An Examination of the Relationship between Urinary Neurotrophin Concentrations and Transcutaneous Electrical Nerve Stimulation (TENS) Used in Pediatric Overactive Bladder Therapy
Abstract
:1. Introduction
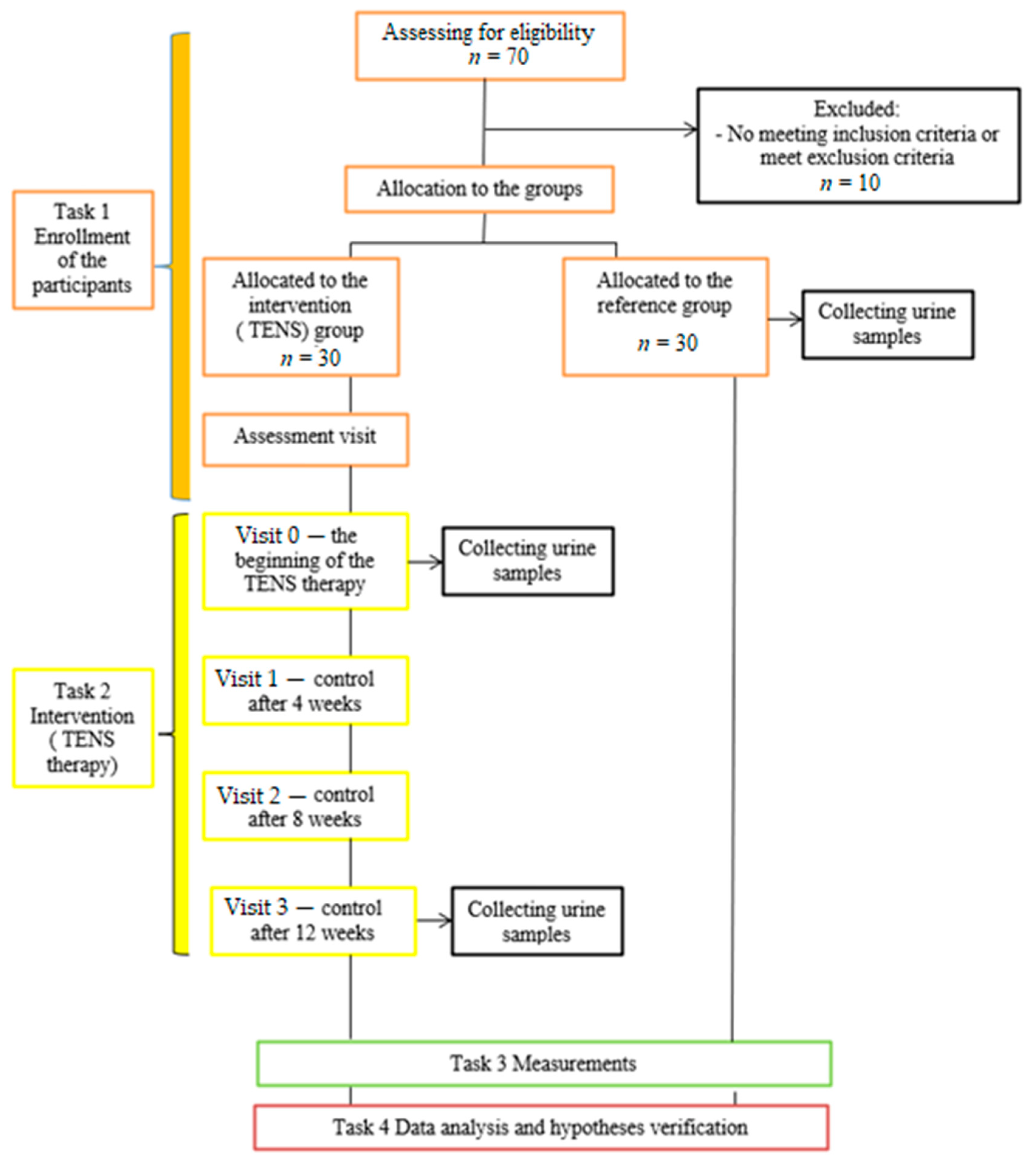
2. Materials and Methods
2.1. Participants
2.2. Intervention—TENS Treatment and Follow-Up Assessment
2.3. Biochemistry
2.4. Statistics
2.5. Ethical Issues
3. Results
4. Discussion
4.1. Neurotrophins at Baseline
4.2. Neurotrophins after TENS Therapy
5. Conclusions
- Urinary NGF, BDNF, and NT4 were increased in OAB refractory to the standard treatment in contrast to NT3;
- The variation of urinary neurotrophins in the course of TENS depended on the age and clinical manifestation of OAB.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nieuwhof-Leppink, A.J.; Schroeder, R.P.J.; van de Putte, E.M.; de Jong, T.P.V.M.; Schappin, R. Daytime urinary incontinence in children and adolescents. Lancet Child. Adolesc. 2019, 3, 492–501. [Google Scholar] [CrossRef]
- Austin, P.F.; Bauer, S.B.; Bower, W.; Chase, J.; Franco, I.; Hoebeke, P.; Rittig, S.; Walle, J.V.; von Gontard, A.; Wright, A.; et al. The standardization of terminology of lower urinary tract function in children and adolescents: Update report from the standardization committee of the International Children’s Continence Society. Neurourol. Urodyn. 2016, 35, 471–481. [Google Scholar] [CrossRef]
- Smith, A.L. Understanding overactive bladder and urgency incontinence: What does the brain have to do with it? F1000Research 2018, 7, F1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apostolidis, A.; Wagg, A.; Rahnama’i, M.S.; Panicker, J.N.; Vrijens, D.; von Gontard, A. Is there “brain OAB” and how can we recognize it? International Consultation on Incontinence-Research Society (ICI-RS) 2017. Neurourol. Urodyn. 2018, 37, S38–S45. [Google Scholar] [CrossRef] [PubMed]
- Franco, I. Overactive bladder in children. Nat. Rev. Urol. 2016, 13, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.D. Neurotrophins in bladder function: What do we know and where do we go from here? Neurourol. Urodyn. 2014, 33, 39–45. [Google Scholar] [CrossRef]
