Low and High Frequency Vibration Perception Thresholds Can Improve the Diagnosis of Diabetic Neuropathy
Abstract
:1. Introduction
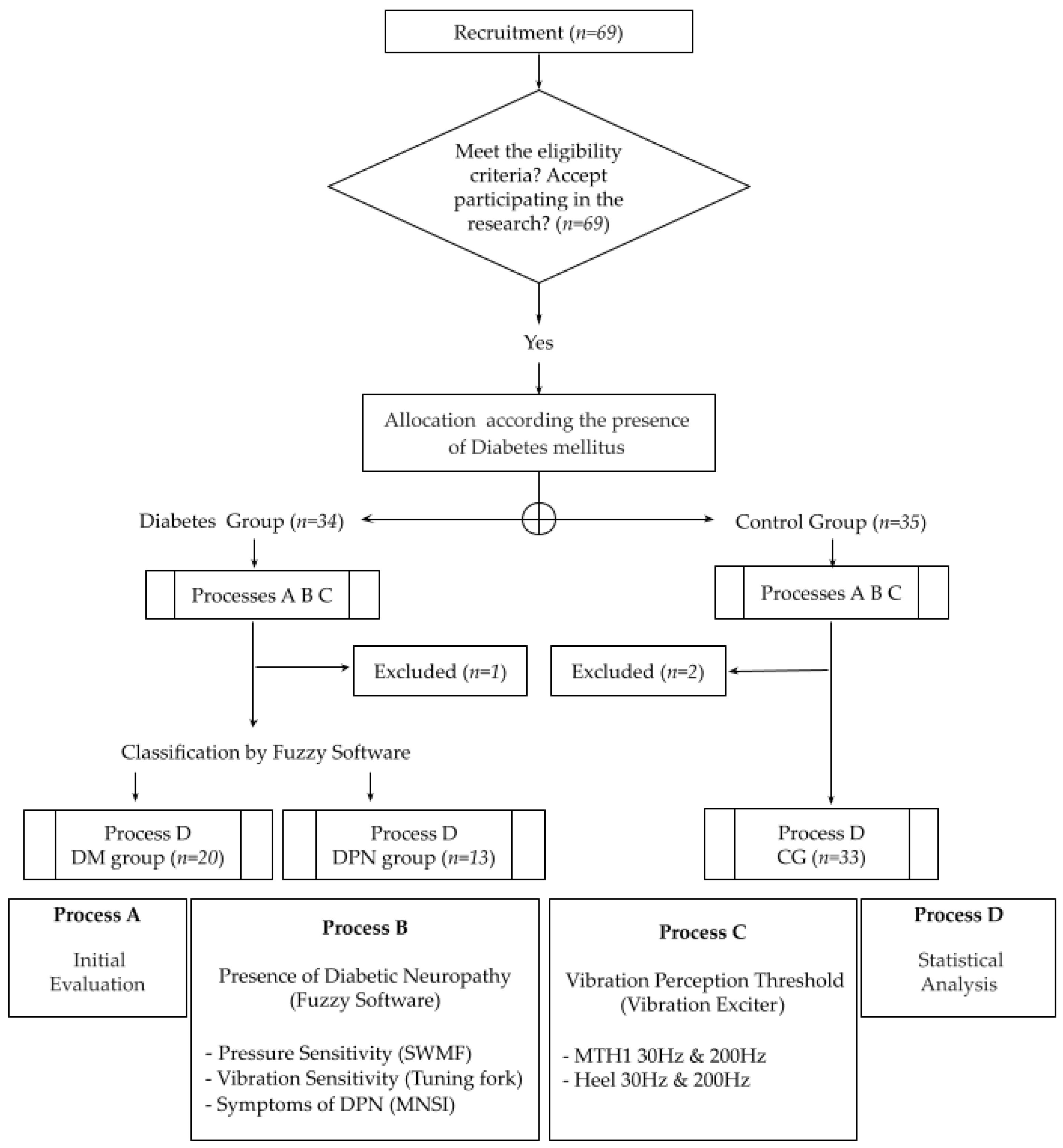
2. Materials and Methods
2.1. Diagnosis and Classification of DPN Severity: Fuzzy Score
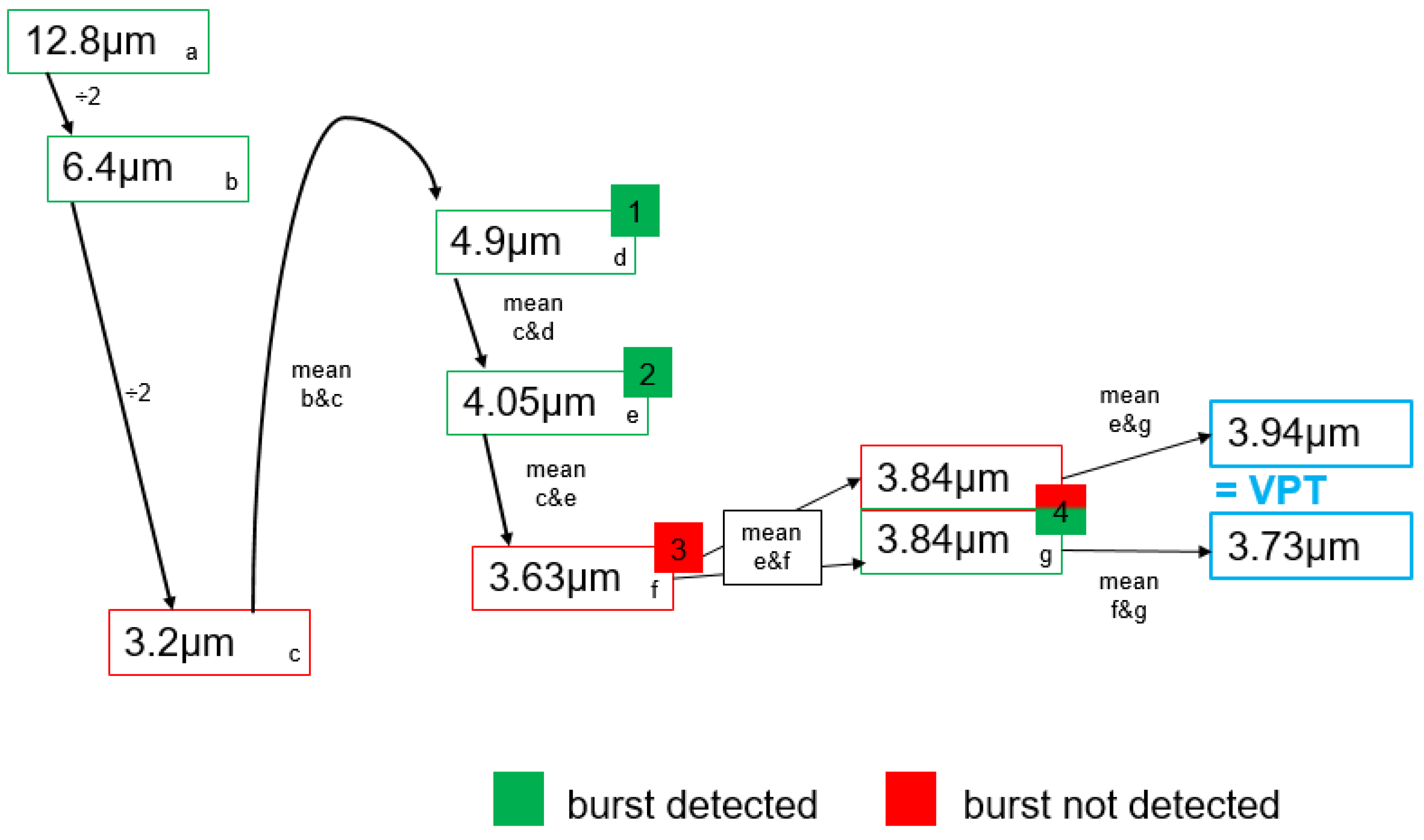
2.2. Vibration Perception Thresholds
2.3. Statistical Analysis
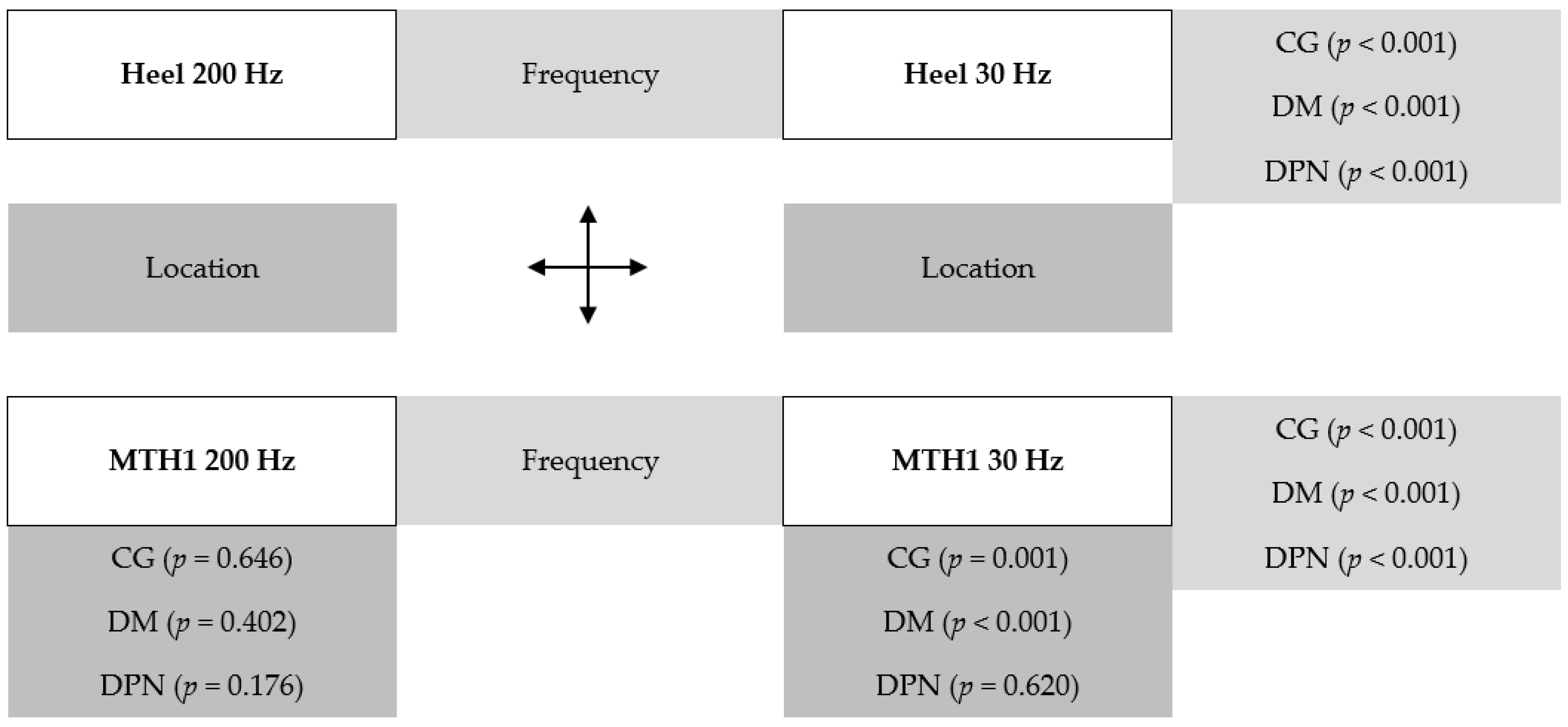
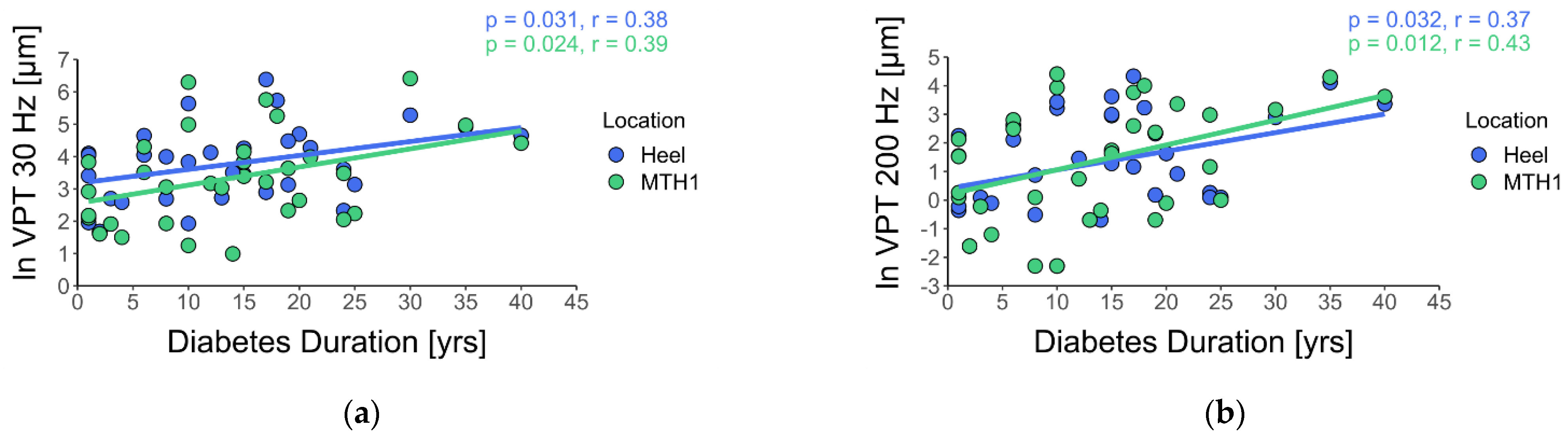
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Group | GLM | Residual Standard Error | Adjusted R-Squared | p-Value GLM | Significant Variables |
|---|---|---|---|---|---|
| CG | Heel 30 Hz ~ age + sex + BMI | 0.641 | 0.577 | p < 0.001 | page < 0.001 psex = 0.037 |
| Heel 200 Hz ~ age + sex + BMI | 1.003 | 0.585 | p < 0.001 | page < 0.001 | |
| MTH1 30 Hz ~ age + sex + BMI | 0.725 | 0.500 | p < 0.001 | page < 0.001 | |
| MTH1 200 Hz ~ age + sex + BMI | 1.322 | 0.458 | p < 0.001 | page < 0.001 | |
| DM | Heel 30 Hz ~ age + sex + BMI | 0.876 | 0.054 | p = 0.290 | - |
| Heel 200 Hz ~ age + sex + BMI | 1.382 | 0.131 | p = 0.161 | page = 0.032 | |
| MTH1 30 Hz ~ age + sex + BMI | 0.951 | 0.040 | p = 0.320 | - | |
| MTH1 200 Hz ~ age + sex + BMI | 1.486 | 0.168 | p = 0.119 | page = 0.034 | |
| DPN | Heel 30 Hz ~ age + sex + BMI | 0.957 | −0.099 | p = 0.609 | - |
| Heel 200 Hz ~ age + sex + BMI | 1.106 | 0.231 | p = 0.158 | - | |
| MTH1 30 Hz ~ age + sex + BMI | 0.873 | 0.459 | p = 0.036 | pBMI = 0.031 | |
| MTH1 200 Hz ~ age + sex + BMI | 1.125 | 0.147 | p = 0.239 | - |
References
- Ziegler, D. Diabetes und Nervenerkrankungen. In Deutscher Gesundheitsbericht Diabetes 2018 Die Bestandsaufnahme; Deutsche Diabetes Gesellschaft (DDG) und diabetesDE—Deutsche Diabetes-Hilfe: Mainz, Germany, 2018; pp. 125–133. [Google Scholar]
- Goddard, K.; Vas, P.; Purves, A.; McMillan, V.; Langford, T.; Reid, F.; Edmonds, M. Comparing the Diagnostic Accuracy of Simple Tests to Screen for Diabetic Peripheral Neuropathy: Protocol for a Cross-Sectional Study. JMIR Res. Protoc. 2018, 7, e72. [Google Scholar] [CrossRef] [Green Version]
