P.; Winter, S.S.; Devidas, M.; Wood, B.L.; Esiashvili, N.; Chen, Z.; Eisenberg, N.; Briegel, N.; Hayashi, R.J.; Gastier Foster, J.M.; et al. Children’s Oncology Group AALL0434: A Phase III Randomized Clinical Trial Testing Nelarabine in Newly Diagnosed T-Cell Acute Lymphoblastic Leukemia. J. Clin. Oncol. 2020, 38, 3282–3293. [Google Scholar] [CrossRef]
- DeAngelo, D.J. Nelarabine for the treatment of patients with relapsed or refractory T-cell acute lymphoblastic leukemia or lymphoblastic lymphoma. Hematol. Oncol. Clin. N. Am. 2009, 23, 1121–1135, vii–viii. [Google Scholar] [CrossRef] [PubMed]
- Ewins, K.; Malone, A.; Phelan, E.; Webb, D.; McHugh, J.C.; Smith, O. Nelarabine-induced peripheral and central neurotoxicity: Can sequential MRI brain imaging help to define its natural history? Br. J. Haematol. 2017, 294–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, J.B.; DeAngelis, L.M. Cancer-treatment-induced neurotoxicity—Focus on newer treatments. Nat. Rev. Clin. Oncol. 2016, 13, 92–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langholz, B.; Skolnik, J.M.; Barrett, J.S.; Renbarger, J.; Seibel, N.L.; Zajicek, A.; Arndt, C.A. Dactinomycin and vincristine toxicity in the treatment of childhood cancer: A retrospective study from the Children’s Oncology Group. Pediatr. Blood Cancer 2011, 57, 252–257. [Google Scholar] [CrossRef] [Green Version]
- Gilchrist, L.S.; Tanner, L.R.; Ness, K.K. Short-term recovery of chemotherapy-induced peripheral neuropathy after treatment for pediatric non-CNS cancer. Pediatr. Blood Cancer 2017, 64, 180–187. [Google Scholar] [CrossRef]
- Moore, R.J.; Groninger, H. Chemotherapy-induced peripheral neuropathy in pediatric cancer patients. Cureus 2013, 5, e124. [Google Scholar] [CrossRef] [Green Version]
- Gilchrist, L. Chemotherapy-induced peripheral neuropathy in pediatric cancer patients. Semin. Pediatr. Neurol. 2012, 19, 9–17. [Google Scholar] [CrossRef]
- Ness, K.K.; Hudson, M.M.; Pui, C.H.; Green, D.M.; Krull, K.R.; Huang, T.T.; Robison, L.L.; Morris, E.B. Neuromuscular impairments in adult survivors of childhood acute lymphoblastic leukemia: Associations with physical performance and chemotherapy doses. Cancer 2012, 118, 828–838. [Google Scholar] [CrossRef]
- Ness, K.K.; Jones, K.E.; Smith, W.A.; Spunt, S.L.; Wilson, C.L.; Armstrong, G.T.; Srivastava, D.K.; Robison, L.L.; Hudson, M.M.; Gurney, J.G. Chemotherapy-related neuropathic symptoms and functional impairment in adult survivors of extracranial solid tumors of childhood: Results from the St. Jude Lifetime Cohort Study. Arch. Phys. Med. Rehabil. 2013, 94, 1451–1457. [Google Scholar] [CrossRef] [Green Version]
- Varedi, M.; McKenna, R.; Lamberg, E.M. Balance in children with acute lymphoblastic leukemia. Pediatr. Int. 2017, 59, 293–302. [Google Scholar] [CrossRef]
- Tay, C.G.; Lee, V.W.M.; Ong, L.C.; Goh, K.J.; Ariffin, H.; Fong, C.Y. Vincristine-induced peripheral neuropathy in survivors of childhood acute lymphoblastic leukaemia. Pediatr. Blood Cancer 2017, 64. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.A.; Wilson, L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Cavaletti, G.; Alberti, P.; Argyriou, A.A.; Lustberg, M.; Staf, N.P.; Tamburin, S. Chemotherapy-induced peripheral neurotoxicity: A multifaceted, still unsolved issue. J. Peripher. Nerv. Syst. 2019, 24, S6–S12. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, B.; Henning, S.A.; Leung, J.; Falade-Nwulia, O.; Jarosinski, P.; Penzak, S.R.; Walsh, T.J. Adverse interactions between antifungal azoles and vincristine: Review and analysis of cases. Mycoses 2012, 55, 290–297. [Google Scholar] [CrossRef]
- Teusink, A.C.; Ragucci, D.; Shatat, I.F.; Kalpatthi, R. Potentiation of vincristine toxicity with concomitant fluconazole prophylaxis in children with acute lymphoblastic leukemia. Pediatr. Hematol. Oncol. 2012, 29, 62–67. [Google Scholar] [CrossRef]
- Bhushan, B.; Bhargava, A.; Kasundra, G.M.; Shubhakaran, K.; Sood, I. Guillain-Barre syndrome in acute lymphoblastic leukemia: Causal or coincidental. J. Pediatr. Neurosci. 2015, 10, 64–66. [Google Scholar] [CrossRef]
- Beutler, A.S.; Kulkarni, A.A.; Kanwar, R.; Klein, C.J.; Therneau, T.M.; Qin, R.; Banck, M.S.; Boora, G.K.; Ruddy, K.J.; Wu, Y.; et al. Sequencing of Charcot-Marie-Tooth disease genes in a toxic polyneuropathy. Ann. Neurol. 2014, 76, 727–737. [Google Scholar] [CrossRef] [Green Version]
- van de Velde, M.E.; Kaspers, G.L.; Abbink, F.C.H.; Wilhelm, A.J.; Ket, J.C.F.; van den Berg, M.H. Vincristine-induced peripheral neuropathy in children with cancer: A systematic review. Crit. Rev. Oncol. Hematol. 2017, 114, 114–130. [Google Scholar] [CrossRef]
- Kuehl, P.; Zhang, J.; Lin, Y.; Lamba, J.; Assem, M.; Schuetz, J.; Watkins, P.B.; Daly, A.; Wrighton, S.A.; Hall, S.D.; et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat. Genet. 2001, 27, 383–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brigo, F.; Balter, R.; Marradi, P.; Ferlisi, M.; Zaccaron, A.; Fiaschi, A.; Frasson, E.; Bertolasi, L. Vincristine-related neuropathy versus acute inflammatory demyelinating polyradiculoneuropathy in children with acute lymphoblastic leukemia. J. Child. Neurol. 2012, 27, 867–874. [Google Scholar] [CrossRef]
- Aghajan, Y.; Yoon, J.M.; Crawford, J.R. Severe vincristine-induced polyneuropathy in a teenager with anaplastic medulloblastoma and undiagnosed Charcot-Marie-Tooth disease. BMJ Case Rep. 2017, 2017, bcr2016218981. [Google Scholar] [CrossRef] [PubMed]
- Chauvenet, A.R.; Shashi, V.; Selsky, C.; Morgan, E.; Kurtzberg, J.; Bell, B. Pediatric Oncology Group Study. Vincristine-induced neuropathy as the initial presentation of charcot-marie-tooth disease in acute lymphoblastic leukemia: A Pediatric Oncology Group study. J. Pediatr. Hematol. Oncol. 2003, 25, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Kandula, T.; Farrar, M.A.; Kiernan, M.C.; Krishnan, A.V.; Goldstein, D.; Horvath, L.; Grimison, P.; Boyle, F.; Baron-Hay, S.; Park, S.B. Neurophysiological and clinical outcomes in chemotherapy-induced neuropathy in cancer. Clin. Neurophysiol. 2017, 128, 1166–1175. [Google Scholar] [CrossRef]
- Li, Y.; Womer, R.B.; Silber, J.H. Predicting cisplatin ototoxicity in children: The influence of age and the cumulative dose. Eur. J. Cancer 2004, 40, 2445–2451. [Google Scholar] [CrossRef]
