The Inflammatory Milieu of Amniotic Fluid Increases with Chorio-Deciduitis Grade in Inflammation-Restricted to Choriodecidua, but Not Amnionitis, of Extra-Placental Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Clinical Characteristics and Pregnancy Outcomes
2.3. Diagnosis of Chorio-Deciduitis and Amnionitis
2.4. The Studies of Amniotic Fluid (AF)
2.5. Early Onset Neonatal Sepsis
2.6. Statistical Analysis
3. Results
3.1. Clinical Characteristics and Pregnancy Outcomes According to Chorio-Deciduitis Grade in the Context of Inflammation Restricted to Chorio-Decidua (CD) and Amnionitis
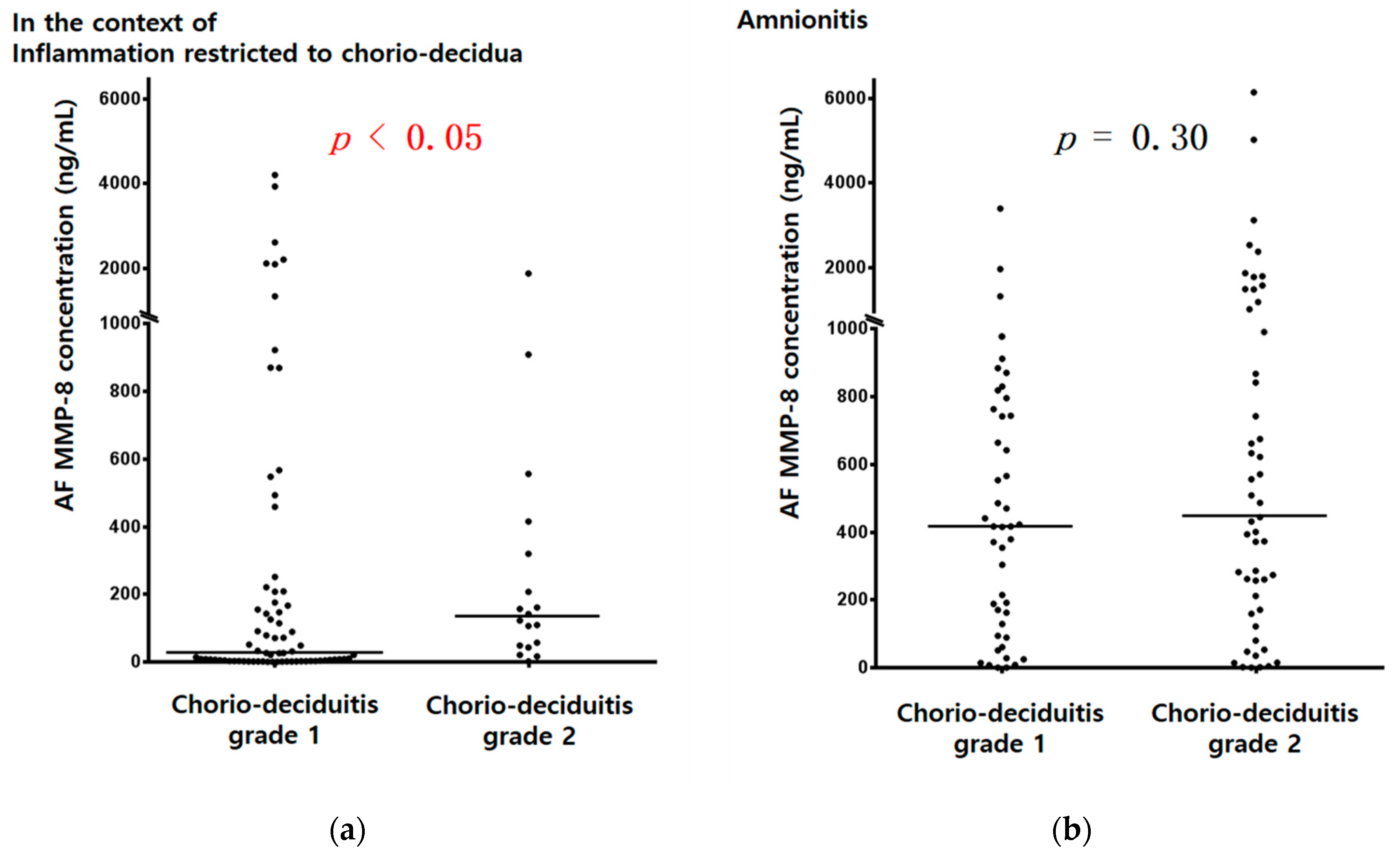
3.2. Amniotic Fluid (AF) MMP-8 Concentrations and AF WBC Counts According to Chorio-Deciduitis Grade in the Context of Inflammation Restricted to Chorio-Decidua (CD) and Amnionitis
3.3. Early Onset Neonatal Sepsis According to Chorio-Deciduitis Grade in the Context of Inflammation Restricted to Chorio-Decidua (CD) and Amnionitis
3.4. Positive Amniotic Fluid (AF) Culture According to Chorio-Deciduitis Grade in the Context of Inflammation Restricted to Chorio-Decidua (CD) and Amnionitis
3.5. Histopathology According to Chorio-Deciduitis Grade in the Context of Inflammation Restricted to Chorio-Decidua (CD) and Amnionitis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goldenberg, R.L.; Hauth, J.C.; Andrews, W.W. Intrauterine infection and preterm delivery. N. Engl. J. Med. 2000, 342, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- Helmo, F.R.; Alves, E.A.R.; Moreira, R.A.A.; Severino, V.O.; Rocha, L.P.; Monteiro, M.L.G.D.R.; Reis, M.A.D.; Etchebehere, R.M.; Machado, J.R.; Corrêa, R.R.M. Intrauterine infection, immune system and premature birth. J. Matern. Fetal Neonatal Med. 2018, 31, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, V.; Hirsch, E. Intrauterine infection and preterm labor. Semin. Fetal Neonatal Med. 2012, 17, 12–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kemp, M.W. Preterm birth, intrauterine infection, and fetal inflammation. Front. Immunol. 2014, 5, 574. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.J.; Gur, T.L. Intrauterine Microbiota: Missing, or the Missing Link? Trends Neurosci. 2019, 42, 402–413. [Google Scholar] [CrossRef]
- Romero, R.; Mazor, M. Infection and preterm labor. Clin. Obstet. Gynecol. 1988, 31, 553–584. [Google Scholar] [CrossRef] [PubMed]
- Cappelletti, M.; Presicce, P.; Kallapur, S.G. Immunobiology of Acute Chorioamnionitis. Front. Immunol. 2020, 11, 649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petit, E.; Abergel, A.; Dedet, B. The role of infection in preterm birth. J. Gynecol. Obstet. Biol. Reprod. 2012, 41, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.; Dunlop, A.L.; Kramer, M.R.; Fortunato, S.J.; Hogue, C.J. An overview of racial disparities in preterm birth rates: Caused by infection or inflammatory response? Acta Obstet. Gynecol. Scand. 2011, 90, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, L.F.; Chaiworapongsa, T.; Romero, R. Intrauterine infection and prematurity. Ment. Retard. Dev. Disabil. Res. Rev. 2002, 8, 3–13. [Google Scholar] [CrossRef]