- Schaper, N.C.; Van Netten, J.J.; Apelqvist, J.; Lipsky, B.A.; Bakker, K. Prevention and management of foot problems in diabetes: A Summary Guidance for Daily Practice 2015, based on the IWGDF Guidance Documents. Diabetes Metab. Res. Rev. 2016, 32, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Gordois, A.; Scuffham, P.; Shearer, A.; Oglesby, A.; Tobian, J.A. The Health Care Costs of Diabetic Peripheral Neuropathy in the U.S. Diabetes Care 2003, 26, 1790–1795. [Google Scholar] [CrossRef] [Green Version]
- Asad, A.; Hameed, M.A.; Khan, U.A.; Ahmed, N.; Butt, M.-R.A. Reliability of the neurological scores for assessment of sensorimotor neuropathy in type 2 diabetics. J. Pak. Med. Assoc. 2010, 60, 166–170. [Google Scholar]
- Dyck, P.J.; Albers, J.W.; Andersen, H.; Arezzo, J.C.; Biessels, G.-J.; Bril, V.; Feldman, E.L.; Litchy, W.J.; O’Brien, P.C.; Russell, J.W.; et al. Diabetic polyneuropathies: Update on research definition, diagnostic criteria and estimation of severity. Diabetes Metab. Res. Rev. 2011, 27, 620–628. [Google Scholar] [CrossRef] [Green Version]
- Bakker, K.; Apelqvist, J.; Schaper, N.C. Practical guidelines on the management and prevention of the diabetic foot 2011. Diabetes Metab. Res. Rev. 2012, 28, 225–231. [Google Scholar] [CrossRef]
- Perkins, B.A.; Olaleye, D.; Zinman, B.; Bril, V. Simple Screening Tests for Peripheral Neuropathy in the Diabetes Clinic. Diabetes Care 2001, 24, 250–256. [Google Scholar] [CrossRef] [Green Version]
- Garrow, A.P.; Boulton, A.J.M. Vibration perception threshold—A valuable assessment of neural dysfunction in people with diabetes. Diabetes Metab. Res. Rev. 2006, 22, 411–419. [Google Scholar] [CrossRef]
- Küper, M.; Buchner, H. Polyneuropathien: Axonale versus demyelinisierende Neuropathie Polyneuropathies: Axonal versus demyelinating neuropathy. Das Neurophysiol. Labor 2014, 36, 80–89. [Google Scholar] [CrossRef]
- O’Neill, J.; Mccann, S.M.; Lagan, K.M. Tuning fork (128 Hz) versus Neurothesiometer: A comparison of methods of assessing vibration sensation in patients with diabetes mellitus. Int. J. Clin. Pract. 2005, 60, 174–178. [Google Scholar] [CrossRef]
