Cognitive Assessment in GNAO1 Neurodevelopmental Disorder Using an Eye Tracking System
Abstract
:1. Introduction
2. Methods
2.1. Participants
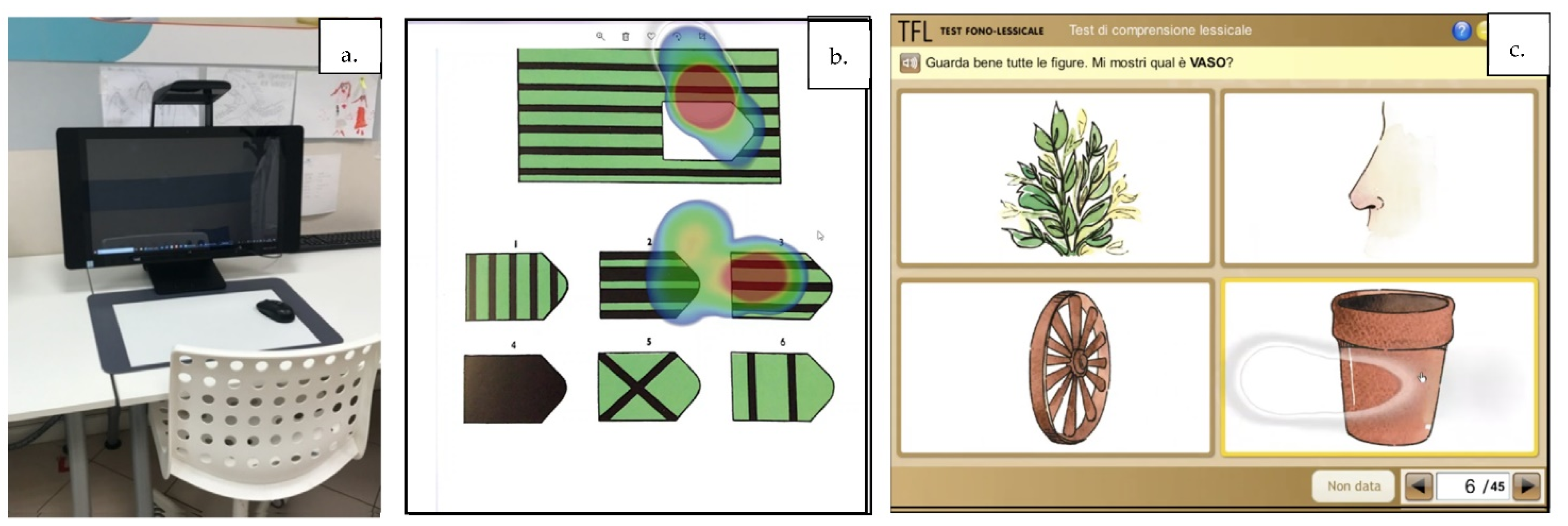
2.2. Language Assessment
2.3. Cognitive Assessment
2.4. Eye Tracking System
3. Results
3.1. Participants
3.2. Cognitive and Language Assessment
3.3. Quality of Life and Parental Stress
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Talvik, I.; Møller, R.S.; Vaher, M.; Vaher, U.; Larsen, L.H.G.; Dahl, H.A.; Ilves, P.; Talvik, T. Clinical Phenotype of De Novo GNAO1 Mutation: Case Report and Review of Literature. Child Neurol. 2015, 2, 2329048X15583717. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, K.; Kodera, H.; Akita, T.; Shiina, M.; Kato, M.; Hoshino, H.; Terashima, H.; Osaka, H.; Nakamura, S.; Tohyama, J.; et al. De Novo mutations in GNAO1, encoding a Galphao subunit of heterotrimeric G proteins, cause epileptic encephalopathy. Am. J. Hum. Genet. 2013, 93, 496–505. [Google Scholar] [CrossRef] [Green Version]
- Schirinzi, T.; Garone, G.; Travaglini, L.; Vasco, G.; Galosi, S.; Rios, L.; Castiglioni, C.; Barassi, C.; Battaglia, D.; Gambardella, M.L.; et al. Phenomenology and clinical course of movement disorder in GNAO1 variants: Results from an analytical review. Parkinsonism Relat. Disord. 2019, 61, 19–25. [Google Scholar] [CrossRef]
- Kelly, M.; Park, M.; Mihalek, I.; Rochtus, A.; Gramm, M.; Perez-Palma, E.; Axeen, E.T.; Hung, C.Y.; Olson, H.; Swanson, L.; et al. Spectrum of neurodevelopmental disease associated with the GNAO1 guanosine triphosphate–binding region. Epilepsia 2019, 60, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Light, J. Toward a definition of communicative competence by individuals using augmentative and alternative communication systems. Augment. Altern. Commun. 1989, 5, 137–144. [Google Scholar] [CrossRef]
- American Speech-Language-Hearing Association. Guidelines for speech-language pathologists serving persons with language, socio-communicative, and/or cognitive-communicative impairments. Committee on Language American Speech-Language-Hearing Association. ASHA 1991, 5, 21–28. [Google Scholar]
- Wilkinson, M.; Madel, M. Eye Tracking Measures Reveal How Changes in the Design of Displays for Augmentative and Alternative Communication Influence Visual Search in Individuals with Down Syndrome or Autism Spectrum Disorder. Am. J. Speech-Lang. Pathol. 2019, 28, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Karatekin, C. Eye tracking studies of normative and atypical development. Dev. Rev. 2007, 27, 283–348. [Google Scholar] [CrossRef]
- Vessoyan, K.; Steckle, G.; Easton, B.; Nichols, M.; Mok Siu, V.; McDougall, J. Using eye-tracking technology for communication in Rett syndrome: Perceptions of impact. Augment. Altern. Commun. 2018, 34, 230–241. [Google Scholar] [CrossRef]
- Branson, D.; Demchak, M. The use of augmentative and alternative communication methods with infants and toddlers with disabilities: A research review. Augment. Altern. Commun. 2009, 25, 274–286. [Google Scholar] [CrossRef]
- Hildebrand, M.S.; Jackson, V.E.; Scerri, T.S.; Van Reyk, O.; Coleman, M.; Braden, R.O.; Turner, S.; Rigbye, K.A.; Boys, A.; Barton, S.; et al. Severe childhood speech disorder. Gene discovery highlights transcriptional dysregulation. Am. Acad. Neurol. 2020, 94, 1–20. [Google Scholar]
