Nonalcoholic Fatty Liver Disease-Related Hepatocellular Carcinoma: Clinical Patterns, Outcomes, and Prognostic Factors for Overall Survival—A Retrospective Analysis of a Slovak Cohort
Abstract
:1. Introduction
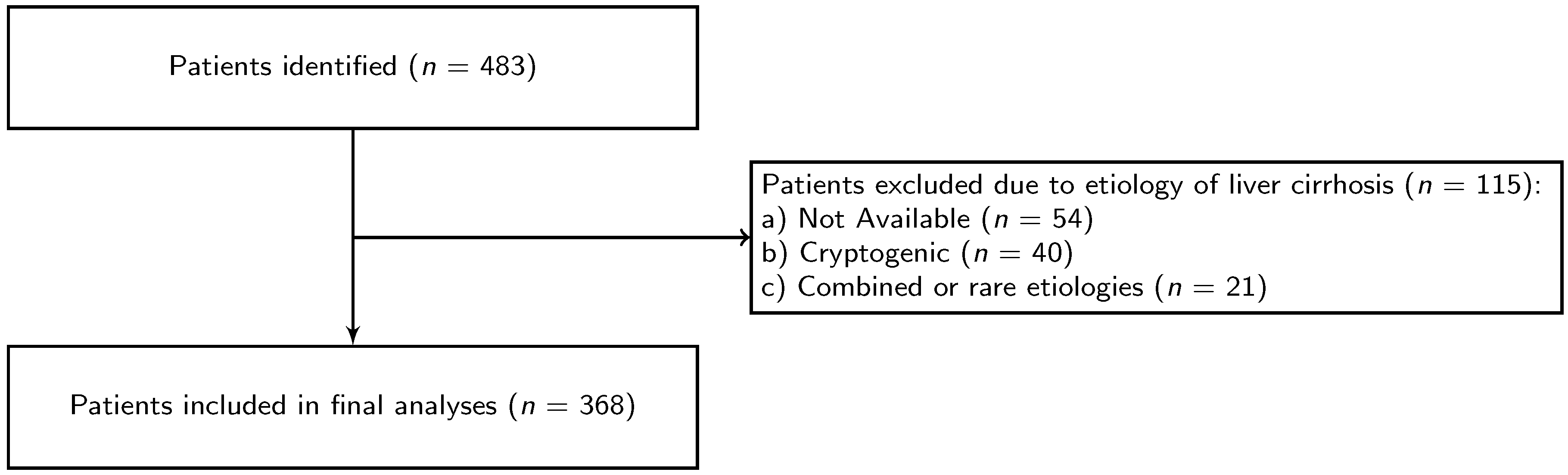
2. Materials and Methods
Statistical Analyses
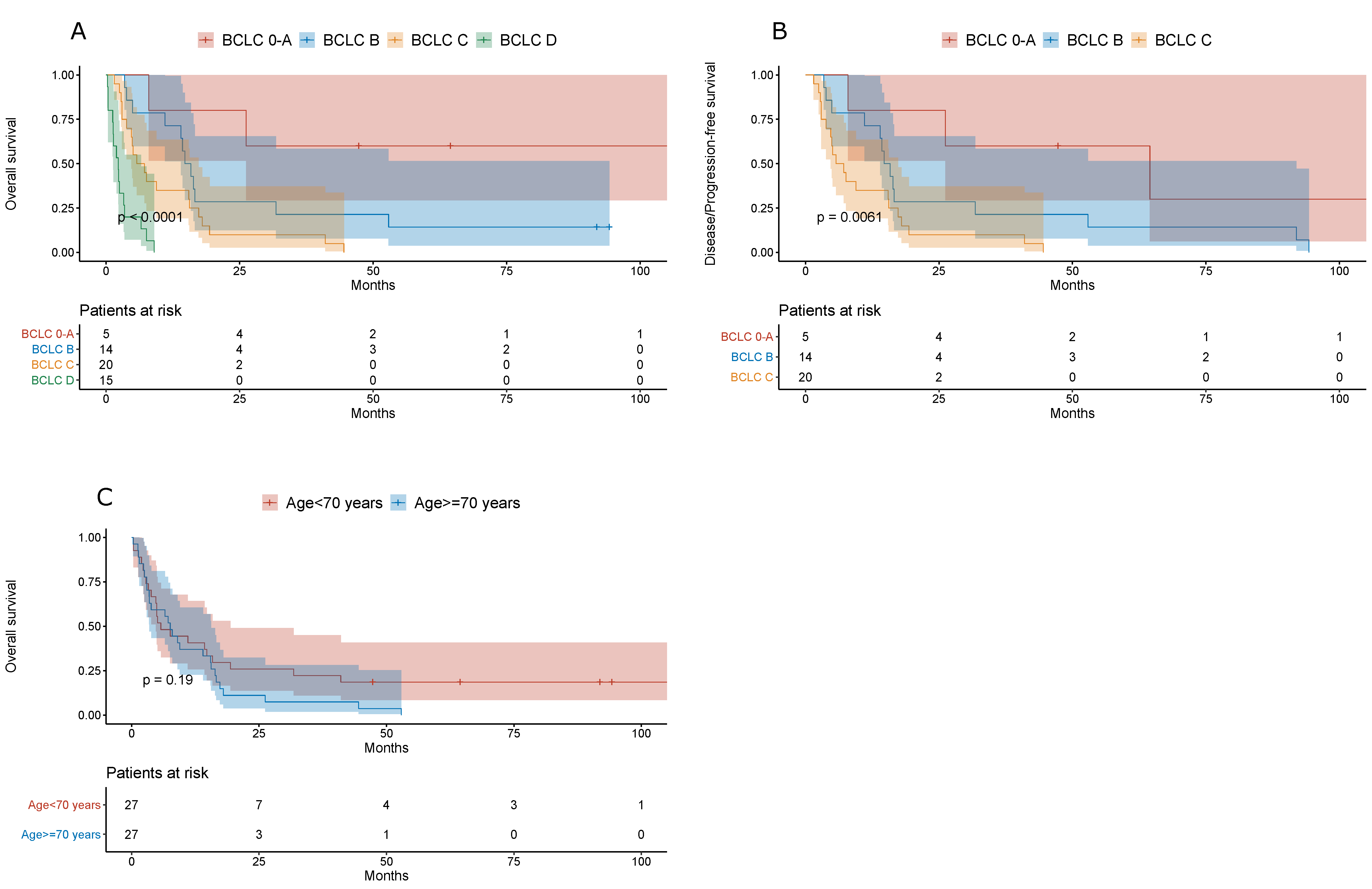
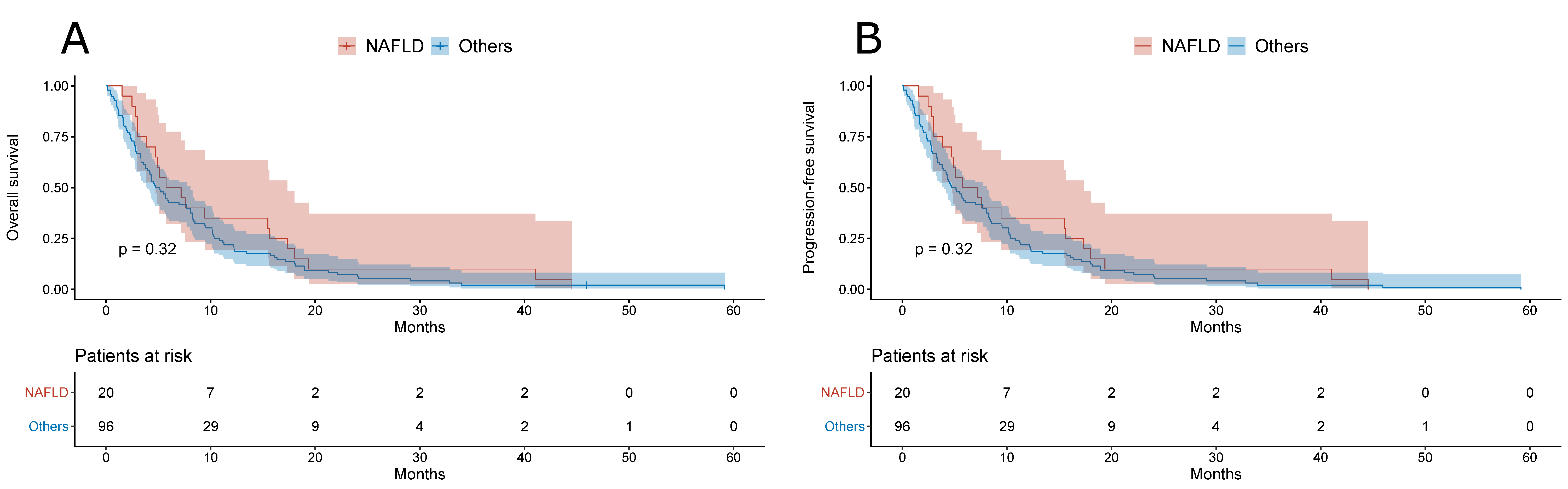
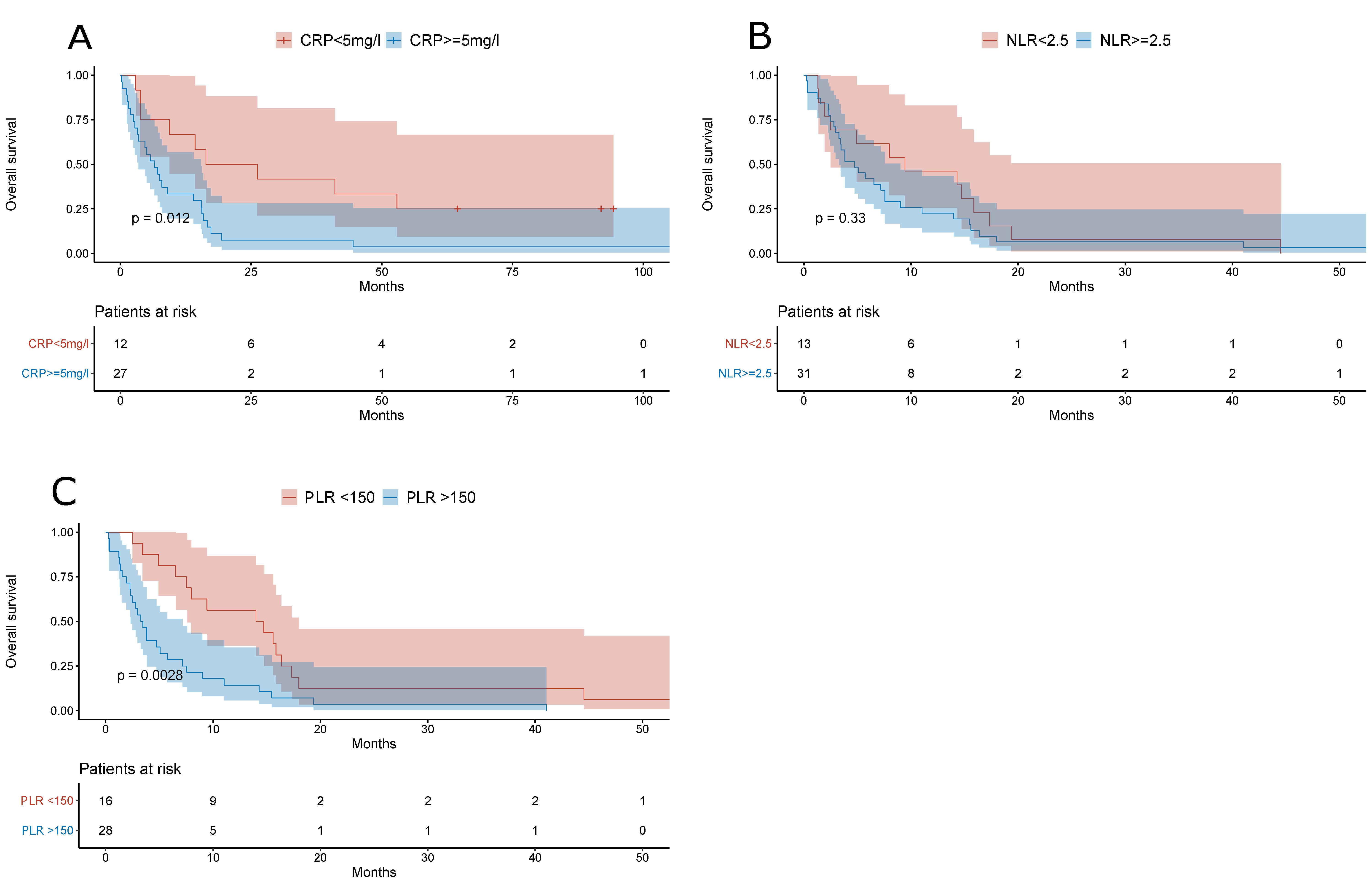
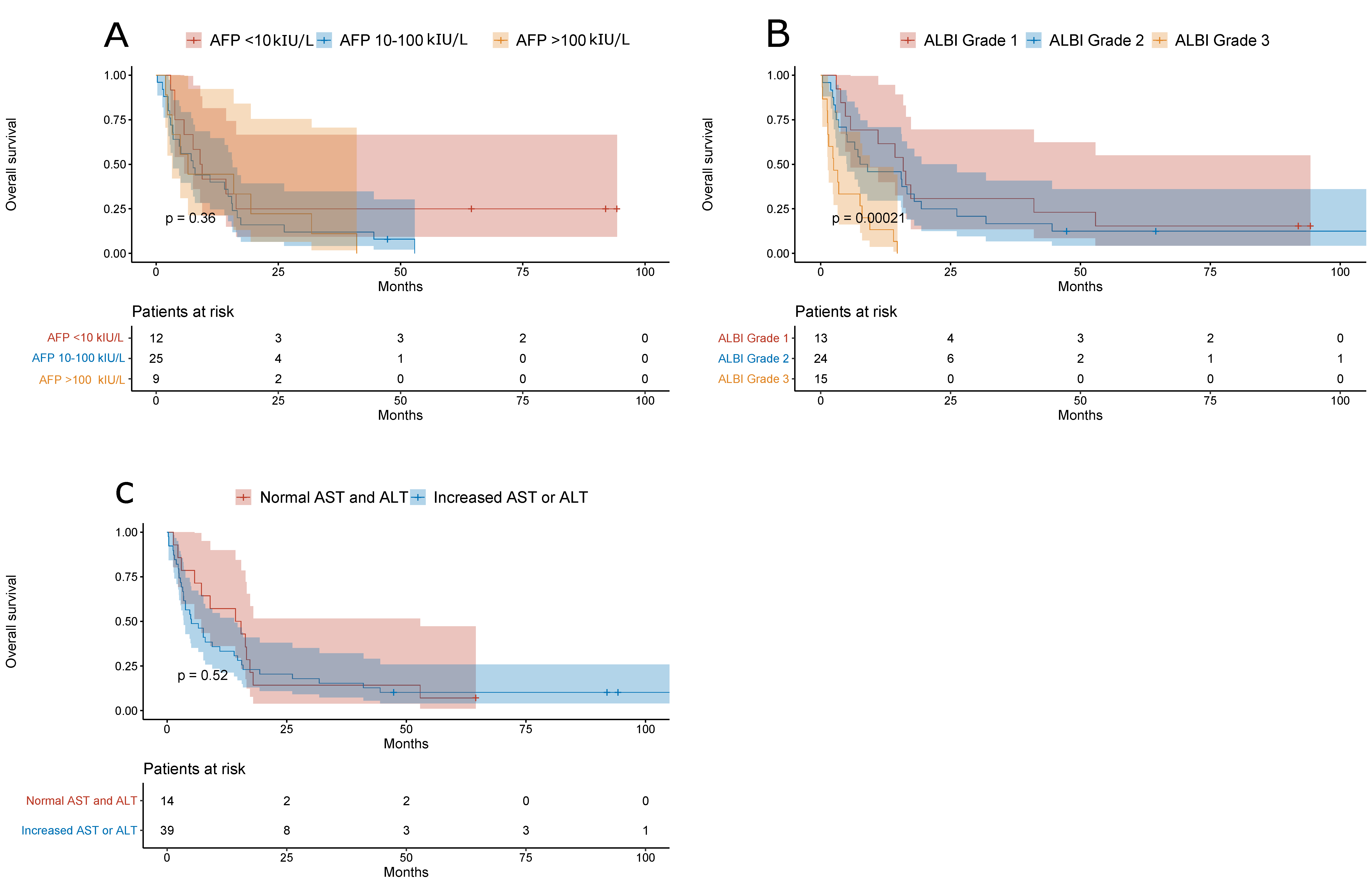
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.-L.; Schirmacher, P.; Vilgrain, V. Easl clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [Green Version]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calzadilla Bertot, L.; Adams, L. The natural course of non-alcoholic fatty liver disease. Int. J. Mol. Sci. 2016, 17, 774. [Google Scholar] [CrossRef] [Green Version]
- Pais, R.; Maurel, T. Natural history of nafld. J. Clin. Med. 2021, 10, 1161. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.A.; Lymp, J.F.; St. Sauver, J.; Sanderson, S.O.; Lindor, K.D.; Feldstein, A.; Angulo, P. The natural history of nonalcoholic fatty liver disease: A population-based cohort study. Gastroenterology 2005, 129, 113–121. [Google Scholar] [CrossRef]
- Alexander, M.; Loomis, A.K.; van der Lei, J.; Duarte-Salles, T.; Prieto-Alhambra, D.; Ansell, D.; Pasqua, A.; Lapi, F.; Rijnbeek, P.; Mosseveld, M.; et al. Risks and clinical predictors of cirrhosis and hepatocellular carcinoma diagnoses in adults with diagnosed nafld: Real-world study of 18 million patients in four european cohorts. BMC Med. 2019, 17, 95. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Kramer, J.R.; Mapakshi, S.; Natarajan, Y.; Chayanupatkul, M.; Richardson, P.A.; Li, L.; Desiderio, R.; Thrift, A.P.; Asch, S.M.; et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology 2018, 155, 1828–1837.e1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, H.; Williams, B.; Schnabl, B. Gut microbiota, fatty liver disease, and hepatocellular carcinoma. Liver Res. 2018, 2, 43–51. [Google Scholar] [CrossRef]