- Paré, M.; Albrecht, P.J.; Noto, C.J.; Bodkin, N.L.; Pittenger, G.L.; Schreyer, D.J.; Tigno, X.T.; Hansen, B.C.; Rice, F.L. Differential hypertrophy and atrophy among all types of cutaneous innervation in the glabrous skin of the monkey hand during aging and naturally occurring type 2 diabetes. J. Comp. Neurol. 2007, 501, 543–567. [Google Scholar] [CrossRef]
- Shun, C.; Chang, Y.; Wu, H.; Hsieh, S.; Lin, W.; Lin, Y.; Tai, T.; Hsieh, S. Skin denervation in type 2 diabetes: Correlations with diabetic duration and functional impairments. Brain 2004, 127, 1593–1605. [Google Scholar] [CrossRef]
- Johansson, R.S.; Landström, U.; Lundström, R. Responses of mechanoreceptive afferent units in the glabrous skin of the human hand to sinusoidal skin displacements. Brain Res. 1982, 244, 17–25. [Google Scholar] [CrossRef]
- Toma, S.; Nakajima, Y. Response characteristics of cutaneous mechanoreceptors to vibratory stimuli in human glabrous skin. Neurosci. Lett. 1995, 195, 61–63. [Google Scholar] [CrossRef]
- Löfvenberg, J.; Johansson, R.S. Regional differences and interindividual variability in sensitivity to vibration in the glabrous skin of the human hand. Brain Res. 1984, 301, 65–72. [Google Scholar] [CrossRef]
- Johansson, R.S.; Vallbo, Å.B. Tactile sensory coding in the glabrous skin of the human hand. Trends Neurosci. 1983, 6, 27–32. [Google Scholar] [CrossRef]
- Holowka, N.B.; Wynands, B.; Drechsel, T.J.; Yegian, A.K.; Tobolsky, V.A.; Okutoyi, P.; Mang’eni Ojiambo, R.; Haile, D.W.; Sigei, T.K.; Zippenfennig, C.; et al. Foot callus thickness does not trade off protection for tactile sensitivity during walking. Nature 2019, 571, 261–264. [Google Scholar] [CrossRef]
- Watari, R.; Sartor, C.D.; Picon, A.P.; Butugan, M.K.; Amorim, C.F.; Ortega, N.R.; Sacco, I.C. Effect of diabetic neuropathy severity classified by a fuzzy model in muscle dynamics during gait. J. Neuroeng. Rehabil. 2014, 11, 11. [Google Scholar] [CrossRef] [Green Version]