- Flores, M.; Musgrove, K.; Renner, S.; Hinton, V.; Strozier, S.; Franklin, S.; Hill, D. A comparison of communication using the Apple iPad and a picture-based system. Augment. Altern. Commun. 2021, 28, 74–84. [Google Scholar] [CrossRef] [Green Version]
- Kagohara, D.M.; van der Meer, L.; Ramdoss, S.; O’Reilly, M.F.; Lancioni, G.E.; Davis, T.N.; Sigafoos, J. Using iPods and iPads in teaching programs for individuals with developmental disabilities: A systematic review. Res. Dev. Disabil. 2013, 34, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Ramdoss, S.; Lang, R.; Mulloy, A.; Franco, J.; O’Reilly, M.; Didden, R.; Lancioni, G. Use of computer-based interventions to teach communication skills to children with autism spectrum disorders: A systematic review. J. Behav. Educ. 2011, 20, 55–76. [Google Scholar] [CrossRef] [Green Version]
- Baptista, P.; Mercadante, M.; Macedo, E.; Schwartzman, J. Cognitive performance in Rett syndrome girls: A pilot study using eye-tracking technology. J. Intellect. Disabil. Res. 2006, 50, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Hirano, D.; Taniguchi, T. Application of eye-tracker to individuals with Rett syndrome: A systematic review. Int. J. Phys. Med. Rehabil. 2015, 3, 1–9. [Google Scholar]
- Ahonniska-Assa, J.; Polack, O.; Saraf, E.; Wine, J.; Silberg, T.; Nissenkorn, A.; Ben-Zeev, B. Assessing cognitive functioning in females with Rett syndrome by eye-tracking methodology. Eur. J. Paediat. Neurol. 2017, 3, 1–7. [Google Scholar] [CrossRef]
- Townend, G.; Marschik, P.; Smeets, E.; van de Berg, R.; van den Berg, M.; Curfs, L. Eye gaze technology as a form of augmentative and alternative communication for individuals with Rett syndrome: Experiences of families in the Netherlands. J. Dev. Phys. Disabil. 2016, 21, 101–112. [Google Scholar] [CrossRef] [Green Version]
- Palisano, R.; Rosenbaum, P.; Walter, S.; Russell, D.; Wood, E.; Galuppi, B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev. Med. Child Neurol. 1997, 39, 214–223. [Google Scholar] [CrossRef]
- Palisano, R.J.; Rosenbaum, P.; Bartlett, D.; Livingston, M.H. Content validity of the expanded and revised gross motor function classification system. Dev. Med. Child Neurol. 2008, 50, 744–750. [Google Scholar] [CrossRef]
- Eliasson, A.-C.; Krumlinde-Sundholm, L.; Rosblad, B.; Beckung, E.; Arner, M.; Ohrvall, A.M.; Rosenbaum, P. The manual ability classification system (MACS) for children with cerebral palsy: Scale development and evidence of validity and reliability. Dev. Med. Child Neurol. 2006, 48, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.E.; Fahn, S.; Marsden, C.D.; Bressman, S.B.; Moskowitz, C.; Friedman, J. Validity and reliability of a rating scale for the primary torsion dystonias. Neurology 1985, 35, 73–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hidecker, M.J.C.; Paneth, N.; Rosenbaum, P.L.; Kent, R.D.; Lillie, J.; Eulenberg, J.B.; Chester, K., Jr.; Johnson, B.; Michalsen, L.; Evatt, M.; et al. Developing and validating the communication function classification system for individuals with cerebral palsy. Dev. Med. Child Neurol. 2011, 53, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.L.; Oakland, T. ABAS II: Adaptive Behavior Assessment System, 2nd ed.; Psych Corp: San Antonio, TX, USA, 2003. [Google Scholar]