- Geh, D.; Anstee, Q.M.; Reeves, H.L. Nafld-associated hcc: Progress and opportunities. J. Hepatocell. Carcinoma 2021, 8, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Q.; El-Serag, H.B.; Loomba, R. Global epidemiology of nafld-related hcc: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2020, 18, 223–238. [Google Scholar] [CrossRef]
- Negro, F. Natural history of nash and hcc. Liver Int. 2020, 40, 72–76. [Google Scholar] [CrossRef] [Green Version]
- Easl–eortc clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2012, 56, 908–943. [CrossRef] [PubMed] [Green Version]
- Zahorec, R. Ratio of neutrophil to lymphocyte counts—Rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl. Lek. Listy 2001, 102, 5–14. [Google Scholar] [PubMed]
- Li, X.; Chen, Z.-H.; Xing, Y.-F.; Wang, T.-T.; Wu, D.-H.; Wen, J.-Y.; Chen, J.; Lin, Q.; Dong, M.; Wei, L.; et al. Platelet-to-lymphocyte ratio acts as a prognostic factor for patients with advanced hepatocellular carcinoma. Tumor Biol. 2014, 36, 2263–2269. [Google Scholar] [CrossRef]
- Wai, C. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis c. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef] [Green Version]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach—The albi grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2003, 27, S5–S10. [Google Scholar]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Zubrod, C.G.; Schneiderman, M.; Frei, E.; Brindley, C.; Lennard Gold, G.; Shnider, B.; Oviedo, R.; Gorman, J.; Jones, R.; Jonsson, U.; et al. Appraisal of methods for the study of chemotherapy of cancer in man: Comparative therapeutic trial of nitrogen mustard and triethylene thiophosphoramide. J. Chronic Dis. 1960, 11, 7–33. [Google Scholar] [CrossRef]
- Llovet, J.; Brú, C.; Bruix, J. Prognosis of hepatocellular carcinoma: The bclc staging classification. Semin. Liver Dis. 2008, 19, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Camacho, J.C.; Kokabi, N.; Xing, M.; Prajapati, H.J.; El-Rayes, B.; Kim, H.S. Modified response evaluation criteria in solid tumors and european association for the study of the liver criteria using delayed-phase imaging at an early time point predict survival in patients with unresectable intrahepatic cholangiocarcinoma following yttrium-90 radioembolization. J. Vasc. Interv. Radiol. 2014, 25, 256–265. [Google Scholar] [PubMed]