- Strzalkowski, N.D.J.; Mildren, R.L.; Bent, L.R. Thresholds of cutaneous afferents related to perceptual threshold across the human foot sole. J. Neurophysiol. 2015, 114, 2144–2151. [Google Scholar] [CrossRef]
- Boulton, A.J.M.; Armstrong, D.G.; Albert, S.F.; Frykberg, R.G.; Hellman, R.; Kirkman, M.S.; Lavery, L.A.; Lemaster, J.W.; Mills, J.L.; Mueller, M.J.; et al. Comprehensive foot examination and risk assessment: A report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care 2008, 31, 1679–1685. [Google Scholar] [CrossRef] [Green Version]
- Sartor, C.D.; Oliveira, M.D.; Campos, V.; Ferreira, J.S.S.P.; Sacco, I.C.N. Cross-cultural adaptation and measurement properties of the Brazilian Version of the Michigan Neuropathy Screening Instrument. Braz. J. Phys. Ther. 2018, 22, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.A.; Suda, E.Y.; Sartor, C.D.; Ortega, N.R.S.; Watari, R.; Vigneron, V.; Sacco, I.C. Use of artificial intelligence methods for classifying diabetic patients with polyneuropathy. Diabetol. Metab. Syndr. 2015, 7, A4. [Google Scholar] [CrossRef] [Green Version]
- Zippenfennig, C.; Wynands, B.; Milani, T.L. Vibration perception thresholds of skin mechanoreceptors are influenced by different contact forces. J. Clin. Med. 2021. [Google Scholar]
- Schlee, G.; Sterzing, T.; Milani, T.L. Foot sole skin temperature affects plantar foot sensitivity. Clin. Neurophysiol. 2009, 120, 1548–1551. [Google Scholar] [CrossRef]
- Mildren, R.L.; Strzalkowski, N.D.J.; Bent, L.R. Foot sole skin vibration perceptual thresholds are elevated in a standing posture compared to sitting. Gait Posture 2016, 43, 87–92. [Google Scholar] [CrossRef]
- Schmidt, D.; Germano, A.M.C.; Milani, T.L. Subjective sensitivity data: Considerations to treat heteroscedasticity. Cogent Med. 2019, 6, 1673086. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing 2018; R Core Team: Vienna, Austria, 2018. [Google Scholar]