- Paulino, A.C.; Lobo, M.; The, B.S.; Okcu, M.F.; South, M.; Butler, E.B.; Su, J.; Chintagumpala, M. Ototoxicity after intensity-modulated radiation therapy and cisplatin-based chemotherapy in children with medulloblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1445–1450. [Google Scholar] [CrossRef]
- Freyer, D.R.; Brock, P.R.; Chang, K.W.; Dupuis, L.L.; Epelman, S.; Knight, K.; Mills, D.; Phillips, R.; Potter, E.; Risby, D.; et al. Prevention of cisplatin-induced ototoxicity in children and adolescents with cancer: A clinical practice guideline. Lancet Child. Adolesc. Health 2020, 4, 141–150. [Google Scholar] [CrossRef]
- Podratz, J.L.; Knight, A.M.; Ta, L.E.; Staff, N.P.; Gass, J.M.; Genelin, K.; Schlattau, A.; Lathroum, L.; Windebank, A.J. Cisplatin induced mitochondrial DNA damage in dorsal root ganglion neurons. Neurobiol. Dis. 2011, 41, 661–668. [Google Scholar] [CrossRef] [Green Version]
- Canta, A.; Pozzi, E.; Carozzi, V.A. Mitochondrial dysfunction in chemotherapy-induced peripheral neuropathy (CIPN). Toxics 2015, 3, 198–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carozzi, V.A.; Canta, A.; Chiorazzi, A. Chemotherapy-induced peripheral neuropathy: What do we know about mechanisms? Neurosci. Lett. 2015, 596, 90–107. [Google Scholar] [CrossRef]
- Wang, J.T.; Medress, Z.A.; Barres, B.A. Axon degeneration: Molecular mechanisms of a self-destruction pathway. J. Cell Biol. 2012, 196, 7–18. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.; Gereau, R.W., 4th. Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J. Neurosci. 2006, 26, 246–255. [Google Scholar] [CrossRef] [PubMed]
- van der Vijgh, W.J. Clinical pharmacokinetics of carboplatin. Clin. Pharmacol. 1991, 21, 242–261. [Google Scholar] [CrossRef]
- Loss, J.F.; Santos, P.P.; Leone, L.D.; Brunetto, A.L. Outcome of pediatric recurrent and refractory malignant solid tumors following ifosfamide/carboplatin/etoposide (ICE): A phase II study in a pediatric oncology centre in Brazil. Pediatr. Blood Cancer 2004, 42, 139–144. [Google Scholar] [CrossRef]
- Staff, N.P.; Podratz, J.L.; Grassner, L.; Bader, M.; Paz, J.; Knight, A.M.; Loprinzi, C.L.; Trushina, E.; Windebank, A.J. Bortezomib alters microtubule polymerization and axonal transport in rat dorsal root ganglion neurons. Neurotoxicology 2013, 39, 124–131. [Google Scholar] [CrossRef] [Green Version]
- Sandler, S.G.; Tobin, W.; Henderson, E.S. Vincristine-induced neuropathy. A clinical study of fifty leukemic patients. Neurology 1969, 19, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Lobert, S.; Vulevic, B.; Correia, J.J. Interaction of vinca alkaloids with tubulin: A comparison of vinblastine, vincristine, and vinorelbine. Biochemistry 1996, 35, 6806–6814. [Google Scholar] [CrossRef]
- Chan, S.Y.; Worth, R.; Ochs, S. Block of axoplasmic transport in vitro by vinca alkaloids. J. Neurobiol. 1980, 11, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Hudson, M.M.; Neglia, J.P.; Woods, W.G.; Sandlund, J.T.; Pui, C.H.; Kun, L.E.; Robison, L.L.; Green, D.M. Lessons from the past: Opportunities to improve childhood cancer survivor care through outcomes investigations of historical therapeutic approaches for pediatric hematological malignancies. Pediatr. Blood Cancer 2012, 58, 334–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, D.M.; Kun, L.E.; Matthay, K.K.; Meadows, A.T.; Meyer, W.H.; Meyers, P.A.; Spunt, S.L.; Robison, L.L.; Hudson, M.M. Relevance of historical therapeutic approaches to the contemporary treatment of pediatric solid tumors. Pediatr. Blood Cancer 2013, 60, 1083–1094. [Google Scholar] [CrossRef] [Green Version]
- Yildiz, F.G.; Temucin, Ç.M. Vincristine-induced neurotoxicity: Electrophysiological features in children. Neurol. Res. 2016, 38, 124–129. [Google Scholar] [CrossRef]
- Kavcic, M.; Koritnik, B.; Krzan, M.; Velikonja, O.; Prelog, T.; Stefanovic, M.; Debeljak, M.; Jazbec, J. Electrophysiological studies to detect peripheral neuropathy in children treated with vincristine. J. Pediatr. Hematol. Oncol. 2017, 39, 266–271. [Google Scholar] [CrossRef]
- Harila-Saari, A.H.; Vainionpää, L.K.; Kovala, T.T.; Tolonen, E.U.; Lanning, B.M. Nerve lesions after therapy for childhood acute lymphoblastic leukemia. Cancer 1998, 82, 200–207. [Google Scholar] [CrossRef] [Green Version]
- Lehtinen, S.S.; Huuskonen, U.E.; Harila-Saari, A.H.; Tolonen, U.; Vainionpää, L.K.; Lanning, B.M. Motor nervous system impairment persists in long-term survivors of childhood acute lymphoblastic leukemia. Cancer 2002, 94, 2466–2473. [Google Scholar] [CrossRef]
- Jain, P.; Gulati, S.; Seth, R.; Bakhshi, S.; Toteja, G.S.; Pandey, R.M. Vincristine-induced neuropathy in childhood ALL (acute lymphoblastic leukemia) survivors: Prevalence and electrophysiological characteristics. J. Child. Neurol. 2014, 29, 932–937. [Google Scholar] [CrossRef]
- Ramchandren, S.; Leonard, M.; Mody, R.J.; Donohue, J.E.; Moyer, J.; Hutchinson, R.; Gurney, J.G. Peripheral neuropathy in survivors of childhood acute lymphoblastic leukemia. J. Peripher. Nerv. Syst. 2009, 14, 184–189. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, R.J.; Blaney, S.; Sullivan, J.; Weitman, S.; Vietti, T.; Bernstein, M.L. Pediatric Oncology Group Study. Phase 1 study of Paclitaxel administered twice weekly to children with refractory solid tumors: A pediatric oncology group study. J. Pediatr. Hematol. Oncol. 2003, 25, 539–542. [Google Scholar] [CrossRef]
- Shemesh, O.A.; Spira, M.E. Paclitaxel induces axonal microtubules polar reconfiguration and impaired organelle transport: Implications for the pathogenesis of paclitaxel-induced polyneuropathy. Acta Neuropathol. 2010, 119, 235–248. [Google Scholar] [CrossRef] [PubMed]
- LaPointe, N.E.; Morfini, G.; Brady, S.T.; Feinstein, S.C.; Wilson, L.; Jordan, M.A. Effects of eribulin, vincristine, paclitaxel and ixabepilone on fast axonal transport and kinesin-1 driven microtubule gliding: Implications for chemotherapy-induced peripheral neuropathy. Neurotoxicology 2013, 37, 231–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bober, B.G.; Shah, S.B. Paclitaxel alters sensory nerve biomechanical properties. J. Biomech. 2015, 48, 3559–3567. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.; Xiao, W.H.; Bennett, G.J. Mitotoxicity and bortezomib-induced chronic painful peripheral neuropathy. Exp. Neurol. 2012, 238, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Messinger, Y.H.; Gaynon, P.S.; Sposto, R.; van der Giessen, J.; Eckroth, E.; Malvar, J.; Bostrom, B.C. Therapeutic Advances in Childhood Leukemia & Lymphoma (TACL) Consortium. Bortezomib with chemotherapy is highly active in advanced B-precursor acute lymphoblastic leukemia: Therapeutic Advances in Childhood Leukemia & Lymphoma (TACL) Study. Blood 2012, 120, 285–290. [Google Scholar] [CrossRef] [Green Version]