- Dancey, J.E.; Dodd, L.E.; Ford, R.; Kaplan, R.; Mooney, M.; Rubinstein, L.; Schwartz, L.H.; Shankar, L.; Therasse, P. Recommendations for the assessment of progression in randomised cancer treatment trials. Eur. J. Cancer 2009, 45, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Cheng, A.-L.; Meinhardt, G.; Nakajima, K.; De Sanctis, Y.; Llovet, J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: Analysis of two phase iii studies. J. Hepatol. 2017, 67, 999–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrne, C.D.; Targher, G. What’s new in nafld pathogenesis, biomarkers and treatment? Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 70–71. [Google Scholar] [CrossRef]
- Margini, C.; Dufour, J.F. The story of hcc in nafld: From epidemiology, across pathogenesis, to prevention and treatment. Liver Int. 2016, 36, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Thuluvath, P.J.; Kantsevoy, S.; Thuluvath, A.J.; Savva, Y. Is cryptogenic cirrhosis different from nash cirrhosis? J. Hepatol. 2018, 68, 519–525. [Google Scholar] [CrossRef]
- Dyson, J.; Jaques, B.; Chattopadyhay, D.; Lochan, R.; Graham, J.; Das, D.; Aslam, T.; Patanwala, I.; Gaggar, S.; Cole, M.; et al. Hepatocellular cancer: The impact of obesity, type 2 diabetes and a multidisciplinary team. J. Hepatol. 2014, 60, 110–117. [Google Scholar] [CrossRef]
- Pais, R.; Fartoux, L.; Goumard, C.; Scatton, O.; Wendum, D.; Rosmorduc, O.; Ratziu, V. Temporal trends, clinical patterns and outcomes of nafld-related hcc in patients undergoing liver resection over a 20-year period. Aliment. Pharmacol. Ther. 2017, 46, 856–863. [Google Scholar] [CrossRef] [Green Version]
- Yasui, K.; Hashimoto, E.; Komorizono, Y.; Koike, K.; Arii, S.; Imai, Y.; Shima, T.; Kanbara, Y.; Saibara, T.; Mori, T.; et al. Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. Clin. Gastroenterol. Hepatol. 2011, 9, 428–433. [Google Scholar] [CrossRef]
- Bengtsson, B.; Stål, P.; Wahlin, S.; Björkström, N.K.; Hagström, H. Characteristics and outcome of hepatocellular carcinoma in patients with nafld without cirrhosis. Liver Int. 2019, 39, 1098–1108. [Google Scholar] [CrossRef]