- Liu, H.T.; Kuo, H.C. Urinary nerve growth factor levels are increased in patients with bladder outlet obstruction with overactive bladder symptoms and reduced after successful medical treatment. J. Urol. 2008, 72, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; Chancellor, M.B.; Kuo, H.C. Decrease of urinary nerve growth factor levels after antimuscarinic therapy in patients with overactive bladder. BJU Int. 2009, 103, 1668–1672. [Google Scholar] [CrossRef]
- Cho, K.J.; Kim, H.S.; Koh, J.S.; Kim, J.C. Changes in urinary nerve growth factor and prostaglandin E2 in women with overactive bladder after anticholinergics. Int. Urogynecol. J. 2013, 24, 325–330. [Google Scholar] [CrossRef]
- Antunes-Lopes, T.; Pinto, R.; Barros, S.C.; Botelho, F.; Silva, C.M.; Cruz, C.D.; Cruz, F. Urinary neurotrophic factors in healthy individuals and patients with overactive bladder. J. Urol. 2013, 189, 359–365. [Google Scholar] [CrossRef]
- Oktar, T.; Kocak, T.; Oner-Iyidogan, Y. Urinary nerve growth factor in children with overactive bladder: A promising, non-invasive and objective biomarker. J. Pediatr. Urol. 2013, 9, 617–621. [Google Scholar] [CrossRef]
- Korzeniecka-Kozerska, A.; Wasilewska, A. Urinary nerve growth factor in patients with detrusor overactivity. Ir. J. Med. Sci. 2015, 184, 737–743. [Google Scholar] [CrossRef] [Green Version]
- Özdemir, K.; Dinçel, N.; Berdeli, A.; Mir, S. Can Urinary Nerve Growth Factor and Brain-Derived Neurotrophic Factor be used in the Diagnosis and Follow-Up of Voiding Dysfunction in Children? Urol. J. 2016, 13, 2690–2696. [Google Scholar]
- Liu, H.T.; Chancellor, M.B.; Kuo, H.C. Urinary nerve growth factor levels are elevated in patients with detrusor overactivity and decreased in responders to detrusor botulinum toxin-A injection. Eur. Urol. 2009, 56, 700–706. [Google Scholar] [CrossRef]
- Wright, A.J.; Haddad, M. Electroneurostimulation for the management of bladder bowel dysfunction in childhood. Eur. J. Paediatr. Neurol. 2017, 21, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Barroso, U., Jr. Superficial stimulation therapy for the treatment of functional voiding problems. In Pediatric Incontinence: Evaluation and Clinical Management, 1st ed.; Franco, I., Austin, P.F., Bauer, S.B., von Gontard, A., Homsy, Y., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 183–188. [Google Scholar] [CrossRef]
- Hoebeke, P.; Van Laecke, E.; Everaert, K.; Renson, C.; De Paepe, H.; Raes, A.; Vande Walle, J. Transcutaneous neuromodulation for the urge syndrome in children: A pilot study. J. Urol. 2001, 166, 2416–2419. [Google Scholar] [CrossRef]