- Tesfaye, S.; Boulton, A.J.M.; Dyck, P.J.; Freeman, R.; Horowitz, M.; Kempler, P.; Lauria, G.; Malik, R.A.; Spallone, V.; Vinik, A.; et al. Diabetic Neuropathies: Update on Definitions, Diagnostic Criteria, Estimation of Severity, and Treatments. Diabetes Care 2010, 33, 2285–2293. [Google Scholar] [CrossRef] [Green Version]
- Nelander, J.; Speidel, T.; Björkman, A.; Dahlin, L.B. Vibration thresholds are increased at low frequencies in the sole of the foot in diabetes-a novel multi-frequency approach. Diabet. Med. 2012, 29, e449–e456. [Google Scholar] [CrossRef]
- Lindholm, E.; Löndahl, M.; Fagher, K.; Apelqvist, J.; Dahlin, L.B. Strong association between vibration perception thresholds at low frequencies (4 and 8 Hz), neuropathic symptoms and diabetic foot ulcers. PLoS ONE 2019, 14, e0212921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medakkel, A.A.; Sheela, P. Vibration Perception Threshold Values and Clinical Symptoms of Diabetic Peripheral Neuropathy. J. Clin. Diagn. Res. 2018, 12, 20–23. [Google Scholar] [CrossRef]
- Zochodne, D.W. Diabetes mellitus and the peripheral nervous system: Manifestations and mechanisms. Muscle Nerve 2007, 36, 144–166. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.-T.; Chiang, H.-Y.; Lin, W.-M. Pathology of Nerve Terminal Degeneration in the Skin. J. Neuropathol. Exp. Neurol. 2000, 59, 297–307. [Google Scholar] [CrossRef] [Green Version]
- Myers, M.I.; Peltier, A.C. Uses of Skin Biopsy for Sensory and Autonomic Nerve Assessment. Curr. Neurol. Neurosci. Rep. 2013, 13, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paré, M.; Elde, R.; Mazurkiewicz, J.E.; Smith, A.M.; Rice, F.L. The Meissner Corpuscle Revised: A Multiafferented Mechanoreceptor with Nociceptor Immunochemical Properties. J. Neurosci. 2001, 21, 7236–7246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strzalkowski, N.D.J.; Peters, R.M.; Inglis, J.T.; Bent, L.R. Cutaneous afferent innervation of the human foot sole: What can we learn from single-unit recordings? J. Neurophysiol. 2018, 120, 1233–1246. [Google Scholar] [CrossRef] [PubMed]
- Wells, C.; Ward, L.M.; Chua, R.; Inglis, J.T. Regional Variation and Changes With Ageing in Vibrotactile Sensitivity in the Human Footsole. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2003, 58, B680–B686. [Google Scholar] [CrossRef] [PubMed]