- Lonardo, A.; Nascimbeni, F.; Ballestri, S.; Fairweather, D.; Win, S.; Than, T.A.; Abdelmalek, M.F.; Suzuki, A. Sex differences in nonalcoholic fatty liver disease: State of the art and identification of research gaps. Hepatology 2019, 70, 1457–1469. [Google Scholar] [CrossRef]
- Dukat, A.; Lietava, J.; Krahulec, B.; Caprnda, M.; Vacula, I.; Sirotiakova, J.; Kosmalova, V.; Minarik, P.; Slovakia, I. The prevalence of abdominal obesity in slovakia. The idea slovakia study. Vnitr. Lek. 2007, 53, 326–330. [Google Scholar] [PubMed]
- Griswold, M.G.; Fullman, N.; Hawley, C.; Arian, N.; Zimsen, S.R.M.; Tymeson, H.D.; Venkateswaran, V.; Tapp, A.D.; Forouzanfar, M.H.; Salama, J.S.; et al. Alcohol use and burden for 195 countries and territories, 1990–2016: A systematic analysis for the global burden of disease study 2016. Lancet 2018, 392, 1015–1035. [Google Scholar] [CrossRef] [Green Version]
- Trépo, E.; Romeo, S.; Zucman-Rossi, J.; Nahon, P. Pnpla3 gene in liver diseases. J. Hepatol. 2016, 65, 399–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starley, B.Q.; Calcagno, C.J.; Harrison, S.A. Nonalcoholic fatty liver disease and hepatocellular carcinoma: A weighty connection. Hepatology 2010, 51, 1820–1832. [Google Scholar] [CrossRef]
- Kikuchi, L.; Oliveira, C.P.; Alvares-da-Silva, M.R.; Tani, C.M.; Diniz, M.A.; Stefano, J.T.; Chagas, A.L.; Alencar, R.S.S.M.; Vezozzo, D.C.P.; Santos, G.R.; et al. Hepatocellular carcinoma management in nonalcoholic fatty liver disease patients. Am. J. Clin. Oncol. 2016, 39, 428–432. [Google Scholar] [CrossRef] [Green Version]
- Janicko, M.; Drazilova, S.; Pella, D.; Fedacko, J.; Jarcuska, P. Pleiotropic effects of statins in the diseases of the liver. World J. Gastroenterol. 2016, 22, 6201. [Google Scholar] [CrossRef]
- Zhang, Z.-J.; Zheng, Z.-J.; Shi, R.; Su, Q.; Jiang, Q.; Kip, K.E. Metformin for liver cancer prevention in patients with type 2 diabetes: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2012, 97, 2347–2353. [Google Scholar] [CrossRef] [Green Version]