- Bower, W.F.; Moore, K.H.; Adams, R.D. A pilot study of the home application of transcutaneous neuromodulation in children with urgency or urge incontinence. J. Urol. 2001, 166, 2420–2422. [Google Scholar] [CrossRef]
- Malm-Buatsi, E.; Nepple, K.G.; Boyt, M.A.; Austin, J.C.; Cooper, C.S. Efficacy of trancutaneous electrical nerve stimulation in children with overactive bladder refractory to pharmacotherapy. Urology 2007, 70, 980–983. [Google Scholar] [CrossRef] [PubMed]
- Hagstroem, S.; Mahler, B.; Madsen, B.; Djurhuus, J.C.; Rittig, S. Transcutaneous electrical nerve stimulation for refractory daytime urinary urge incontinence. J. Urol. 2009, 182, 2072–2078. [Google Scholar] [CrossRef] [PubMed]
- Sillén, U.; Arwidsson, C.; Doroszkiewicz, M.; Antonsson, H.; Jansson, I.; Stålklint, M.; Abrahamsson, K.; Sjöström, S. Effects of transcutaneous neuromodulation (TENS) on overactive bladder symptoms in children: A randomized controlled trial. J. Pediatr. Urol. 2014, 10, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Boudaoud, N.; Binet, A.; Line, A.; Chaouadi, D.; Jolly, C.; Fiquet, C.F.; Ripert, T.; Merol, M.L. Management of refractory overactive bladder in children by transcutaneous posterior tibial nerve stimulation: A controlled study. J. Pediatr. Urol. 2015, 11, 138.e1–138.e10. [Google Scholar] [CrossRef] [PubMed]
- Lordelo, P.; Teles, A.; Veiga, M.L.; Correia, L.C.; Barroso, U., Jr. Transcutaneous electrical nerve stimulation in children with overactive bladder: A randomized clinical trial. J. Urol. 2010, 184, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Barroso, U., Jr.; Carvalho, M.T.; Veiga, M.L.; Moraes, M.M.; Cunha, C.C.; Lordêlo, P. Urodynamic outcome of parasacral transcutaneous electrical neural stimulation for overactive bladder in children. Int. Braz. J. Urol. 2015, 41, 739–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bower, W.F.; Yeung, C.K. A review of non-invasive electro neuromodulation as an intervention for non-neurogenic bladder dysfunction in children. Neurourol. Urodyn. 2004, 23, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Suh, Y.S.; Ko, K.J.; Kim, T.H.; Lee, H.S.; Sung, H.H.; Cho, W.J.; Lee, K.S. Urinary Nerve Growth Factor as a Potential Biomarker of Treatment Outcomes in Overactive Bladder Patients. Int. Neurourol. J. 2017, 21, 270–281. [Google Scholar] [CrossRef] [Green Version]