- Stinson, L.F.; Payne, M.S. Infection-mediated preterm birth: Bacterial origins and avenues for intervention. Aust. N. Z. J. Obstet. Gynaecol. 2019, 59, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Pavlidis, I.; Spiller, O.B.; Demarco, G.S.; MacPherson, H.; Howie, S.E.M.; Norman, J.E.; Stock, S.J. Cervical epithelial damage promotes Ureaplasma parvum ascending infection, intrauterine inflammation and preterm birth induction in mice. Nat. Commun. 2020, 11, 199. [Google Scholar] [CrossRef] [PubMed]
- Bayar, E.; Bennett, P.R.; Chan, D.; Sykes, L.; MacIntyre, D.A. The pregnancy microbiome and preterm birth. Semin. Immunopathol. 2020, 42, 487–499. [Google Scholar] [CrossRef]
- Kim, C.J.; Romero, R.; Chaemsaithong, P.; Chaiyasit, N.; Yoon, B.H.; Kim, Y.M. Acute chorioamnionitis and funisitis: Definition, pathologic features, and clinical significance. Am. J. Obstet. Gynecol. 2015, 213, S29–S52. [Google Scholar] [CrossRef] [Green Version]
- Nadeau, H.C.; Subramaniam, A.; Andrews, W.W. Infection and preterm birth. Semin. Fetal Neonatal Med. 2016, 21, 100–105. [Google Scholar] [CrossRef]
- Park, C.W.; Kim, S.M.; Park, J.S.; Jun, J.K.; Yoon, B.H. Fetal, amniotic and maternal inflammatory responses in early stage of ascending intrauterine infection, inflammation restricted to chorio-decidua, in preterm gestation. J. Matern. Fetal Neonatal Med. 2014, 27, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Abehsera, D.; Rodrigues, Y.; Mingorance, J.; Suárez, A.; Magdaleno, F.; Bartha, J.L. Prediction and clinical relevance of pathologic patterns of injury associated with chorioamnionitis. Placenta 2014, 35, 70–71. [Google Scholar] [CrossRef]
- Buhimschi, I.A.; Zambrano, E.; Pettker, C.M.; Bahtiyar, M.O.; Paidas, M.; Rosenberg, V.A.; Thung, S.; Salafia, C.M.; Buhimschi, C.S. Using proteomic analysis of the human amniotic fluid to identify histologic chorioamnionitis. Obstet. Gynecol. 2008, 111, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Hockney, R.; Waring, G.J.; Taylor, G.; Cummings, S.P.; Robson, S.C.; Orr, C.H.; Nelson, A. Fetal membrane bacterial load is increased in histologically confirmed inflammatory chorioamnionitis: A retrospective cohort study. Placenta 2020, 91, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Park, C.W.; Moon, K.C.; Park, J.S.; Jun, J.K.; Romero, R.; Yoon, B.H. The involvement of human amnion in histologic chorioamnionitis is an indicator that a fetal and an intra-amniotic inflammatory response is more likely and severe: Clinical implications. Placenta 2009, 30, 56–61. [Google Scholar] [CrossRef] [Green Version]