- Keller, S.; Seipel, K.; Novak, U.; Mueller, B.U.; Taleghani, B.M.; Leibundgut, K.; Pabst, T. Neurotoxicity of stem cell mobilization chemotherapy with vinorelbine in myeloma patients after bortezomib treatment. Leuk. Res. 2015, 39, 786–792. [Google Scholar] [CrossRef] [Green Version]
- Salvemini, D.; Doyle, T.; Kress, M.; Nicol, G. Therapeutic targeting of the ceramide-to- sphingosine 1-phosphate pathway in pain. Trends. Pharmacol. Sci. 2013, 34, 110–118. [Google Scholar] [CrossRef]
- Stockstill, K.; Doyle, T.M.; Yan, X.; Chen, Z.; Janes, K.; Little, J.W.; Braden, K.; Lauro, F.; Giancotti, L.A.; Harada, C.M.; et al. Dysregulation of sphingolipid metabolism contributes to bortezomib-induced neuropathic pain. J. Exp. Med. 2018, 215, 1301–1313. [Google Scholar] [CrossRef]
- Emery, E.C.; Wood, J.N. Gaining on pain. N. Engl. J. Med. 2018, 379, 485–487. [Google Scholar] [CrossRef]
- Zajączkowska, R.; Kocot-Kępska, M.; Leppert, W.; Wrzosek, A.; Mika, J.; Wordliczek, J. Mechanisms of chemotherapy-induced peripheral neuropathy. Int. J. Mol. Sci. 2019, 20, 1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casafont, I.; Berciano, M.T.; Lafarga, M. Bortezomib induces the formation of nuclear poly(A) RNA granules enriched in Sam68 and PABPN1 in sensory ganglia neurons. Neurotox. Res. 2010, 17, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Palanca, A.; Casafont, I.; Berciano, M.T.; Lafarga, M. Proteasome inhibition induces DNA damage and reorganizes nuclear architecture and protein synthesis machinery in sensory ganglion neurons. Cell Mol. Life Sci. 2014, 71, 1961–1975. [Google Scholar] [CrossRef]
- Meregalli, C.; Chiorazzi, A.; Carozzi, V.A.; Canta, A.; Sala, B.; Colombo, M.; Oggioni, N.; Ceresa, C.; Foudah, D.; La Russa, F.; et al. Evaluation of tubulin polymerization and chronic inhibition of proteasome as citotoxicity mechanisms in bortezomib-induced peripheral neuropathy. Cell Cycle 2014, 13, 612–621. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.K.; Berdeja, J.G.; Niesvizky, R.; Lonial, S.; Laubach, J.P.; Hamadani, M.; Stewart, A.K.; Hari, P.; Roy, V.; Vescio, R.; et al. Safety and tolerability of ixazomib, an oral proteasome inhibitor, in combination with lenalidomide and dexamethasone in patients with previously untreated multiple myeloma: An open-label phase 1/2 study. Lancet Oncol. 2014, 15, 1503–1512. [Google Scholar] [CrossRef]
- Berg, S.L.; Blaney, S.M.; Devidas, M.; Lampkin, T.A.; Murgo, A.; Bernstein, M.; Billett, A.; Kurtzberg, J.; Reaman, G.; Gaynon, P.; et al. Phase II study of nelarabine (compound 506U78) in children and young adults with refractory T-cell malignancies: A report from the Children’s Oncology Group. J. Clin. Oncol. 2005, 23, 3376–3382. [Google Scholar] [CrossRef]
- Ngo, D.; Patel, S.; Kim, E.J.; Brar, R.; Koontz, M.Z. Nelarabine neurotoxicity with concurrent intrathecal chemotherapy: Case report and review of literature. J. Oncol. Pharm. Pract. 2015, 21, 296–300. [Google Scholar] [CrossRef]
- Kurtzberg, J.; Ernst, T.J.; Keating, M.J.; Gandhi, V.; Hodge, J.P.; Kisor, D.F.; Lager, J.J.; Stephens, C.; Levin, J.; Krenitsky, T.; et al. Phase I study of 506U78 administered on a consecutive 5-day schedule in children and adults with refractory hematologic malignancies. J. Clin. Oncol. 2005, 23, 3396–3403. [Google Scholar] [CrossRef]
- Papayannidis, C.; Iacobucci, I.; Abbenante, M.C.; Curti, A.; Paolini, S.; Parisi, S.; Baccarani, M.; Martinelli, G. Complete paraplegia after nelarabine treatment in a T-cell acute lymphoblastic leukemia adult patient. Am. J. Hematol. 2010, 85, 608. [Google Scholar] [CrossRef]
- Gollard, R.P.; Selco, S. Irreversible myelopathy associated with nelaribine in T-cell acute lymphoblastic leukemia. J. Clin. Oncol. 2013, 3, e327–e331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pui, C.H.; Carroll, W.L.; Meshinchi, S.; Arceci, R.J. Biology, risk stratification, and therapy of pediatric acute leukemias: An update. J. Clin. Oncol. 2011, 29, 551–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilchrist, L.S.; Tanner, L. The pediatric-modified total neuropathy score: A reliable and valid measure of chemotherapy-induced peripheral neuropathy in children with non-CNS cancers. Support. Care Cancer 2013, 2, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, L.S.; Marais, L.; Tanner, L. Comparison of two chemotherapy-induced peripheral neuropathy measurement approaches in children. Support. Care Cancer 2014, 22, 359–366. [Google Scholar] [CrossRef]
- Lavoie Smith, E.M.; Li, L.; Hutchinson, R.J.; Ho, R.; Burnette, W.B.; Wells, E.; Bridges, C.; Renbarger, J. Measuring vincristine-induced peripheral neuropathy in children with acute lymphoblastic leukemia. Cancer Nurs. 2013, 36, E49–E60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavaletti, G.; Cornblath, D.R.; Merkies, I.S.J.; Postma, T.J.; Rossi, E.; Frigeni, B.; Alberti, P.; Bruna, J.; Velasco, R.; Argyriou, A.A.; et al. The chemotherapy-induced peripheral neuropathy outcome measures standardization study: From consensus to the first validity and reliability findings. Ann. Oncol. 2013, 24, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Haggiagi, A.; Tzatha, E.; DeAngelis, L.M.; Santomasso, B. Electrophysiological findings in immune checkpoint inhibitor-related peripheral neuropathy. Clin. Neurophysiol. 2019, 130, 1440–1445. [Google Scholar] [CrossRef]
- Griffith, K.A.; Dorsey, S.G.; Renn, C.L.; Zhu, S.; Johantgen, M.E.; Cornblath, D.R.; Argyriou, A.A.; Cavaletti, G.; Merkies, I.S.; Alberti, P.; et al. Correspondence between neurophysiological and clinical measurements of chemotherapy-induced peripheral neuropathy: Secondary analysis of data from the CI-PeriNomS study. J. Peripher. Nerv. Syst. 2014, 19, 127–135. [Google Scholar] [CrossRef]