- Božin, T. Albi score as a predictor of survival in patients with compensated cirrhosis resected for hepatocellular carcinoma: Exploratory evaluation in relationship to palbi and meld liver function scores. Acta Clin. Croat. 2018, 57, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Gui, B.; Weiner, A.A.; Nosher, J.; Lu, S.-E.; Foltz, G.M.; Hasan, O.; Kim, S.K.; Gendel, V.; Mani, N.B.; Carpizo, D.R.; et al. Assessment of the albumin-bilirubin (albi) grade as a prognostic indicator for hepatocellular carcinoma patients treated with radioembolization. Am. J. Clin. Oncol. 2018, 41, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Sieghart, W.; Pinter, M.; Hucke, F.; Graziadei, I.; Schöniger-Hekele, M.; Müller, C.; Vogel, W.; Trauner, M.; Peck-Radosavljevic, M. Single determination of c-reactive protein at the time of diagnosis predicts long-term outcome of patients with hepatocellular carcinoma. Hepatology 2013, 57, 2224–2234. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Cai, J.; Li, H.; Zeng, K.; He, L.; Fu, H.; Zhang, J.; Chen, L.; Yao, J.; Zhang, Y.; et al. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio as prognostic predictors for hepatocellular carcinoma patients with various treatments: A meta-analysis and systematic review. Cell. Physiol. Biochem. 2017, 44, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-B.; Zhao, W.; Liu, B.; Lu, L.-G.; He, X.; Huang, J.-W.; Li, Y.; Hu, B.-S. The blood neutrophil-to-lymphocyte ratio predicts survival in patients with advanced hepatocellular carcinoma receiving sorafenib. Asian Pac. J. Cancer Prev. 2013, 14, 5527–5531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratziu, V.; Sanyal, A.J.; Loomba, R.; Rinella, M.; Harrison, S.; Anstee, Q.M.; Goodman, Z.; Bedossa, P.; MacConell, L.; Shringarpure, R.; et al. Regenerate: Design of a pivotal, randomised, phase 3 study evaluating the safety and efficacy of obeticholic acid in patients with fibrosis due to nonalcoholic steatohepatitis. Contemp. Clin. Trials 2019, 84, 105803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younossi, Z.M.; Ratziu, V.; Loomba, R.; Rinella, M.; Anstee, Q.M.; Goodman, Z.; Bedossa, P.; Geier, A.; Beckebaum, S.; Newsome, P.N.; et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: Interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2019, 394, 2184–2196. [Google Scholar] [CrossRef] [Green Version]