- Yoneda, S.; Shiozaki, A.; Ito, M.; Yoneda, N.; Inada, K.; Yonezawa, R.; Kigawa, M.; Saito, S. Accurate Prediction of the Stage of Histological Chorioamnionitis before Delivery by Amniotic Fluid IL-8 Level. Am. J. Reprod. Immunol. 2015, 73, 568–576. [Google Scholar] [CrossRef]
- Kidokoro, K.; Furuhashi, M.; Kuno, N.; Ishikawa, K. Amniotic fluid neutrophil elastase and lactate dehydrogenase: Association with histologic chorioamnionitis. Acta Obstet. Gynecol. Scand. 2006, 85, 669–674. [Google Scholar] [CrossRef]
- Miura, H.; Ogawa, M.; Hirano, H.; Sanada, H.; Sato, A.; Obara, M.; Terada, Y. Neutrophil elastase and interleukin-6 in amniotic fluid as indicators of chorioamnionitis and funisitis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 158, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Park, C.W.; Moon, K.C.; Park, J.S.; Jun, J.K.; Yoon, B.H. The frequency and clinical significance of intra-uterine infection and inflammation in patients with placenta previa and preterm labor and intact membranes. Placenta 2009, 30, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.W.; Park, C.W.; Moon, K.C.; Park, J.S.; Jun, J.K. Acute Chorioamnionitis and Intra-amniotic Inflammation are More Severe according to Outside-in Neutrophil Migration within the Same Chorio-decidua. Taiwan J. Obstet. Gynecol. accepted.
- Mauri, A.; Perrini, M.; Mateos, J.M.; Maake, C.; Ochsenbein-Koelble, N.; Zimmermann, R.; Ehrbar, M.; Mazza, E. Second harmonic generation microscopy of fetal membranes under deformation: Normal and altered morphology. Placenta 2013, 34, 1020–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.; Kedige, S.D.; Jain, K. Amnion and Chorion Membranes: Potential Stem Cell Reservoir with Wide Applications in Periodontics. Int. J. Biomater. 2015, 2015, 274082. [Google Scholar] [CrossRef] [Green Version]
- Avila, C.; Santorelli, J.; Mathai, J.; Ishkin, S.; Jabsky, M.; Willins, J.; Figueroa, R.; Kaplan, C. Anatomy of the fetal membranes using optical coherence tomography: Part 1. Placenta 2014, 35, 1065–1069. [Google Scholar] [CrossRef]
- Park, C.W.; Oh, J.W.; Moon, K.C.; Park, J.S.; Jun, J.K. Amniotic necrosis is associated with severe and advanced acute histologic chorioamnionitis. Placenta 2017, 57, 285. [Google Scholar] [CrossRef]
- Seong, J.S.; Park, C.W.; Moon, K.C.; Park, J.S.; Jun, J.K. Necrotizing funisitis is an indicator that intra-amniotic inflammatory response is more severe and amnionitis is more frequent in the context of the extension of inflammation into Wharton’s jelly. Taiwan J. Obstet. Gynecol. accepted.