- Mallik, A.; Weir, I. Nerve conduction studies: Essentials and pitfalls in practice. J. Neurol. Neurosurg. Psychiatry 2005, 76 (Suppl. 2), ii23–ii31. [Google Scholar] [CrossRef] [Green Version]
- Cavaletti, G.; Frigeni, B.; Lanzani, F.; Mattavelli, L.; Susani, E.; Alberti, P.; Cortinovis, D.; Bidoli, P. Chemotherapy-Induced Peripheral Neurotoxicity assessment: A critical revision of the currently available tools. Eur. J. Cancer 2010, 463, 479–494. [Google Scholar] [CrossRef]
- Kokotis, P.; Schmelz, M.; Kostouros, E.; Karandreas, N.; Dimopoulos, M.A. Oxaliplatin-induced neuropathy: A long-term clinical and neurophysiologic follow-up study. Clin. Colorectal. Cancer 2016, 15, e133–e140. [Google Scholar] [CrossRef]
- Chen, X.; Stubblefield, M.D.; Custodio, C.M.; Hudis, C.A.; Seidman, A.D.; DeAngelis, L.M. Electrophysiological features of taxane-induced polyneuropathy in patients with breast cancer. J. Clin. Neurophysiol. 2013, 30, 199–203. [Google Scholar] [CrossRef]
- Mileshkin, L.; Stark, R.; Day, B.; Seymour, J.F.; Zeldis, J.B.; Prince, H.M. Development of neuropathy in patients with myeloma treated with thalidomide: Patterns of occurrence and the role of electrophysiologic monitoring. J. Clin. Oncol. 2006, 24, 4507–4514. [Google Scholar] [CrossRef]
- Fuglsang-Frederiksen, A.; Pugdahl, K. Current status on electrodiagnostic standards and guidelines in neuromuscular disorders. Clin. Neurophysiol. 2011, 122, 440–455. [Google Scholar] [CrossRef]
- Hershman, D.L.; Lacchetti, C.; Dworkin, R.H.; Lavoie Smith, E.M.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Gavin, P.; Lavino, A.; Lustberg, M.B.; et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 2014, 32, 1941–1967. [Google Scholar] [CrossRef] [Green Version]
- Akbayram, S.; Akgun, C.; Doğan, M.; Sayin, R.; Caksen, H.; Oner, A.F. Use of pyridoxine and pyridostigmine in children with vincristine-induced neuropathy. Indian J. Pediatr. 2010, 77, 681–683. [Google Scholar] [CrossRef] [PubMed]
- Vondracek, P.; Oslejskova, H.; Kepak, T.; Mazanek, P.; Sterba, J.; Rysava, M.; Gal, P. Efficacy of pregabalin in neuropathic pain in paediatric oncological patients. Eur. J. Paediatr. Neurol. 2009, 13, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Anghelescu, D.L.; Faughnan, L.G.; Jeha, S.; Relling, M.V.; Hinds, P.S.; Sandlund, J.T.; Cheng, C.; Pei, D.; Hankins, G.; Pauley, J.L.; et al. Neuropathic pain during treatment for childhood acute lymphoblastic leukemia. Pediatr. Blood Cancer 2011, 57, 1147–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedrichsdorf, S.J.; Nugent, A.P. Management of neuropathic pain in children with cancer. Curr. Opin. Support. Palliat. Care 2013, 7, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Windsor, R.B.; Tham, S.W.; Adams, T.L.; Anderson, A. The use of opioids for treatment of pediatric neuropathic pain. Clin. J. Pain. 2019, 35, 509–514. [Google Scholar] [CrossRef]
- Anghelescu, D.L.; Tesney, J.M. Neuropathic pain in pediatric oncology—A clinical decision algorithm. Paediatr. Drugs 2019, 21, 59–70. [Google Scholar] [CrossRef]
- Wacker, K.; Tanner, L.; Ovans, J.; Mason, J.; Gilchrist, L. Improving functional mobility in children and adolescents undergoing treatment for non-central nervous system cancers: A systematic review. PM&R 2017, 9, S385–S397. [Google Scholar] [CrossRef]
- Gilchrist, L.S.; Tanner, L.R. Short-term recovery of balance control: Association with chemotherapy-induced peripheral neuropathy in pediatric oncology. Pediatr. Phys. Ther. 2018, 30, 119–124. [Google Scholar] [CrossRef]
- Tomasello, C.; Pinto, R.M.; Mennini, C.; Conicella, E.; Stoppa, F.; Raucci, U. Scrambler therapy efficacy and safety for neuropathic pain correlated with chemotherapy-induced peripheral neuropathy in adolescents: A preliminary study. Pediatr. Blood Cancer 2018, 65, e27064. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Capozza, M.A.; Mastrangelo, S.; Maurizi, P.; Triarico, S.; Rolesi, R.; Attinà, G.; Fetoni, A.R.; Ruggiero, A. Assessment and management of platinum-related ototoxicity in children treated for cancer. Cancers 2020, 12, 1266. [Google Scholar] [CrossRef] [PubMed]
- Triarico, S.; Romano, A.; Attinà, G.; Capozza, M.A.; Maurizi, P.; Mastrangelo, S.; Ruggiero, A. Vincristine-induced peripheral neuropathy (VIPN) in pediatric tumors: Mechanisms, risk factors, strategies of prevention and treatment. Int. J. Mol. Sci. 2021, 22, 4112. [Google Scholar] [CrossRef] [PubMed]
- Freyer, D.R.; Chen, L.; Krailo, M.D.; Knight, K.; Villaluna, D.; Bliss, B.; Pollock, B.H.; Ramdas, J.; Lange, B.; Van Hoff, D.; et al. Effects of sodium thiosulfate versus observation on development of cisplatin-induced hearing loss in children with cancer (ACCL0431): A multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2017, 18, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Pachman, D.R.; Barton, D.L.; Watson, J.C.; Loprinzi, C.L. Chemotherapy-induced peripheral neuropathy: Prevention and treatment. Clin. Pharmacol. Ther. 2011, 90, 377–387. [Google Scholar] [CrossRef]
- Loprinzi, C.L.; Lacchetti, C.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Hertz, D.L.; Kelley, M.R.; Lavino, A.; Lustberg, M.B.; Paice, J.A.; et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO Guideline Update. J. Clin. Oncol. 2020, 38, 3325–3348. [Google Scholar] [CrossRef] [PubMed]
- Kerckhove, N.; Collin, A.; Condé, S.; Chaleteix, C.; Pezet, D.; Balayssac, D. Long-term effects, pathophysiological mechanisms, and risk factors of chemotherapy-induced peripheral neuropathies: A comprehensive literature review. Front. Pharmacol. 2017, 8, 86. [Google Scholar] [CrossRef] [Green Version]
- Mols, F.; van de Poll-Franse, L.V.; Vreugdenhil, G.; Beijers, A.J.; Kieffer, J.M.; Aaronson, N.K.; Husson, O. Reference data of the European organisation for research and treatment of cancer (EORTC) QLQ-CIPN20 questionnaire in the general dutch population. Eur. J. Cancer 2016, 69, 28–38. [Google Scholar] [CrossRef]
- Kolb, N.A.; Smith, A.G.; Singleton, J.R.; Beck, S.L.; Stoddard, G.J.; Brown, S.; Mooney, K. The association of chemotherapy-induced peripheral neuropathy symptoms and the risk of falling. JAMA Neurol. 2016, 73, 860–866. [Google Scholar] [CrossRef] [Green Version]
- Albers, J.W.; Chaudhry, V.; Cavaletti, G.; Donehower, R.C. Interventions for preventing neuropathy caused by cisplatin and related compounds. Cochrane Database Syst. Rev. 2011, 16, CD005228, Update in: Cochrane Database Syst. Rev. 2014, 3, CD005228. [Google Scholar] [CrossRef]