- Lauria, G.; Bakkers, M.; Schmitz, C.; Lombardi, R.; Penza, P.; Devigili, G.; Smith, A.G.; Hsieh, S.-T.; Mellgren, S.I.; Umapathi, T.; et al. Intraepidermal nerve fiber density at the distal leg: A worldwide normative reference study. J. Peripher. Nerv. Syst. 2010, 15, 202–207. [Google Scholar] [CrossRef]
- Collongues, N.; Samama, B.; Schmidt-Mutter, C.; Chamard-Witkowski, L.; Debouverie, M.; Chanson, J.-B.; Antal, M.-C.; Benardais, K.; de Seze, J.; Velten, M.; et al. Quantitative and qualitative normative dataset for intraepidermal nerve fibers using skin biopsy. PLoS ONE 2018, 13, e0191614. [Google Scholar] [CrossRef] [Green Version]
- Divisova, S.; Vlckova, E.; Srotova, I.; Kincova, S.; Skorna, M.; Dusek, L.; Dubovy, P.; Bednarik, J. Intraepidermal nerve-fibre density as a biomarker of the course of neuropathy in patients with Type 2 diabetes mellitus. Diabet. Med. 2016, 33, 650–654. [Google Scholar] [CrossRef]
- Chao, C.Y.L.; Zheng, Y.P.; Cheing, G.L.Y. Epidermal Thickness and Biomechanical Properties of Plantar Tissues in Diabetic Foot. Ultrasound Med. Biol. 2011, 37, 1029–1038. [Google Scholar] [CrossRef]
- Kekoni, J.; Hämäläinen, H.; Rautio, J.; Tukeva, T. Mechanical sensibility of the sole of the foot determined with vibratory stimuli of varying frequency. Exp. Brain Res. 1989, 78. [Google Scholar] [CrossRef] [PubMed]
- Zippenfennig, C.; Drechsel, T.J.; Monteiro, R.L.; Sacco, I.C.N.; Milani, T.L. The mechanoreceptor’s role in plantar skin changes in individuals with diabetes mellitus. J. Clin. Med. 2021, 10, 2537. [Google Scholar] [CrossRef] [PubMed]
- Masson, E.A.; Veves, A.; Fernando, D.; Boulton, A.J.M. Current perception thresholds: A new, quick, and reproducible method for the assessment of peripheral neuropathy in diabetes mellitus. Diabetologia 1989, 32, 724–728. [Google Scholar] [CrossRef] [Green Version]