- Moon, K.C.; Oh, J.W.; Park, C.W.; Park, J.S.; Jun, J.K. The Relationship Among Intra-Amniotic Inflammatory Response, The Progression of Inflammation in Chorionic Plate and Early-Onset Neonatal Sepsis. Front. Pediatr. 2021, 9, 582472:1–582472:9. [Google Scholar] [CrossRef]
- Kim, S.M.; Romero, R.; Park, J.W.; Oh, K.J.; Jun, J.K.; Yoon, B.H. The relationship between the intensity of intraamniotic inflammation and the presence and severity of acute histologic chorioamnionitis in preterm gestation. J. Matern. Fetal Neonatal Med. 2015, 28, 1500–1509. [Google Scholar] [CrossRef] [Green Version]
- Park, C.W.; Yoon, B.H.; Kim, S.M.; Park, J.S.; Jun, J.K. Which is more important for the intensity of intra-amniotic inflammation between total grade or involved anatomical region in preterm gestations with acute histologic chorioamnionitis? Obstet. Gynecol. Sci. 2013, 56, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Yoon, B.H.; Jun, J.K.; Park, K.H.; Syn, H.C.; Gomez, R.; Romero, R. Serum C-reactive protein, white blood cell count, and amniotic fluid white blood cell count in women with preterm premature rupture of membranes. Obstet. Gynecol. 1996, 88, 1034–1040. [Google Scholar] [CrossRef]
- Yoon, B.H.; Yang, S.H.; Jun, J.K.; Park, K.H.; Kim, C.J.; Romero, R. Maternal blood C-reactive protein, white blood cell count, and temperature in preterm labor: A comparison with amniotic fluid white blood cell count. Obstet. Gynecol. 1996, 87, 231–237. [Google Scholar] [CrossRef]
- Yoon, B.H.; Romero, R.; Kim, C.J.; Jun, J.K.; Gomez, R.; Choi, J.H.; Syn, H.C. Amniotic fluid interleukin-6: A sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am. J. Obstet. Gynecol. 1995, 172, 960–970. [Google Scholar] [CrossRef]
- Park, J.S.; Romero, R.; Yoon, B.H.; Moon, J.B.; Oh, S.Y.; Han, S.Y.; Ko, E.M. The relationship between amniotic fluid matrix metalloproteinase-8 and funisitis. Am. J. Obstet. Gynecol. 2001, 185, 1156–1161. [Google Scholar] [CrossRef]
- Romero, R.; Salafia, C.M.; Athanassiadis, A.P.; Hanaoka, S.; Mazor, M.; Sepulveda, W.; Bracken, M.B. The relationship between acute inflammatory lesions of the preterm placenta and amniotic fluid microbiology. Am. J. Obstet. Gynecol. 1992, 166, 1382–1388. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Andrews, W.W.; Hauth, J.C. Choriodecidual infection and preterm birth. Nutr. Rev. 2002, 60, S19–S25. [Google Scholar] [CrossRef] [PubMed]
- Grigsby, P.L.; Novy, M.J.; Waldorf, K.M.A.; Sadowsky, D.W.; Gravett, M.G. Choriodecidual inflammation: A harbinger of the preterm labor syndrome. Reprod. Sci. 2010, 17, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Suff, N.; Karda, R.; Diaz, J.A.; Ng, J.; Baruteau, J.; Perocheau, D.; Tangney, M.; Taylor, P.W.; Peebles, D.; Buckley, S.M.K.; et al. Ascending Vaginal Infection Using Bioluminescent Bacteria Evokes Intrauterine Inflammation, Preterm Birth, and Neonatal Brain Injury in Pregnant Mice. Am. J. Pathol. 2018, 188, 2164–2176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldorf, K.M.A.; Rubens, C.E.; Gravett, M.G. Use of nonhuman primate models to investigate mechanisms of infection-associated preterm birth. BJOG Int. J. Obstet. Gynaecol. 