- Deng, C.; Zhang, W.; Peng, Q.; Hu, X.; Li, M.; Gao, L.; Xu, J.; Su, J.; Xia, X. Urinary nerve growth factor: A biomarker for overactive bladder in children? A meta-analysis and trail sequential analysis. Pediatr. Surg. Int. 2019, 35, 1027–1032. [Google Scholar] [CrossRef]
- Antunes-Lopes, T.; Cruz, F. Urinary Biomarkers in Overactive Bladder: Revisiting the Evidence in 2019. Eur. Urol. Focus 2019, 5, 329–336. [Google Scholar] [CrossRef]
- Ochodnicky, P.; Cruz, C.D.; Yoshimura, N.; Cruz, F. Neurotrophins as regulators of urinary bladder function. Nat. Rev. Urol. 2012, 9, 628–637. [Google Scholar] [CrossRef]
- Pinto, R.; Frias, B.; Allen, S.; Dawbarn, D.; McMahon, S.B.; Cruz, F.; Cruz, C.D. Sequestration of brain derived nerve factor by intravenous delivery of TrkB-Ig2 reduces bladder overactivity and noxious input in animals with chronic cystitis. Neuroscience 2010, 166, 907–916. [Google Scholar] [CrossRef]
- Vizzard, M.A.; Wu, K.H.; Jewett, I.T. Developmental expression of urinary bladder neurotrophic factor mRNA and protein in the neonatal rat. Brain Res. Dev. Brain Res. 2000, 119, 217–224. [Google Scholar] [CrossRef]
- Pradat, P.F.; Kennel, P.; Naimi-Sadaoui, S.; Finiels, F.; Orsini, C.; Revah, F.; Delaere, P.; Mallet, J. Continuous delivery of neurotrophin 3 by gene therapy has a neuroprotective effect in experimental models of diabetic and acrylamide neuropathies. Hum. Gene Ther. 2001, 12, 2237–2249. [Google Scholar] [CrossRef] [PubMed]
- Miyazato, M.; Yoshimura, N.; Chancellor, M.B. The other bladder syndrome: Underactive bladder. Rev. Urol. 2013, 15, 11–22. [Google Scholar] [PubMed]
- Corcos, J.; Przydacz, M. Neurogenic Bladder Pathophysiology. In Consultation in Neurourology, 1st ed.; Corcos, J., Przydacz, M., Eds.; Springer: Cham, Switzerland, 2018; pp. 7–16. [Google Scholar] [CrossRef]
- Ognenovska, S.; Cheng, Y.; Li, A.; Mansfield, K.J.; Moore, K.H. What’s normal? Should urinary creatinine or osmolarity be used to normalise urinary protein measurements? In Proceedings of the International Continence Society, Philadelphia, PA, USA, 28–31 August 2018; p. 138. [Google Scholar]