- André, T.; Boni, C.; Mounedji-Boudiaf, L.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Zaninelli, M.; Clingan, P.; Bridgewater, J.; et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N. Engl. J. Med. 2004, 350, 2343–2351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balayssac, D.; Ferrier, J.; Descoeur, J.; Ling, B.; Pezet, D.; Eschalier, A.; Authier, N. Chemotherapy-induced peripheral neuropathies: From clinical relevance to preclinical evidence. Expert Opin. Drug Saf. 2011, 10, 407–417. [Google Scholar] [CrossRef]
- Osmani, K.; Vignes, S.; Aissi, M.; Wade, F.; Milani, P.; Lévy, B.I.; Kubis, N. Taxane-induced peripheral neuropathy has good long-term prognosis: A 1- to 13-year evaluation. J. Neurol. 2012, 259, 1936–1943. [Google Scholar] [CrossRef] [PubMed]
- Roché, H.; Yelle, L.; Cognetti, F.; Mauriac, L.; Bunnell, C.; Sparano, J.; Kerbrat, P.; Delord, J.P.; Vahdat, L.; Peck, R.; et al. Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, as first-line therapy in patients with metastatic breast cancer previously treated with anthracycline chemotherapy. J. Clin. Oncol. 2007, 25, 3415–3420. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.A.; Lerzo, G.; Pivot, X.; Thomas, E.; Vahdat, L.; Bosserman, L.; Viens, P.; Cai, C.; Mullaney, B.; Peck, R.; et al. Efficacy and safety of ixabepilone (BMS-247550) in a phase II study of patients with advanced breast cancer resistant to an anthracycline, a taxane, and capecitabine. J. Clin. Oncol. 2007, 25, 3407–3414. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, W.W.; Huang, W.J. Chemotherapy-induced peripheral neuropathy. Biomed. Rep. 2017, 6, 267–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
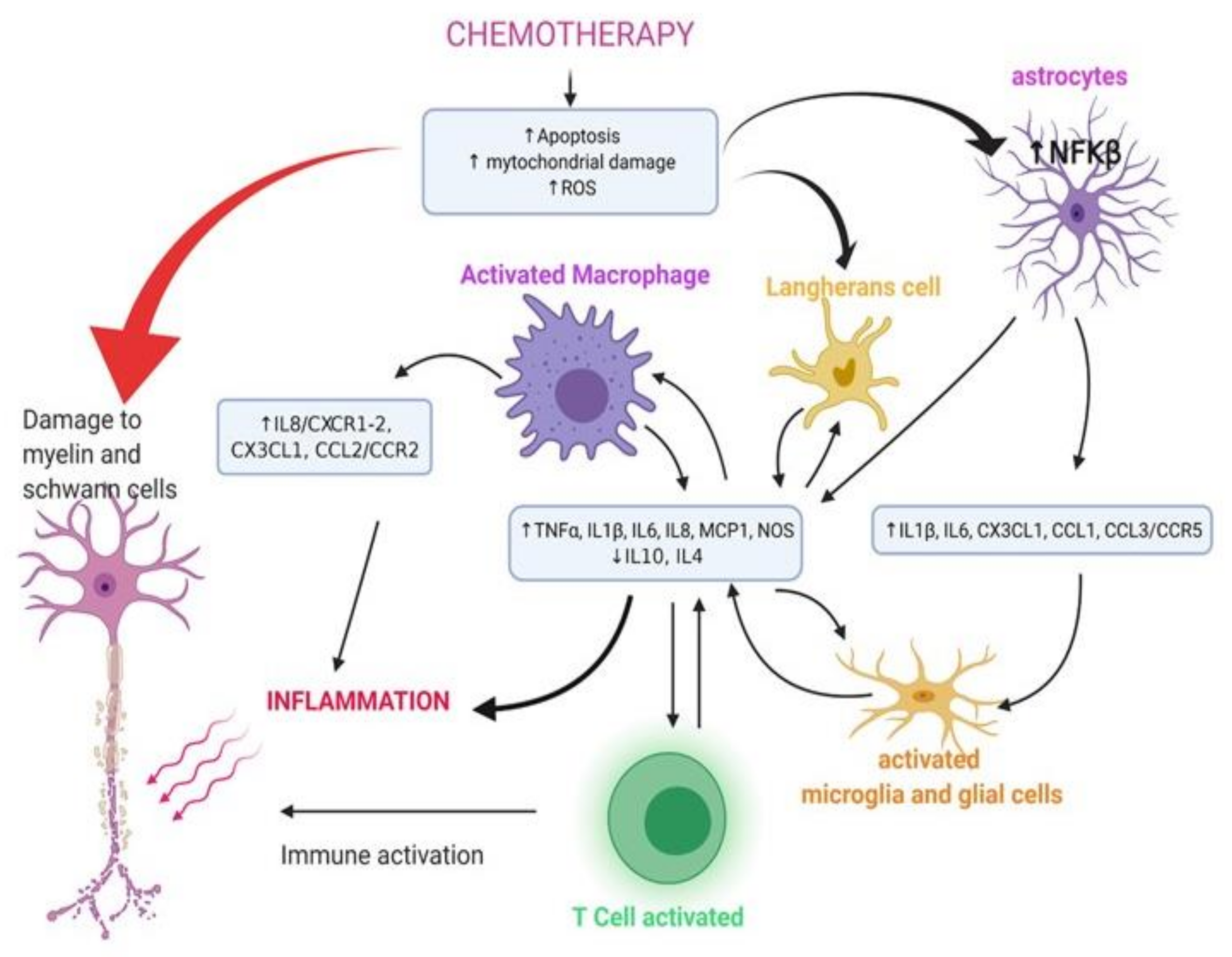
- Brandolini, L.; d’Angelo, M.; Antonosante, A.; Allegretti, M.; Cimini, A. Chemokine signaling in chemotherapy-induced neuropathic pain. Int. J. Mol. Sci. 2019, 20, 2904. [Google Scholar] [CrossRef] [Green Version]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [Green Version]
- Jaggi, A.S.; Singh, N. Mechanisms in cancer-chemotherapeutic drugs-induced peripheral neuropathy. Toxicology 2012, 291, 1–9. [Google Scholar] [CrossRef]
- Wang, Z.C.; Li, L.H.; Bian, C.; Yang, L.; Lv, N.; Zhang, Y.Q. Involvement of NF-κB and the CX3CR1 signaling network in mechanical allodynia induced by tetanic sciatic stimulation. Neurosci. Bull. 2018, 34, 64–73. [Google Scholar] [CrossRef]
- Fukuda, Y.; Li, Y.; Segal, R.A. A mechanistic understanding of axon degeneration in chemotherapy-induced peripheral neuropathy. Front. Neurosci. 2017, 11, 481. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Qin, L.; Liu, B.; Liu, Y.; Wilson, B.; Eling, T.E.; Langenbach, R.; Taniura, S.; Hong, J.S. Role of reactive oxygen species in LPS-induced production of prostaglandin E2 in microglia. J. Neurochem. 2004, 88, 939–947. [Google Scholar] [CrossRef]
- Salvemini, D.; Little, J.W.; Doyle, T.; Neumann, W.L. Roles of reactive oxygen and nitrogen species in pain. Free Radic Biol. Med. 2011, 51, 951–966. [Google Scholar] [CrossRef] [Green Version]
- Lees, J.; Makker, P.G.; Tonkin, R.S.; Abdulla, M.; Park, S.B.; Goldstein, D.; Moalem-Taylor, G. Immune-mediated processes implicated in chemotherapy-induced peripheral neuropathy. Eur. J. Cancer 2017, 73, 22–29. [Google Scholar] [CrossRef]
- So, Y.T. Immune-mediated neuropathies. Continuum. Minneap. Minn. 2012, 18, 85–105. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.; Shroff, S.; Kamiya-Matsuoka, C.; Tummala, S. Atypical neurological complications of ipilimumab therapy in patients with metastatic melanoma. Neuro Oncol. 2014, 16, 589–593. [Google Scholar] [CrossRef]
- Thaipisuttikul, I.; Chapman, P.; Avila, E.K. Peripheral neuropathy associated with ipilimumab: A report of 2 cases. J. Immunother. 2015, 38, 77–79. [Google Scholar] [CrossRef] [Green Version]
- Johnson, D.B.; Manouchehri, A.; Haugh, A.M.; Quach, H.T.; Balko, J.M.; Lebrun-Vignes, B.; Mammen, A.; Moslehi, J.J.; Salem, J.E. Neurologic toxicity associated with immune checkpoint inhibitors: A pharmacovigilance study. J. Immunother. Cancer 2019, 7, 134. [Google Scholar] [CrossRef] [Green Version]
- Gaudy-Marqueste, C.; Monestier, S.; Franques, J.; Cantais, E.; Richard, M.A.; Grob, J.J. A severe case of ipilimumab-induced guillain-barré syndrome revealed by an occlusive enteric neuropathy: A differential diagnosis for ipilimumab-induced colitis. J. Immunother. 2013, 36, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Kemar, E.; Green, D.O.; Anna, M.; Levine, B.S.; Jayne, H.; Ward, D.O.; David, I.; Kaufman, D.O. GQ1b-Seronegative Miller Fisher Syndrome Associated with Pembrolizumab. J. Neuro Ophthalmol. 2019, 39, 394–396. [Google Scholar] [CrossRef]
- Kolb, N.A.; Trevino, C.R.; Waheed, W.; Sobhani, F.; Landry, K.K.; Thomas, A.A.; Hehir, M. Neuromuscular complications of immune checkpoint inhibitor therapy. Muscle Nerve 2018. [Google Scholar] [CrossRef]
- Moudgil, S.S.; Riggs, J.E. Fulminant peripheral neuropathy with severe quadriparesis associated with vincristine therapy. Ann. Pharmacother. 2000, 34, 1136–1138. [Google Scholar] [CrossRef]
- Jariwal, R.; Shoua, B.; Sabetian, K.; Natarajan, P.; Cobos, E. Unmasking a case of asymptomatic Charcot-Marie-Tooth disease (CMT1A) with vincristine. J. Investig. Med. High. Impact. Case Rep. 2018, 6, 2324709618758349. [Google Scholar] [CrossRef] [Green Version]
- Stübgen, J.P. Tumor necrosis factor-alpha antagonists and neuropathy. Muscle Nerve 2008, 37, 281–292. [Google Scholar] [CrossRef]
- Ravaglia, S.; Corso, A.; Piccolo, G.; Lozza, A.; Alfonsi, E.; Mangiacavalli, S.; Varettoni, M.; Zappasodi, P.; Moglia, A.; Lazzarino, M.; et al. Immune-mediated neuropathies in myeloma patients treated with bortezomib. Clin. Neurophysiol. 2008, 119, 2507–2512. [Google Scholar] [CrossRef]
- Christodoulou, C.; Anastasopoulos, D.; Visvikis, A.; Mellou, S.; Detsi, I.; Tsiakalos, G.; Pateli, A.; Klouvas, G.; Papadimitriou, A.; Skarlos, D.V. Guillain-Barré syndrome in a patient with metastatic colon cancer receiving oxaliplatin-based chemotherapy. Anticancer Drugs 2004, 15, 997–999. [Google Scholar] [CrossRef] [PubMed]