2011, 118, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Fortner, K.B.; Grotegut, C.A.; Ransom, C.E.; Bentley, R.C.; Feng, L.; Lan, L.; Heine, R.P.; Seed, P.C.; Murtha, A.P. Bacteria localization and chorion thinning among preterm premature rupture of membranes. PLoS ONE 2014, 9, e83338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.J.; Romero, R.; Gervasi, M.T.; Kim, J.S.; Yoo, W.; Lee, D.C.; Mittal, P.; Erez, O.; Kusanovic, J.P.; Hassan, S.S.; et al. Widespread microbial invasion of the chorioamniotic membranes is a consequence and not a cause of intra-amniotic infection. Lab. Investig. 2009, 89, 924–936. [Google Scholar] [CrossRef]
- Jefferson, K.K. The bacterial etiology of preterm birth. Adv. Appl. Microbiol. 2012, 80, 1–22. [Google Scholar] [CrossRef]
- Romero, R.; Gómez, R.; Chaiworapongsa, T.; Conoscenti, G.; Kim, J.C.; Kim, Y.M. The role of infection in preterm labour and delivery. Paediatr. Perinat. Epidemiol. 2001, 15 (Suppl. S2), 41–56. [Google Scholar] [CrossRef]
- Stranik, J.; Kacerovsky, M.; Andrys, C.; Soucek, O.; Bolehovska, R.; Holeckova, M.; Matulova, J.; Jacobsson, B.; Musilova, I. Intra-amniotic infection and sterile intra-amniotic inflammation are associated with elevated concentrations of cervical fluid interleukin-6 in women with spontaneous preterm labor with intact membranes. J. Matern. Fetal Neonatal Med. 2021. [Google Scholar] [CrossRef]
- Yoon, B.H.; Romero, R.; Moon, J.B.; Shim, S.S.; Kim, M.; Kim, G.; Jun, J.K. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am. J. Obstet. Gynecol. 2001, 185, 1130–1136. [Google Scholar] [CrossRef]
- Combs, C.A.; Gravett, M.; Garite, T.J.; Hickok, D.E.; Lapidus, J.; Porreco, R.; Rael, J.; Grove, T.; Morgan, T.K.; Clewell, W.; et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am. J. Obstet. Gynecol. 2014, 210, 125.e1–125.e15. [Google Scholar] [CrossRef]
- Shim, S.S.; Romero, R.; Hong, J.S.; Park, C.W.; Jun, J.K.; Kim, B.I.; Yoon, B.H. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am. J. Obstet. Gynecol. 2004, 191, 1339–1345. [Google Scholar] [CrossRef]
- Cobo, T.; Kacerovsky, M.; Palacio, M.; Hornychova, H.; Hougaard, D.M.; Skogstrand, K.; Jacobsson, B. Intra-amniotic inflammatory response in subgroups of women with preterm prelabor rupture of the membranes. PLoS ONE 2012, 7, e43677. [Google Scholar] [CrossRef] [Green Version]
- Cobo, T.; Kacerovsky, M.; Holst, R.M.; Hougaard, D.M.; Skogstrand, K.; Wennerholm, U.B.; Hagberg, H.; Jacobsson, B. Intra-amniotic inflammation predicts microbial invasion of the amniotic cavity but not spontaneous preterm delivery in preterm prelabor membrane rupture. Acta Obstet. Gynecol. Scand. 2012, 91, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.W.; Shen, T.; Chung, P.; Buhimschi, I.A.; Buhimschi, C.S. Uncultivated bacteria as etiologic agents of intra-amniotic inflammation leading to preterm birth. J. Clin. Microbiol. 2009, 47, 38–47. [Google Scholar] [CrossRef] [Green Version]