- Barroso, U., Jr.; Tourinho, R.; Lordêlo, P.; Hoebeke, P.; Chase, J. Electrical stimulation for lower urinary tract dysfunction in children: A systematic review of the literature. Neurourol. Urodyn. 2011, 30, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Lordêlo, P.; Benevides, I.; Kerner, E.G.; Teles, A.; Lordêlo, M.; Barroso, U., Jr. Treatment of non-monosymptomatic nocturnal enuresis by transcutaneous parasacral electrical nerve stimulation. J. Pediatr. Urol. 2010, 6, 486–489. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, L.F.; de Oliveira, D.M.; da Silva de Paula, L.I.; de Figueiredo, A.A.; de Bessa, J., Jr.; de Sá, C.A.; Bastos Netto, J.M. Transcutaneous parasacral electrical neural stimulation in children with primary monosymptomatic enuresis: A prospective randomized clinical trial. J. Urol. 2013, 190, 1359–1363. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, C.S.; Kamperis, K.; Borch, L.; Borg, B.; Rittig, S. Transcutaneous Electrical Nerve Stimulation in Children with Monosymptomatic Nocturnal Enuresis: A Randomized, Double-Blind, Placebo Controlled Study. J. Urol. 2017, 198, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Sampaio, C.; Nascimento, A.A.; Veiga, M.L.; Barroso, U. Predictors of outcome in children and adolescents with overactive bladder treated with parasacral transcutaneous electrical nerve stimulation. J. Pediatr. Urol. 2018, 14, 54.e1–54.e6. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| Study group |
|
|
| Reference group |
|
|
| OAB Patients | Group C: Reference Group | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Group A: Before TENS | Group B: After TENS | A&B | A&C | B&C | |||||
| Median, Range | |||||||||
| Girls/boys (n) | 15/15 | 19/11 | - | 0.39 | 0.39 | ||||
| Age (years) | 8.7 (5.25–12.3) | 8.83 (5.5–12.6) | 8.4 (5–17) | 0.55 | 0.85 | 0.6 | |||
| Body weight (kg) | 29.5 (17–62) | 31.5 (15.5–70) | - | 0.55 | 0.55 | ||||
| Height (cm) | 134 (110–158) | 135 (104–174) | - | 0.81 | 0.81 | ||||
| NGF (pg/mL) | 16.7 (5.4–101.3) | 17.9 (6.33–103) | 12.7 (6.3–73.7) | 0.98 | 0.14 | 0.04 * | |||
| BDNF (pg/mL) | 12.7 (11.3–189.9) | 24.6 (11.3–515.3) | 12.7 (11.3–40.8) | 0.03 * | 0.01 * | <0.001 * | |||
| NT4 (pg/mL) | 107.9 (6–1245) | 107.9 (6–2095) | 79.4 (6.0–1096) | 0.71 | 0.94 | 0.52 | |||
| NT3 (pg/mL) | 87 (5.7–1854) | 72 (15.9–2934) | 216 (17.9–4483) | 0.81 | 0.03 * | 0.04 * | |||
| Urinary Cr (mg/mL) | 0.39 (0.04–2.4) | 0.13 (0.04–1.5) | 1.29 (0.49–3.32) | 0.049 * | <0.001 * | <0.001 * | |||