- Pappa, E.; Berzero, G.; Herlin, B.; Ricard, D.; Tafani, C.; Devic, P.; Maillet, D.; Borden, A.; Viala, K.; Maisonobe, T.; et al. Guillain-Barré syndrome during platinum-based chemotherapy: A case series and review of the literature. Oncologist 2020, 25, e194–e197. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.Y.; Nam, T.S.; Kim, M.K.; Hwang, J.E.; Shim, H.J.; Cho, S.H.; Chung, I.J.; Bae, W.K. Acute inflammatory demyelinating polyradiculoneuropathy in a patient receiving oxaliplatin-based chemotherapy. Asia Pac. J. Clin. Oncol. 2012, 8, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Kusunoki, S. Autoimmune neuropathies: Diagnosis, treatment, and recent topics. Brain Nerve 2011, 63, 549–555. [Google Scholar] [PubMed]
- Hanewinckel, R.; Ikram, M.A.; Van Doorn, P.A. Peripheral neuropathies. Handb. Clin. Neurol. 2016, 138, 263–282. [Google Scholar] [CrossRef] [PubMed]
- Broers, M.C.; Bunschoten, C.; Nieboer, D.; Lingsma, H.F.; Jacobs, B.C. Incidence and prevalence of chronic inflammatory demyelinating polyradiculoneuropathy: A systematic review and meta-analysis. Neuroepidemiology 2019, 52, 161–172. [Google Scholar] [CrossRef]
- Aral, Y.Z.; Gursel, T.; Ozturk, G.; Serdaroglu, A. Guillain-Barré syndrome in a child with acute lymphoblastic leukemia. Pediatr. Hematol. Oncol. 2001, 18, 343–346. [Google Scholar] [CrossRef]
- Miller, S.D.; Olson, J.K.; Croxford, J.L. Multiple pathways to induction of virus-induced autoimmune demyelination: Lessons from Theiler’s virus infection. J. Autoimmun. 2001, 16, 219–227. [Google Scholar] [CrossRef]
- Olson, J.K.; Eagar, T.N.; Miller, S.D. Functional activation of myelin-specific T cells by virus-induced molecular mimicry. J. Immunol. 2002, 169, 2719–2726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, R.A.; Hadden, R.D.; Gregson, N.A.; Smith, K.J. Pathogenesis of Guillain-Barré syndrome. J. Neuroimmunol. 1999, 100, 74–97. [Google Scholar] [CrossRef]
- Dalakas, M.C. Neurological complications of immune checkpoint inhibitors: What happens when you ‘take the brakes off’ the immune system. Ther. Adv. Neurol. Disord. 2018, 14, 1756286418799864. [Google Scholar] [CrossRef] [Green Version]
- Zimmer, L.; Goldinger, S.M.; Hofmann, L.; Loquai, C.; Ugurel, S.; Thomas, I.; Schmidgen, M.I.; Gutzmer, R.; Utikal, J.S.; Göppner, D.; et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur. J. Cancer 2016, 60, 210–225. [Google Scholar] [CrossRef]
- Re, D.; Schwenk, A.; Hegener, P.; Bamborschke, S.; Diehl, V.; Tesch, H. Guillain-Barré syndrome in a patient with non-Hodgkin’s lymphoma. Ann. Oncol. 2000, 11, 217–220. [Google Scholar] [CrossRef]
- Tazi, I.; Nafil, H.; Zaoui, S.; Mahmal, L. Fatal vincristine-induced acute neurotoxicity mimicking Guillain-Barré syndrome. J. Cancer Res. Ther. 2013, 9, 335–336. [Google Scholar] [CrossRef]
- Bahl, A.; Chakrabarty, B.; Gulati, S.; Raju, K.N.; Raja, A.; Bakhshi, S. Acute onset flaccid quadriparesis in pediatric non-Hodgkin lymphoma: Vincristine induced or Guillain-Barré syndrome? Pediatr. Blood Cancer 2010, 55, 1234–1235. [Google Scholar] [CrossRef]
- Hogan-Dann, C.M.; Fellmeth, W.G.; McGuire, S.A.; Kiley, V.A. Polyneuropathy following vincristine therapy in two patients with Charcot-Marie-Tooth syndrome. JAMA 1984, 252, 2862–2863. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Thompson, E.I.; Rivera, G.K. Vincristine neuropathy in type I and type II Charcot-Marie-Tooth disease (hereditary motor sensory neuropathy). Med. Pediatr. Oncol. 1995, 25, 113–116. [Google Scholar] [CrossRef]
- Okuhara, Y.; Shinomiya, R.; Peng, F.; Kamei, N.; Kurashige, T.; Yokota, K.; Ochi, M. Direct effect of radiation on the peripheral nerve in a rat model. J. Plast. Surg. Hand. Surg. 2014, 48, 276–280. [Google Scholar] [CrossRef]
- Killer, H.E.; Hess, K. Natural history of radiation-induced brachial plexopathy compared with surgically treated patients. J. Neurol. 1990, 237, 247–250. [Google Scholar] [CrossRef]
- Gosk, J.; Rutowski, R.; Reichert, P.; Rabczyński, J. Radiation-induced brachial plexus neuropathy—Aetiopathogenesis, risk factors, differential diagnostics, symptoms and treatment. Folia Neuropathol. 2007, 45, 26–30. [Google Scholar] [PubMed]
- Delanian, S.; Lefaix, J.L.; Pradat, P.F. Radiation-induced neuropathy in cancer survivors. Radiother. Oncol. 2012, 105, 273–282. [Google Scholar] [CrossRef] [Green Version]
- Ducray, F.; Guillevin, R.; Psimaras, D.; Sanson, M.; Mokhtari, K.; Delanian, S.; Navarro, S.; Maisonobe, T.; Cornu, P.; Hoang-Xuan, K.; et al. Postradiation lumbosacral radiculopathy with spinal root cavernomas mimicking carcinomatous meningitis. Neuro Oncol. 2008, 10, 1035–1039. [Google Scholar] [CrossRef] [Green Version]
- Wadd, N.J.; Lucraft, H.H. Brachial plexus neuropathy following mantle radiotherapy. Clin. Oncol. R Coll. Radiol. 1998, 10, 399–400. [Google Scholar] [CrossRef]
- Kori, S.H.; Foley, K.M.; Posner, J.B. Brachial plexus lesions in patients with cancer: 100 cases. Neurology 1981, 31, 45–50. [Google Scholar] [CrossRef]
- Olsen, N.K.; Pfeiffer, P.; Johannsen, L.; Schrøder, H.; Rose, C. Radiation-induced brachial plexopathy: Neurological follow-up in 161 recurrence-free breast cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 1993, 26, 43–49. [Google Scholar] [CrossRef]
- Glass, J.P.; Pettigrew, L.C.; Maor, M. Plexopathy induced by radiation therapy. Neurology 1985, 35, 1261. [Google Scholar] [CrossRef] [PubMed]
- Bowen, J.; Gregory, R.; Squier, M.; Donaghy, M. The post-irradiation lower motor neuron syndrome neuronopathy or radiculopathy? Brain 1996, 119, 1429–1439. [Google Scholar] [CrossRef] [Green Version]
- Thomas, J.E.; Cascino, T.L.; Earle, J.D. Differential diagnosis between radiation and tumor plexopathy of the pelvis. Neurology 1985, 35, 1–7. [Google Scholar] [CrossRef]
- Dropcho, E.J. Neurotoxicity of radiation therapy. Neurol. Clin. 2010, 28, 217–234. [Google Scholar] [CrossRef] [PubMed]
- Soto, O. Radiation-induced conduction block: Resolution following anticoagulant therapy. Muscle Nerve 2005, 31, 642–645. [Google Scholar] [CrossRef]
- Bennett, M.H.; Feldmeier, J.; Hampson, N.B.; Smee, R.; Milross, C. Hyperbaric oxygen therapy for late radiation tissue injury. Cochrane Database Syst. Rev. 2016, 4, CD005005. [Google Scholar] [CrossRef]
- Hamama, S.; Gilbert-Sirieix, M.; Vozenin, M.C.; Delanian, S. Radiation-induced enteropathy: Molecular basis of pentoxifylline-vitamin E anti-fibrotic effect involved TGF-β1 cascade inhibition. Radiother. Oncol. 2012, 105, 305–312. [Google Scholar] [CrossRef]