- Varni, J.; Seid, M.; Rode, C. The PedsQL™: Measurement Model for the Pediatric Quality of Life Inventory. Med. Care 1999, 37, 126–139. [Google Scholar] [CrossRef]
- Abidin, R.R. Parenting Stress Index-Manual, 3rd ed.; Psychological Assessment Resources: Odessa, FL, USA, 1995. [Google Scholar]
- Vicari, S.; Marotta, L.; Luci, A. TFL Test Fono-Lessicale: Valutazione Delle Abilitaà Lessicali in età Prescolare; Edizione Centro Studi Erickson: Trento, Italy, 2007. [Google Scholar]
- Raven, J. Raven Progressive Matrices. In Handbook of Nonverbal Assessment; McCallum, R.S., Ed.; Giunti Psychometrics: Firenze, Italy, 2003. [Google Scholar]
| Patient | Age at Evaluation (y) | Sex | DNA Substitution | Aminoacidic Substitution | Dystonia | BFMDRS | Epilepsy | Sleep Disturbance | Status Dystonicus | PEG | DBS | Previous ACC System | Ongoing Usual Drugs | Drugs Stopped | Rehabilitation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 15 | F | c.709G > A | p.Glu237Lys | Generalized | 84 | N | N | Y (ICU) | N | Y (14 y) | VOCA with tablet | TXP | CBZ, TBZ, CLD, BZPs and PB | PT, SLT, PSY |
| 2 | 3 | F | c.709G > A | p.Glu237Lys | Generalized | 16 | N | N | Y (ICU) | N | N | no | TXP TBZ | no | PT, NDT, SLT, Swa |
| 3 | 4 | M | c.736G > A | p.Glu246Lys | Generalized | 33 | N | Y | N | N | N | VOCA with double communication buttons | TXP NPR | no | PT, SLT, Swa |
| 4 | 8 | M | c.625C > T | p.Arg209Cys | Generalized | 28 | focal | N | Y | N | Y (8.5 y) | no | CBZ, TBZ, CLD, BZP | Unknown | PT, VT |
| 5 | 7 | M | c.607G > A | p.Gly203Arg | Generalized | 26 | focal | Y | Y | Y (3 y) | N | no | TBZ, CBZ, Baclofen | PB, TXP | NDT and SLT |
| 6 | 3 | F | c.736G > A | p.Glu246Lys | Generalized | 13 | N | N | N | N | N | no | TBZ | Unknown | SLT, NDT |
| Patient | ABAS–General Adaptive Score (M100 ds15) | ABAS–Conceptual Score (M100 ds15) | ABAS–Social Score (M100 ds15) | ABAS–Practical Score (M100 ds15) | PSI–Total Stress % | PedsQ–HRQoL % | RPM Raw Score/% or IQ | TFL–Lexical Comprehension Raw Score/% |
|---|---|---|---|---|---|---|---|---|
| 1 | 45 | 45 | 51 | 45 | 75 | 26 | 23 na | 33/45 <5% |
| 2 | 45 | 45 | 51 | 45 | 90 | 42 | 25 >95%/130 | 31/45 75–90% |
| 3 | 45 | 45 | 56 | 45 | 80 | 43 | 17 >95%/130 | 30/45 10–25% |
| 4 | 45 | 45 | 55 | 45 | 90 | 22 | na na | 24/45 <5% |
| 5 | 45 | 45 | 55 | 45 | 80 | 36 | 12 na | 36/45 10–25% |
| 6 | 45 | 45 | 56 | 45 | 90 | 83 | 30 >95%/130 | 42/45 >95% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graziola, F.; Garone, G.; Grasso, M.; Capuano, A. Cognitive Assessment in GNAO1 Neurodevelopmental Disorder Using an Eye Tracking System. J. Clin. Med. 2021, 10, 3541. https://doi.org/10.3390/jcm10163541
Graziola F, Garone G, Grasso M, Capuano A. Cognitive Assessment in GNAO1 Neurodevelopmental Disorder Using an Eye Tracking System. Journal of Clinical Medicine. 2021; 10(16):3541. https://doi.org/10.3390/jcm10163541
Chicago/Turabian StyleGraziola, Federica, Giacomo Garone, Melissa Grasso, and Alessandro Capuano. 2021. "Cognitive Assessment in GNAO1 Neurodevelopmental Disorder Using an Eye Tracking System" Journal of Clinical Medicine 10, no. 16: 3541. https://doi.org/10.3390/jcm10163541
APA StyleGraziola, F., Garone, G., Grasso, M., & Capuano, A. (2021). Cognitive Assessment in GNAO1 Neurodevelopmental Disorder Using an Eye Tracking System. Journal of Clinical Medicine, 10(16), 3541. https://doi.org/10.3390/jcm10163541