- Yoon, B.H.; Romero, R.; Kim, M.; Kim, E.C.; Kim, T.; Park, J.S.; Jun, J.K. Clinical implications of detection of Ureaplasma urealyticum in the amniotic cavity with the polymerase chain reaction. Am. J. Obstet. Gynecol. 2000, 183, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Stinson, L.; Hallingström, M.; Barman, M.; Viklund, F.; Keelan, J.; Kacerovsky, M.; Payne, M.; Jacobsson, B. Comparison of Bacterial DNA Profiles in Mid-Trimester Amniotic Fluid Samples from Preterm and Term Deliveries. Front. Microbiol. 2020, 11, 415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keskin, F.; Ciftci, S.; Keceli, S.A.; Koksal, M.O.; Caliskan, E.; Cakiroglu, Y.; Agacfidan, A. Comparison of culture and real-time polymerase chain reaction methods for detection of Mycoplasma hominis in amniotic fluids samples. Niger. J. Clin. Pract. 2018, 21, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, N.; Fernandez, C.; Zamora, Y.; Berdasquera, D.; Rivera, J.A. Detection of Ureaplasma urealyticum and Ureaplasma parvum in amniotic fluid: Association with pregnancy outcomes. J. Matern. Fetal Neonatal Med. 2011, 24, 47–50. [Google Scholar] [CrossRef]
- Yoon, B.H.; Romero, R.; Lim, J.H.; Shim, S.S.; Hong, J.S.; Shim, J.Y.; Jun, J.K. The clinical significance of detecting Ureaplasma urealyticum by the polymerase chain reaction in the amniotic fluid of patients with preterm labor. Am. J. Obstet. Gynecol. 2003, 189, 919–924. [Google Scholar] [CrossRef]
- Morimoto, S.; Usui, H.; Kobayashi, T.; Katou, E.; Goto, S.; Tanaka, H.; Shozu, M. Bacterial-Culture-Negative Subclinical Intra-Amniotic Infection Can Be Detected by Bacterial 16S Ribosomal-DNA-Amplifying Polymerase Chain Reaction. Jpn. J. Infect. Dis. 2018, 71, 274–280. [Google Scholar] [CrossRef]
- Marconi, C.; de Andrade Ramos, B.R.; Peraçoli, J.C.; Donders, G.G.; da Silva, M.G. Amniotic fluid interleukin-1 beta and interleukin-6, but not interleukin-8 correlate with microbial invasion of the amniotic cavity in preterm labor. Am. J. Reprod. Immunol. 2011, 65, 549–656. [Google Scholar] [CrossRef] [PubMed]
- DiGiulio, D.B. Diversity of microbes in amniotic fluid. Semin. Fetal Neonatal Med. 2012, 17, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Park, C.W.; Yoon, B.H.; Kim, S.M.; Park, J.S.; Jun, J.K. The frequency and clinical significance of intra-amniotic inflammation defined as an elevated amniotic fluid matrix metalloproteinase-8 in patients with preterm labor and low amniotic fluid white blood cell counts. Obstet. Gynecol. Sci. 2013, 56, 167–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Revello, R.; Alcaide, M.J.; Dudzik, D.; Abehsera, D.; Bartha, J.L. Differential amniotic fluid cytokine profile in women with chorioamnionitis with and without funisitis. J. Matern. Fetal Neonatal Med. 2016, 29, 2161–2165. [Google Scholar] [CrossRef]
- Angus, S.R.; Segel, S.Y.; Hsu, C.D.; Locksmith, G.J.; Clark, P.; Sammel, M.D.; Macones, G.A.; Strauss, J.F., 3rd; Parry, S. Amniotic fluid matrix metalloproteinase-8 indicates intra-amniotic infection. Am. J. Obstet. Gynecol. 2001, 185, 1232–1238. [Google Scholar] [CrossRef]
- Kim, A.; Lee, E.S.; Shin, J.C.; Kim, H.Y. Identification of biomarkers for preterm delivery in mid-trimester amniotic fluid. Placenta 2013, 34, 873–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myntti, T.; Rahkonen, L.; Nupponen, I.; Pätäri-Sampo, A.; Tikkanen, M.; Sorsa, T.; Juhila, J.; Andersson, S.; Paavonen, J.; Stefanovic, V. Amniotic Fluid Infection in Preterm Pregnancies with Intact Membranes. Dis. Markers 2017, 2017, 8167276. [Google Scholar] [CrossRef]