- Williamson, R.; Kondziolka, D.; Kanaan, H.; Lunsford, L.D.; Flickinger, J.C. Adverse radiation effects after radiosurgery may benefit from oral vitamin E and pentoxifylline therapy: A pilot study. Stereotact. Funct. Neurosurg. 2008, 86, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Delanian, S.; Lefaix, J.L.; Maisonobe, T.; Salachas, F.; Pradat, P.F. Significant clinical improvement in radiation-induced lumbosacral polyradiculopathy by a treatment combining pentoxifylline, tocopherol, and clodronate (Pentoclo). J. Neurol. Sci. 2008, 275, 164–166. [Google Scholar] [CrossRef]
- Spencer, N.J.; Hu, H. Enteric nervous system: Sensory transduction, neural circuits and gastrointestinal motility. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 338–351. [Google Scholar] [CrossRef]
- Di Nardo, G.; Blandizzi, C.; Volta, U.; Colucci, R.; Stanghellini, V.; Barbara, G.; Del Tacca, M.; Tonini, M.; Corinaldesi, R.; De Giorgio, R. Review article: Molecular, pathological and therapeutic features of human enteric neuropathies. Aliment. Pharmacol. Ther. 2008, 28, 25–42. [Google Scholar] [CrossRef]
- Fleming, M.A., 2nd; Ehsan, L.; Moore, S.R.; Levin, D.E. The Enteric Nervous System and Its Emerging Role as a Therapeutic Target. Gastroenterol. Res. Pract. 2020, 2020, 8024171. [Google Scholar] [CrossRef]
- McQuade, R.M.; Al Thaalibi, M.; Nurgali, K. Impact of chemotherapy-induced enteric nervous system toxicity on gastrointestinal mucositis. Curr. Opin. Support. Palliat. Care. 2020, 14, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Wafai, L.; Taher, M.; Jovanovska, V.; Bornstein, J.C.; Dass, C.R.; Nurgali, K. Effects of oxaliplatin on mouse myenteric neurons and colonic motility. Front. Neurosci. 2013, 7, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pini, A.; Garella, R.; Idrizaj, E.; Calosi, L.; Baccari, M.C.; Vannucchi, M.G. Glucagon-like peptide 2 counteracts the mucosal damage and the neuropathy induced by chronic treatment with cisplatin in the mouse gastric fundus. Neurogastroenterol. Motil. 2016, 28, 206–216. [Google Scholar] [CrossRef]
- Vera, G.; Castillo, M.; Cabezos, P.A.; Chiarlone, A.; Martín, M.I.; Gori, A.; Pasquinelli, G.; Barbara, G.; Stanghellini, V.; Corinaldesi, R.; et al. Enteric neuropathy evoked by repeated cisplatin in the rat. Neurogastroenterol. Motil. 2011, 23, 370–378, e162–e163. [Google Scholar] [CrossRef]
- McQuade, R.M.; Carbone, S.E.; Stojanovska, V.; Rahman, A.; Gwynne, R.M.; Robinson, A.M.; Goodman, C.A.; Bornstein, J.C.; Nurgali, K. Role of oxidative stress in oxaliplatin-induced enteric neuropathy and colonic dysmotility in mice. Br. J. Pharmacol. 2016, 173, 3502–3521. [Google Scholar] [CrossRef] [Green Version]
- Robinson, A.M.; Stojanovska, V.; Rahman, A.A.; McQuade, R.M.; Senior, P.V.; Nurgali, K. Effects of oxaliplatin treatment on the enteric glial cells and neurons in the mouse ileum. J. Histochem. Cytochem. 2016, 64, 530–545. [Google Scholar] [CrossRef] [Green Version]
- McQuade, R.M.; Stojanovska, V.; Stavely, R.; Timpani, C.; Petersen, A.C.; Abalo, R.; Bornstein, J.C.; Rybalka, E.; Nurgali, K. Oxaliplatin-induced enteric neuronal loss and intestinal dysfunction is prevented by co-treatment with BGP-15. Br. J. Pharmacol. 2018, 175, 656–677. [Google Scholar] [CrossRef] [Green Version]
- Donald, E.L.; Stojanovska, L.; Apostolopoulos, V.; Nurgali, K. Resveratrol alleviates oxidative damage in enteric neurons and associated gastrointestinal dysfunction caused by chemotherapeutic agent oxaliplatin. Maturitas 2017, 105, 100–106. [Google Scholar] [CrossRef]
- McQuade, R.M.; Stojanovska, V.; Donald, E.; Abalo, R.; Bornstein, J.C.; Nurgali, K. Gastrointestinal dysfunction and enteric neurotoxicity following treatment with anticancer chemotherapeutic agent 5-fluorouracil. Neurogastroenterol. Motil. 2016, 28, 1861–1875. [Google Scholar] [CrossRef] [Green Version]
- McQuade, R.M.; Stojanovska, V.; Donald, E.L.; Rahman, A.A.; Campelj, D.G.; Abalo, R.; Rybalka, E.; Bornstein, J.C.; Nurgali, K. Irinotecan-induced gastrointestinal dysfunction Is associated with enteric neuropathy, but increased numbers of cholinergic myenteric neurons. Front. Physiol. 2017, 8, 391. [Google Scholar] [CrossRef] [Green Version]
- López-Gómez, L.; Díaz-Ruano, S.; Girón, R.; López-Pérez, A.E.; Vera, G.; Herradón Pliego, E.; López-Miranda, V.; Nurgali, K.; Martín-Fontelles, M.I.; Uranga, J.A.; et al. Preclinical evaluation of the effects on the gastrointestinal tract of the antineoplastic drug vincristine repeatedly administered to rats. Neurogastroenterol. Motil. 2018, 30, e13399. [Google Scholar] [CrossRef]
- Hooper, L.V.; Littman, D.R.; Macpherson, A.J. Interactions between the microbiota and the immune system. Science 2012, 336, 1268–1273. [Google Scholar] [CrossRef] [Green Version]
- Al-Chaer, E.D.; Kawasaki, M.; Pasricha, P.J. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology 2000, 119, 1276–1285. [Google Scholar] [CrossRef] [Green Version]
- Bolton, C.F.; Gilbert, J.J.; Hahn, A.F.; Sibbald, W.J. Polyneuropathy in critically ill patients. J. Neurol. Neurosurg. Psychiatry 1984, 47, 1223–1231. [Google Scholar] [CrossRef]
- Charisius, J.; Stiefel, M.; Merkel, N.; Kornhuber, M.; Haase, R.; Kramm, C.M. Critical illness polyneuropathy: A rare but serious adverse event in pediatric oncology. Pediatr. Blood Cancer 2010, 54, 161–165. [Google Scholar] [CrossRef]
- Williams, S.; Horrocks, I.A.; Ouvrier, R.A.; Gillis, J.; Ryan, M.M. Critical illness polyneuropathy and myopathy in pediatric intensive care: A review. Pediatr. Crit. Care Med. 2007, 8, 18–22. [Google Scholar] [CrossRef]
- Bolton, C.F. Neuromuscular manifestations of critical illness. Muscle Nerve 2005, 32, 140–163. [Google Scholar] [CrossRef]
- Tabarki, B.; Coffiniéres, A.; Van Den Bergh, P.; Huault, G.; Landrieu, P.; Sébire, G. Critical illness neuromuscular disease: Clinical, electrophysiological, and prognostic aspects. Arch. Dis. Child. 2002, 86, 103–107. [Google Scholar] [CrossRef]
- Hirabayashi, K.; Shiohara, M.; Suzuki, T.; Saito, S.; Tanaka, M.; Yanagisawa, R.; Tsuruta, G.; Fukuyama, T.; Hidaka, Y.; Nakazawa, Y.; et al. Critical illness polyneuropathy and myopathy caused by Bacillus cereus sepsis in acute lymphoblastic leukemia. J. Pediatr. Hematol. Oncol. 2012, 34, 10–113. [Google Scholar] [CrossRef]