- Deshpande, N.; Metter, E.J.; Ling, S.; Conwit, R.; Ferrucci, L. Physiological correlates of age-related decline in vibrotactile sensitivity. Neurobiol. Aging 2008, 29, 765–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergenheim, T.; Borssén, B.; Lithner, F. Sensory thresholds for vibration, perception and pain in diabetic patients aged 15–50 years. Diabetes Res. Clin. Pract. 1992, 16, 47–52. [Google Scholar] [CrossRef]
- Sohn, M.-W.; Budiman-Mak, E.; Lee, T.A.; Oh, E.; Stuck, R.M. Significant J-shaped association between body mass index (BMI) and diabetic foot ulcers. Diabetes Metab. Res. Rev. 2011, 27, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Viseux, F.; Lemaire, A.; Barbier, F.; Charpentier, P.; Leteneur, S.; Villeneuve, P. How can the stimulation of plantar cutaneous receptors improve postural control? Review and clinical commentary. Neurophysiol. Clin. 2019, 49, 263–268. [Google Scholar] [CrossRef] [PubMed]

| CG (n = 33) | DM Group (n = 20) | DPN Group (n = 13) | p-Value | |
|---|---|---|---|---|
| Age (years) | 55.7 ± 15.4 | 53.3 ± 15.1 | 61.0 ± 14.5 | 0.241 |
| Body height (m) | 1.7 ± 0.1 | 1.6 ± 0.1 | 1.6 ± 0.1 | 0.049 |
| Body mass (kg) | 70.1 ± 11.9 | 77.9 ± 13.3 | 81.8 ± 17.9 | 0.055 |
| Body Mass Index (kg/m2) | 24.5 ± 3.3 *,# | 29.5 ± 5.1 * | 30.1 ± 5.8 # | <0.001 |
| Sex (female:male) | 20:13 | 13:7 | 6:7 | 0.543 |
| Duration of DM (years) | - | 11.8 ± 10.3 | 17.5 ± 8.7 | 0.070 |
| DM Type (Type 1:Type 2) | - | 4:16 | 1:12 | - |
| DPN severity (mild:moderate:severe) | - | - | 7:5:1 | - |
| Fuzzy score | - | - | 5.3 ± 1.8 | - |
| MNSI score | - | 3.7 ± 2.5 | 4.1 ± 2.9 | 1.000 |
| Group | VPT (µm) | p-Value ANOVA | Eta-Squared ANOVA | Pairwise Comparisons | ||
|---|---|---|---|---|---|---|
| Heel 200 Hz | CG | 2.0 (0.6–5.9) | p < 0.001 | 0.23 | CG vs. DM | p = 1.0 |
| DM | 1.1 (0.7–4.4) | DM vs. DPN | p < 0.001 | |||
| DPN | 18.0 (5.1–31.0) | CG vs. DPN | p < 0.001 | |||
| MTH1 200 Hz | CG | 1.9 (0.4–8.6) | p < 0.001 | 0.29 | CG vs. DM | p = 1.0 |
| DM | 1.1 (0.5–4.7) | DM vs. DPN | p < 0.001 | |||
| DPN | 23.7 (16.5–51.1) | CG vs. DPN | p < 0.001 | |||
| Heel 30 Hz | CG | 15.4 (7.3–31.3) | p < 0.001 | 0.37 | CG vs. DM | p = 0.256 |