- Myntti, T.; Rahkonen, L.; Pätäri-Sampo, A.; Tikkanen, M.; Sorsa, T.; Juhila, J.; Helve, O.; Andersson, S.; Paavonen, J.; Stefanovic, V. Comparison of amniotic fluid matrix metalloproteinase-8 and cathelicidin in the diagnosis of intra-amniotic infection. J. Perinatol. 2016, 36, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Quintero, R.; Nores, J.; Avila, C.; Mazor, M.; Hanaoka, S.; Hagay, Z.; Merchant, L.; Hobbins, J.C. Amniotic fluid white blood cell count: A rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am. J. Obstet. Gynecol. 1991, 165, 821–830. [Google Scholar] [CrossRef]
- Fan, S.R.; Liu, P.; Yan, S.M.; Peng, J.Y.; Liu, X.P. Diagnosis and Management of Intraamniotic Infection. Matern. Fetal Med. 2020, 2, 223–230. [Google Scholar] [CrossRef]
- Romero, R.; Yoon, B.H.; Mazor, M.; Gomez, R.; Diamond, R.X.; Kenney, J.S.; Ramirez, M.; Fidel, P.L.; Sorokin, Y.; Cotton, D.; et al. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and gram stain in patients with preterm labor and intact membranes. Am. J. Obstet. Gynecol. 1993, 169, 805–816. [Google Scholar] [CrossRef]
- Abdel-Razeq, S.S.; Buhimschi, I.A.; Bahtiyar, M.O.; Rosenberg, V.A.; Dulay, A.T.; Han, C.S.; Werner, E.F.; Thung, S.; Buhimschi, C.S. Interpretation of amniotic fluid white blood cell count in “bloody tap” amniocenteses in women with symptoms of preterm labor. Obstet. Gynecol. 2010, 116, 344–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.W.; Park, J.S.; Jun, J.K.; Yoon, B.H. The inflammatory milieu of amniotic fluid in acute-chorioamnionitis decreases with increasing gestational age. Placenta 2015, 36, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Park, C.W.; Yoon, B.H.; Park, J.S.; Jun, J.K. A fetal and an intra-amniotic inflammatory response is more severe in preterm labor than in preterm PROM in the context of funisitis: Unexpected observation in human gestations. PLoS ONE 2013, 8, e62521. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Chorio-Deciduitis Grade 1 | Chorio-Deciduitis Grade 2 | p † | |
|---|---|---|---|
| Inflammation restricted to CD (n = 93) | (n = 74) | (n = 19) | |
| Maternal age, years (mean ± SD) | 30.0 ± 4.6 | 31.1 ± 3.3 | 0.230 |
| Nulliparity | 51.4% (38/74) | 68.4% (13/19) | 0.207 |
| Causes of preterm birth | 0.071 | ||
| PTL | 44.6% (33/74) | 21.1% (4/19) | |
| Preterm-PROM | 55.4% (41/74) | 78.9% (15/19) | |
| GA at amniocentesis, (weeks) median, range | 32.9 (23.0, 35.6) | 32.6 (23.0, 35.6) | 0.277 |
| GA at delivery, (weeks) median, range | 33.1 (23.4, 35.7) | 32.7 (23.3, 35.7) | 0.466 |
| Amniocentesis-to-delivery interval, (hours) median, range | 19.20 (0.01, 159.80) | 53.30 (0.01, 152.80) | 0.053 |
| Birth weight, g (mean ± SD) | 1854 ± 645 | 1691 ± 643 | 0.282 |
| Male newborn | 58.1% (43/74) | 57.9% (11/19) | 1.000 |
| Cesarean section | 33.8% (25/74) | 36.8% (7/19) | 0.793 |
| Apgar score at 1 min <7 | 45.9% (34/74) | 47.4% (9/19) | 1.000 |
| Apgar score at 5 min <7 | 28.4% (21/74) | 15.8% (3/19) | 0.381 |