- van Mook, W.N.; Hulsewé-Evers, R.P. Critical illness polyneuropathy. Curr. Opin. Crit. Care 2002, 8, 302–310. [Google Scholar] [CrossRef] [PubMed]

| Chemotherapeutic Agent | Mechanism of Action | Clinical Features |
|---|---|---|
| Platinum compounds | Damage on the dorsal root ganglion and consequently a primarily sensory neuropathy [6,7]. | Cisplatin: causes reversible peripheral sensory neuropathy, characterized by numbness, tingling, and paresthesias, sometimes Lhermitte’s sign [8,9,10]. Carboplatin: milder CIPN than cisplatin [11]. Oxaliplatin: cold-induced dysesthesias in the hands and mouth [12]. |
| Anti-microtubule agents | Vinca alkaloids: cause cytoskeletal disorganization and disorientation within axons, leading to inhibition of vesicle-mediated transport of neurotransmitters and axonal degeneration and denervation [13]. | Vincristine: axonal, sensorimotor polyneuropathy, which is generally related to cumulative dose. Manifestations comprise reduced deep tendon reflexes, foot and wrist drop, gait abnormalities, and muscle weakness that may be asymmetrical neurotic pain (jaw pain, muscle cramps), paresthesias and dysesthesia. Cranial motor nerves can be affected, causing hoarse voice, ptosis, eye movement disorders, and rarely optic neuropathy. Autonomic nerve involvement may underlie constipation, paralytic ileus, and urinary retention [14,15,16,17,18]. Vinblastine and Vinorelbine: Neurotoxicity is minimal and is less pronounced than that of vincristine; sometimes constipation. If neurotoxicity is present, vincristine may be considered as an alternative chemotherapeutic drug [4,19]. |
| Proteasome inhibitors | Degradation of intracellular proteins, resulting in accumulation of cytoplasmic aggregates, including neurofilaments in neuronal cells [20,21]. | Bortezomib: causes a dose- and length-dependent sensory axonal peripheral neuropathy [22]. |
| Nelarabine | Nelarabine is an antimetabolite, a water-soluble pro-drug of arabinosylguanine nucleotide triphosphate, a purine deoxyguanosine analog, leading to the inhibition of DNA synthesis [23] | Dose-dependent sensory and motor peripheral neuropathy; also Guillaine-Barrè Syndrome [24,25] |
| Chemotherapy | NCS Findings | EMG Findings | ||
|---|---|---|---|---|
| Distal or Proximal Neuropathy | Axonal or Demyelinating Neuropathy | Sensory and/or Motor Neuropathy (S/M) | ||
| Vincristine | Distal or Distal > Proximal; | Axonal; prolonged DML | SM or S > M | neurogenic pattern |
| Cisplatin | Distal; or Distal and Proximal | Axonal | S | |
| Oxaliplatin | In acute stage: repetitive motor discharges associated with CMAP; In chronic stage: distal S axonal | In acute stage: Fasciculations and repetitive discharges; In chronic stage: no chronic neurogenic pattern | ||
| Bortezomib | Axonal | >S or SM | ||
| Nelarabine | Axonal; GBS-like | S or M | ||
| Chemotherapeutic Agent | Mechanisms of Immunity Dysregulation | APN Features |
|---|---|---|
| Immune checkpoint inhibitors (ICIs) | ICIs enhance production of cytokines (IL-6 and IL-17) and produce an alternate Treg/Th17 with consequent abnormal T-regulatory (Treg) cell function and humoral immunity [128,129,130]. | Demyelination: chronic inflammatory demyelinating polyneuropathy (CIDP), Guillain-Barrè syndrome and Miller Fisher variant. Sensorimotor polyneuropathy, autoimmune myopathy, overlaps of myasthenia gravis with myositis and/or myocarditis, acute motor and sensory axonal neuropathy (AMSAN), and myasthenia gravis (anti-MuSK negative) are also reported [136,137,138,139,140,141]. |
| Vinka alkaloids | Immunodepression secondary to intensive chemotherapy [142]. | Acute inflammatory demyelinating polyradiculoneuropathy: examining findings from nerve conduction velocity studies and performing lumbar puncture helps to differentiate between vinca alkaloid neurotoxicity and acute inflammatory demyelinating polyradiculoneuropathy [143]. |
| Proteasome inhibitors | Both T-cell and humoral immune attack against peripheral nerve myelin, vasculitis-induced nerve ischemia, and inhibition of signaling support for axons [144]. | Severe polyradiculoneuropathy. Cases of demyelinating or mixed axonal-demyelinating neuropathy, with prominent motor involvement, albumin-cytological dissociation and lumbar root enhancement on Magnetic Resonance Imaging have been reported in the literature [144,145]. |
| Platinum Compounds | Increase in proinflammatory cytokines (TNF-α and IL-6) and enhancement of anticancer immune responses, which induce an immune reaction towards myelin antigen [146]. | Anecdotal cases of Guillain-Barrè syndromes [147,148]. |
| Subtype | Antibodies |
|---|---|
| AMAN | Anti-GM1a/b, Anti-GD1a GalNAc-GD1 |
| AMSAN | Anti-GM1 Anti-GD1a Anti-GM1b |
| AIDP | AntiGM2 |
| MFS | Anti-GQ1b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pro, S.; Vinti, L.; Boni, A.; Mastronuzzi, A.; Scilipoti, M.; Velardi, M.; Caroleo, A.M.; Farina, E.; Badolato, F.; Alessi, I.; et al. Peripheral Nervous System Involvement in Non-Primary Pediatric Cancer: From Neurotoxicity to Possible Etiologies. J. Clin. Med. 2021, 10, 3016. https://doi.org/10.3390/jcm10143016
Pro S, Vinti L, Boni A, Mastronuzzi A, Scilipoti M, Velardi M, Caroleo AM, Farina E, Badolato F, Alessi I, et al. Peripheral Nervous System Involvement in Non-Primary Pediatric Cancer: From Neurotoxicity to Possible Etiologies. Journal of Clinical Medicine. 2021; 10(14):3016. https://doi.org/10.3390/jcm10143016
Chicago/Turabian StylePro, Stefano, Luciana Vinti, Alessandra Boni, Angela Mastronuzzi, Martina Scilipoti, Margherita Velardi, Anna Maria Caroleo, Elisa Farina, Fausto Badolato, Iside Alessi, and et al. 2021. "Peripheral Nervous System Involvement in Non-Primary Pediatric Cancer: From Neurotoxicity to Possible Etiologies" Journal of Clinical Medicine 10, no. 14: 3016. https://doi.org/10.3390/jcm10143016
APA StylePro, S., Vinti, L., Boni, A., Mastronuzzi, A., Scilipoti, M., Velardi, M., Caroleo, A. M., Farina, E., Badolato, F., Alessi, I., Di Nardo, G., Carai, A., Valeriani, M., Reale, A., Parisi, P., & Raucci, U. (2021). Peripheral Nervous System Involvement in Non-Primary Pediatric Cancer: From Neurotoxicity to Possible Etiologies. Journal of Clinical Medicine, 10(14), 3016. https://doi.org/10.3390/jcm10143016