- Pfister, D.; Núñez, N.G.; Pinyol, R.; Govaere, O.; Pinter, M.; Szydlowska, M.; Gupta, R.; Qiu, M.; Deczkowska, A.; Weiner, A.; et al. Nash limits anti-tumour surveillance in immunotherapy-treated hcc. Nature 2021, 592, 450–456. [Google Scholar] [CrossRef]
- Estes, C.; Anstee, Q.M.; Arias-Loste, M.T.; Bantel, H.; Bellentani, S.; Caballeria, J.; Colombo, M.; Craxi, A.; Crespo, J.; Day, C.P.; et al. Modeling nafld disease burden in china, france, germany, italy, japan, spain, united kingdom, and united states for the period 2016–2030. J. Hepatol. 2018, 69, 896–904. [Google Scholar] [CrossRef] [PubMed]


| Other Etiology-Related HCC | NAFLD-Related HCC | p | ||
|---|---|---|---|---|
| Patients | 314 (85.33%) | 54 (14.67%) | ||
| Male patients | 224 (71.34%) | 21 (38.89%) | <0.001 | |
| Female patients | 90 (28.66%) | 33 (61.11%) | 0.0007 | |
| Age at diagnosis | Years | 65 (60–71) | 69.5 (64.2–75.8) | |
| BCLC | ||||
| 0–A | 57 (18.15%) | 5 (9.26%) | ||
| B | 94 (29.94%) | 14 (25.93%) | ||
| C | 96 (30.57%) | 20 (37.04%) | ||
| D | 67 (21.34%) | 15 (27.78%) | 0.27 | |
| Cirrhosis at diagnosis | 311 (99.04%) | 46 (85.19) | <0.0001 | |
| Diabetes | 99 (31.53%) | 41 (75.93%) | <0.0001 | |
| Child-Pugh score | 6 (5–8) | 7 (5–8) | 0.14 | |
| Performance status | 1 (1–1) | 1 (1–2) | 0.19 | |
| Metabolic syndrome | 70 (22.29%) | 36 (66.67%) | <0.0001 | |
| Total bilirubin | µmol/L | 24.1 (15.5–40.4) | 18.9 (11.3–27.6) | 0.005 |
| Direct bilirubin | µmol/L | 10.6 (6.1–21.6) | 8.85 (5.05–17.9) | 0.11 |
| AST | µkat/L | 1.10 (0.69–1.94) | 0.91 (0.55–1.56) | 0.08 |
| ALT | µkat/L | 0.69 (0.44–1.28) | 0.69 (0.42–1.19) | 0.41 |
| GMT | µkat/L | 2.55 (1.23–4.64) | 2.44 (1.28–5.85) | 0.38 |
| ALP | µkat/L | 2.34 (1.68–3.58) | 2.26 (1.89–4.03) | 0.36 |
| Albumin | g/L | 33.2 (28–38) | 35 (28.7–39.3) | 0.34 |
| CRP | mg/L | 12.6 (3.43–35.0) | 9.8 (4.21–45.6) | 0.63 |
| Total cholesterol | mmol/L | 4.32 (3.37–4.99) | 4.12 (3.69–5.07) | 0.64 |
| HDL-C | mmol/L | 0.92 (0.69–1.37) | 1.05 (0.81–1.11) | 0.90 |
| LDL-C | mmol/L | 2.61 (1.82–3.2) | 2.16 (1.76–2.92) | 0.56 |