| Gestational diabetes mellitus | 1.4% (1/74) | 0% (0/19) | 1.000 |
| Antenatal use of antibiotics †† | 60.3% (44/73) | 84.2% (16/19) | 0.061 |
| Suspected early onset neonatal sepsis ‡ | 8.6% (6/70) | 10.5% (2/19) | 0.677 |
| Proven early onset neonatal sepsis ‡ | 2.9% (2/70) | 10.5% (2/19) | 0.199 |
| Suspected or proven early onset neonatal sepsis ‡ | 10.0% (7/70) | 21.1% (4/19) | 0.239 |
| Chorio-Deciduitis Grade 1 | Chorio-Deciduitis Grade 2 | p† | |
|---|---|---|---|
| Amnionitis (n = 102) | (n = 49) | (n = 53) | |
| Maternal age, years (mean ± SD) | 30.4 ± 4.5 | 30.9 ± 4.6 | 0.573 |
| Nulliparity | 36.7% (18/49) | 39.6% (21/53) | 0.840 |
| Causes of preterm birth | 0.420 | ||
| PTL | 44.9% (22/49) | 35.8% (19/53) | |
| Preterm-PROM | 55.1% (27/49) | 64.2% (34/53) | |
| GA at amniocentesis, (weeks) median, range | 30.4 (24.1, 35.1) | 29.1 (21.6, 35.1) | 0.135 |
| GA at delivery, (weeks) median, range | 31.1 (24.1, 35.3) | 29.3 (21.6, 35.7) | 0.108 |
| Amniocentesis-to-delivery interval, (h) median, range | 32.80 (0.01, 163.70) | 14.10 (0.01, 161.70) | 0.503 |
| Birth weight, g (mean ± SD) | 1524 ± 501 | 1421 ± 584 | 0.260 |
| Male newborn | 46.9% (23/49) | 39.6% (21/53) | 0.549 |
| Cesarean section | 30.6% (15/49) | 22.6% (12/53) | 0.379 |
| Apgar score at 1 min <7 | 61.2% (30/49) | 64.2% (34/53) | 0.839 |
| Apgar score at 5 min <7 | 36.7% (18/49) | 39.6% (21/53) | 0.840 |
| Gestational diabetes mellitus | 2.0% (1/49) | 5.7% (3/53) | 0.619 |
| Antenatal use of antibiotics †† | 79.2% (38/48) | 78.8% (41/52) | 1.000 |
| Suspected early onset neonatal sepsis ‡ | 25.0% (12/48) | 20.8% (10/48) | 0.809 |
| Proven early onset neonatal sepsis ‡ | 6.2% (3/48) | 6.2% (3/48) | 1.000 |
| Suspected or proven early onset neonatal sepsis ‡ | 31.2% (15/48) | 27.1% (13/48) | 0.823 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.H.; Park, C.-W.; Moon, K.C.; Park, J.S.; Jun, J.K. The Inflammatory Milieu of Amniotic Fluid Increases with Chorio-Deciduitis Grade in Inflammation-Restricted to Choriodecidua, but Not Amnionitis, of Extra-Placental Membranes. J. Clin. Med. 2021, 10, 3041. https://doi.org/10.3390/jcm10143041
Lee JH, Park C-W, Moon KC, Park JS, Jun JK. The Inflammatory Milieu of Amniotic Fluid Increases with Chorio-Deciduitis Grade in Inflammation-Restricted to Choriodecidua, but Not Amnionitis, of Extra-Placental Membranes. Journal of Clinical Medicine. 2021; 10(14):3041. https://doi.org/10.3390/jcm10143041
Chicago/Turabian StyleLee, Joon Hyung, Chan-Wook Park, Kyung Chul Moon, Joong Shin Park, and Jong Kwan Jun. 2021. "The Inflammatory Milieu of Amniotic Fluid Increases with Chorio-Deciduitis Grade in Inflammation-Restricted to Choriodecidua, but Not Amnionitis, of Extra-Placental Membranes" Journal of Clinical Medicine 10, no. 14: 3041. https://doi.org/10.3390/jcm10143041
APA StyleLee, J. H., Park, C.-W., Moon, K. C., Park, J. S., & Jun, J. K. (2021). The Inflammatory Milieu of Amniotic Fluid Increases with Chorio-Deciduitis Grade in Inflammation-Restricted to Choriodecidua, but Not Amnionitis, of Extra-Placental Membranes. Journal of Clinical Medicine, 10(14), 3041. https://doi.org/10.3390/jcm10143041