| NGF/Cr (pg/mg) | 55.9 (3.3–894.6) | 126.5 (5.6–802.5) | 7.8 (2.4–68.7) | 0.36 | <0.001 * | <0.001 * | |||
| BDNF/Cr (pg/mg) | 49.7 (4.7–536.7) | 217.7 (8.7–5725) | 11.2 (3.8–44.9) | 0.004 * | <0.001 * | <0.001 * | |||
| NT4/Cr (pg/mg) | 171.8 (11.2–6316) | 748.6 (5.7–3958) | 52.8 (2.2–664) | 0.11 | <0.001 * | <0.001 * | |||
| NT3/Cr (pg/mg) | 379.6 (3.5–3387) | 687.5 (10.9–3532) | 132.6 (10.8–2312) | 0.18 | 0.36 | 0.03 * | |||
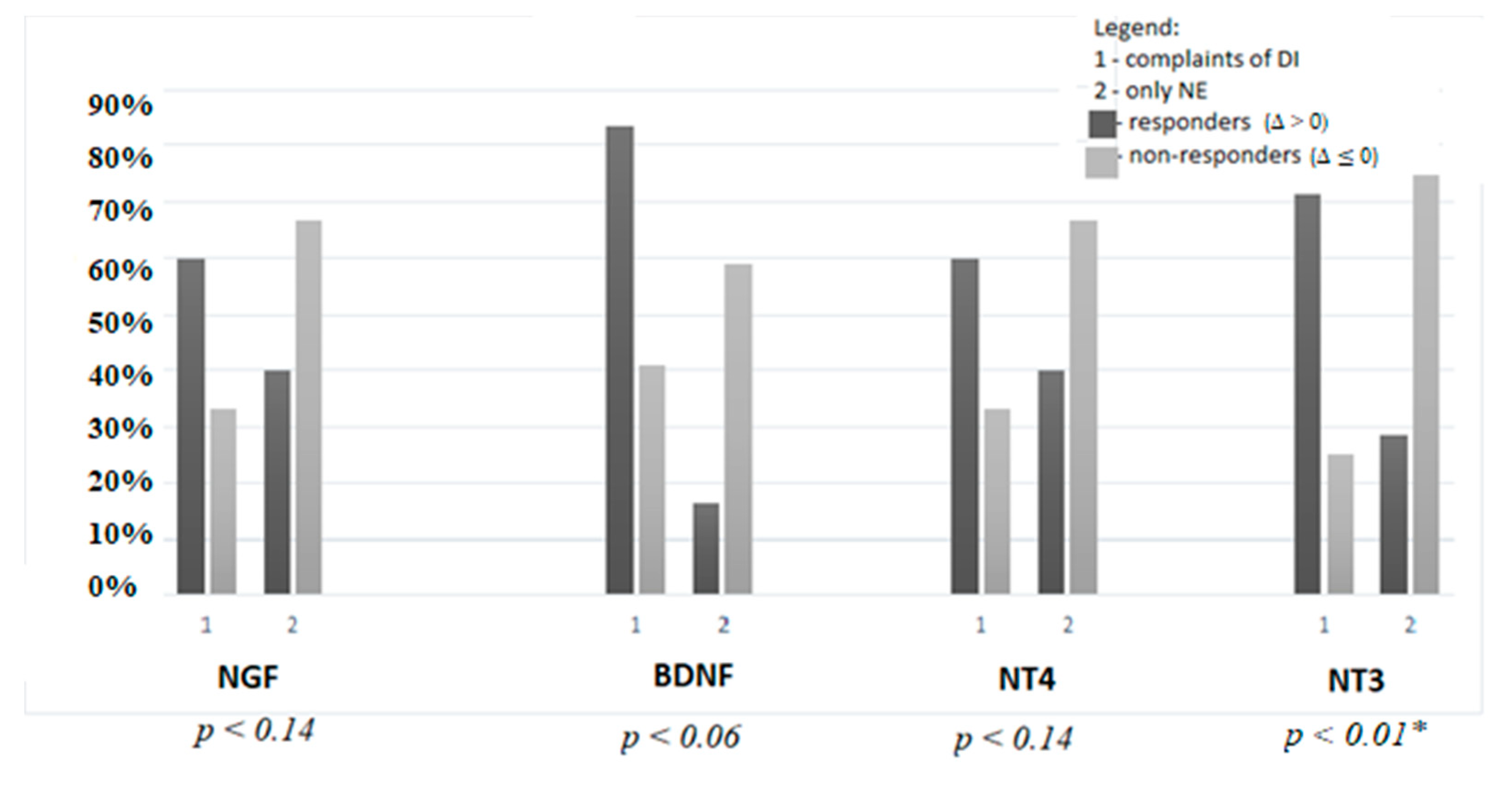
| The Δ Values (Mean; Median, Range) | Responders/Non-Responders n (%) | |
|---|---|---|
| NGF | 4.38; 0.16 (−97.6–78.2) | 15 (50)/15 (50) |
| BDNF | −45.8; −3.26 (−502–177.3) | 7 (23)/23 (77) |
| NT4 | −5.7; 2.63 (−2084–1201) | 15 (50)/15 (50) |
| NT3 | −6.97; −1.22 (−2840–1639) | 14 (47)/16 (53) |
| NGF/Cr | 30.7; 7.04 (−797–820) | 12 (40)/18 (60) |
| BDNF/Cr | −567; −99 (−5693–219) | 7 (23)/23 (77) |
| NT4/Cr | −173; −158 (−3939–5516) | 11 (37)/19 (63) |
| NT3/Cr | −273; −59 (−2486–2293) | 10 (33)/20 (67) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagińska, J.; Sadowska, E.; Korzeniecka-Kozerska, A. An Examination of the Relationship between Urinary Neurotrophin Concentrations and Transcutaneous Electrical Nerve Stimulation (TENS) Used in Pediatric Overactive Bladder Therapy. J. Clin. Med. 2021, 10, 3156. https://doi.org/10.3390/jcm10143156
Bagińska J, Sadowska E, Korzeniecka-Kozerska A. An Examination of the Relationship between Urinary Neurotrophin Concentrations and Transcutaneous Electrical Nerve Stimulation (TENS) Used in Pediatric Overactive Bladder Therapy. Journal of Clinical Medicine. 2021; 10(14):3156. https://doi.org/10.3390/jcm10143156
Chicago/Turabian StyleBagińska, Joanna, Edyta Sadowska, and Agata Korzeniecka-Kozerska. 2021. "An Examination of the Relationship between Urinary Neurotrophin Concentrations and Transcutaneous Electrical Nerve Stimulation (TENS) Used in Pediatric Overactive Bladder Therapy" Journal of Clinical Medicine 10, no. 14: 3156. https://doi.org/10.3390/jcm10143156
APA StyleBagińska, J., Sadowska, E., & Korzeniecka-Kozerska, A. (2021). An Examination of the Relationship between Urinary Neurotrophin Concentrations and Transcutaneous Electrical Nerve Stimulation (TENS) Used in Pediatric Overactive Bladder Therapy. Journal of Clinical Medicine, 10(14), 3156. https://doi.org/10.3390/jcm10143156