| DM | 23.0 (14.4–57.9) | DM vs. DPN | p < 0.001 | |||
| DPN | 104.4 (46.5–196.9) | CG vs. DPN | p < 0.001 | |||
| MTH1 30 Hz | CG | 10.7 (4.9–19.9) | p < 0.001 | 0.41 | CG vs. DM | p = 1.0 |
| DM | 9.9 (6.9–24.1) | DM vs. DPN | p < 0.001 | |||
| DPN | 73.9 (33.6–191.8) | CG vs. DPN | p < 0.001 | |||
| DM Duration | n | Heel 200 Hz | MTH1 200 Hz | Heel 30 Hz | MTH1 30 Hz | ||
|---|---|---|---|---|---|---|---|
| DM and DPN groups | A | ≤4 | 7 | 0.9 [0.8–2.9] | [0.6–3.0] | 14.9 [10.2–43.7] | 8.2 [5.9–13.7] |
| B | >4 ≤ 8 | 4 | 5.4 [1.9–9.7] | 6.6 [0.9–13.1] | 55.9 [44.5–69.2] | 27.5 [17.7–43.7] | |
| C | >8 | 22 | 4.7 [1.2–25.2] | 12.5 [1.3–35.2] | 54.2 [25.1–108.5] | 35.3 [15.8–128.4] | |
| CG | - | 33 | 2.0 [0.6–5.9] | 1.9 [0.4–8.6] | 15.4 [7.3–31.3] | 10.7 [4.9–19.9] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drechsel, T.J.; Monteiro, R.L.; Zippenfennig, C.; Ferreira, J.S.S.P.; Milani, T.L.; Sacco, I.C.N. Low and High Frequency Vibration Perception Thresholds Can Improve the Diagnosis of Diabetic Neuropathy. J. Clin. Med. 2021, 10, 3073. https://doi.org/10.3390/jcm10143073
Drechsel TJ, Monteiro RL, Zippenfennig C, Ferreira JSSP, Milani TL, Sacco ICN. Low and High Frequency Vibration Perception Thresholds Can Improve the Diagnosis of Diabetic Neuropathy. Journal of Clinical Medicine. 2021; 10(14):3073. https://doi.org/10.3390/jcm10143073
Chicago/Turabian StyleDrechsel, Tina J., Renan L. Monteiro, Claudio Zippenfennig, Jane S. S. P. Ferreira, Thomas L. Milani, and Isabel C. N. Sacco. 2021. "Low and High Frequency Vibration Perception Thresholds Can Improve the Diagnosis of Diabetic Neuropathy" Journal of Clinical Medicine 10, no. 14: 3073. https://doi.org/10.3390/jcm10143073
APA StyleDrechsel, T. J., Monteiro, R. L., Zippenfennig, C., Ferreira, J. S. S. P., Milani, T. L., & Sacco, I. C. N. (2021). Low and High Frequency Vibration Perception Thresholds Can Improve the Diagnosis of Diabetic Neuropathy. Journal of Clinical Medicine, 10(14), 3073. https://doi.org/10.3390/jcm10143073