| Triglycerides | mmol/L | 1.13 (0.74–1.5) | 1.32 (1.0–1.65) | 0.12 |
| Na | mmol/L | 139 (134–140) | 138 (137–142) | 0.01 |
| Urea | mmol/L | 5.3 (4.2–7.7) | 5.4 (4.1–6.5) | 0.38 |
| Creatinine | µmol/L | 81.3 (69.2–103) | 81 (70.8–99) | 0.81 |
| Neutrophils | ×109 | 3.85 (2.62–5.59) | 4.58 (3.06–7.34) | 0.04 |
| Lymphocytes | ×109 | 1.09 (0.71–1.72) | 1.35 (1.03–1.61) | 0.11 |
| Platelets | ×109 | 137 (93–208) | 223 (129–317) | <0.0001 |
| NLR | 3.65 (2.23–5.38) | 3.68 (2.35–6.83) | 0.58 | |
| PLR | 125 (82.2–202) | 177 (122–247) | 0.001 | |
| APRI | 0.897 (0.48–1.73) | 0.489 (0.25–0.89) | <0.0001 | |
| APRI ≥1.0 | 146 (46.50%) | 11 (20.37%) | 0.0007 | |
| DFS/PFS | Months | 5.22 (1.97–16.7) | 4.98 (2.78–9.34) | 0.76 |
| OS | Months | 8.38 (2.3–24.4) | 7.37 (2.97–16.5) | 0.68 |
| Other Etiology-Related HCC (n = 314) | NAFLD-Related HCC (n = 54) | |
|---|---|---|
| BCLC 0–A | ||
| Tumor resection | 15 (4.78%) | 5 (9.26%) |
| Radiofrequency ablation | 13 (4.14%) | 0 |
| Transarterial chemoembolization | 9 (2.87%) | 0 |
| Best supportive care | 7 (2.23%) | 0 |
| Liver transplantation | 13 (4.14%) | 0 |
| BCLC B | ||
| Tumor resection | 10 (3.18%) | |
| Radiofrequency ablation | 6 (1.91%) | 1 (1.85%) |
| Transarterial chemoembolization | 56 (17.83%) | 0 |
| Sorafenib | 9 (2.87%) | 10 (18.52%) |
| Best supportive care | 5 (1.59%) | 1 (1.85%) |
| Chemotherapy | 1 (0.32%) | 2 (3.70%) |
| Liver transplantation | 7 (2.23%) | 0 |
| BCLC C | ||
| Tumor resection | 1 (0.32%) | |
| Transarterial chemoembolization | 8 (2.55%) | 0 |
| Sorafenib | 60 (19.11%) | 0 |
| Best supportive care | 24 (7.64%) | 17 (31.48%) |
| Sorafenib + transarterial chemoembolization | 1 (0.32%) | 3 (5.56%) |
| Chemotherapy | 2 (0.64%) | 0 |
| BCLC D | ||
| Sorafenib | 0 | 0 |
| Best supportive care | 67 (21.34%) | 15 (27.78%) |
| Other Etiology-Related HCC (n = 314) | NAFLD-Related HCC (n = 54) | p | |
|---|---|---|---|
| Statins | 12 (3.82%) | 9 (16.67%) | 0.001 |
| Low molecular weight heparin | 14 (4.46%) | 4 (7.41%) | 0.32 |
| Metformin | 24 (7.64%) | 13 (24.07%) | 0.0008 |
| Ursodeoxycholic acid | 76 (24.20%) | 8 (14.81%) | 0.16 |
| Hazard Ratio (95% Confidence Interval) | p-Value | |
|---|---|---|
| Number of liver lesions | ||
| 1 | Ref. | |
| 2 | 1.58 (0.6515–3.8400) | 0.31 |
| ≥3 | 1.38 (0.6976–2.7390) | 0.35 |
| CRP | ||
| <5 mg/L | Ref. | |
| ≥5 mg/L | 1.41 (0.5366–3.706) | 0.49 |
| PLR | ||
| <150 | Ref. | |
| ≥150 | 2.17 (1.0408–4.5440) | 0.04 |
| ALBI | ||
| Grade 1 | Ref. | |
| Grade 2 | 1.37 (0.5899–3.1830) | 0.46 |
| Grade 3 | 3.20 (1.1771–8.7190) | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safcak, D.; Drazilova, S.; Gazda, J.; Andrasina, I.; Adamcova-Selcanova, S.; Barila, R.; Mego, M.; Rac, M.; Skladany, L.; Zigrai, M.; et al. Nonalcoholic Fatty Liver Disease-Related Hepatocellular Carcinoma: Clinical Patterns, Outcomes, and Prognostic Factors for Overall Survival—A Retrospective Analysis of a Slovak Cohort. J. Clin. Med. 2021, 10, 3186. https://doi.org/10.3390/jcm10143186
Safcak D, Drazilova S, Gazda J, Andrasina I, Adamcova-Selcanova S, Barila R, Mego M, Rac M, Skladany L, Zigrai M, et al. Nonalcoholic Fatty Liver Disease-Related Hepatocellular Carcinoma: Clinical Patterns, Outcomes, and Prognostic Factors for Overall Survival—A Retrospective Analysis of a Slovak Cohort. Journal of Clinical Medicine. 2021; 10(14):3186. https://doi.org/10.3390/jcm10143186
Chicago/Turabian StyleSafcak, Dominik, Sylvia Drazilova, Jakub Gazda, Igor Andrasina, Svetlana Adamcova-Selcanova, Radovan Barila, Michal Mego, Marek Rac, Lubomir Skladany, Miroslav Zigrai, and et al. 2021. "Nonalcoholic Fatty Liver Disease-Related Hepatocellular Carcinoma: Clinical Patterns, Outcomes, and Prognostic Factors for Overall Survival—A Retrospective Analysis of a Slovak Cohort" Journal of Clinical Medicine 10, no. 14: 3186. https://doi.org/10.3390/jcm10143186
APA StyleSafcak, D., Drazilova, S., Gazda, J., Andrasina, I., Adamcova-Selcanova, S., Barila, R., Mego, M., Rac, M., Skladany, L., Zigrai, M., Janicko, M., & Jarcuska, P. (2021). Nonalcoholic Fatty Liver Disease-Related Hepatocellular Carcinoma: Clinical Patterns, Outcomes, and Prognostic Factors for Overall Survival—A Retrospective Analysis of a Slovak Cohort. Journal of Clinical Medicine, 10(14), 3186. https://doi.org/10.3390/jcm10143186








