Value of Transnasal Esophagoscopy in the Workup of Laryngo-Pharyngeal Reflux
Abstract
:1. Introduction
2. Materials and Methods
2.1. Transnasal Esophagoscopy (TNE)
2.2. Statistics
3. Results
3.1. Clinical Data
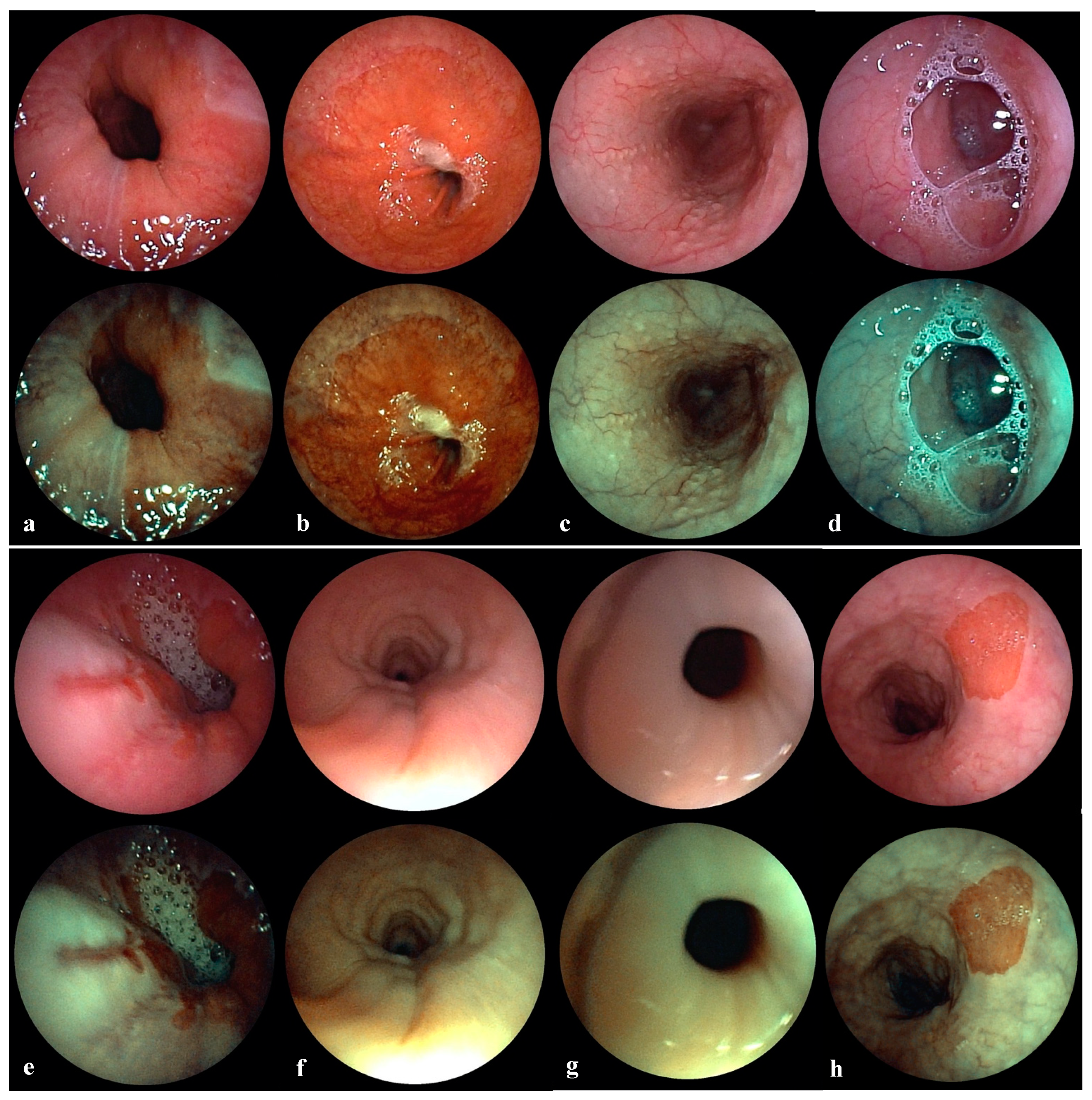
3.2. Transnasal Esophagoscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koufman, J.A.; Aviv, J.E.; Casiano, R.R.; Shaw, G.Y. Laryngopharyngeal Reflux: Position Statement of the Committee on Speech, Voice, and Swallowing Disorders of the American Academy of Otolaryngology-Head and Neck Surgery. YMHN 2016, 127, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Sirin, S.; Öz, F. Laryngopharyngeal reflux concept: What is known and what should we focus on? Braz. J. Otorhinolaryngol. 2019, 85, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Koufman, J.A. The otolaryngologic manifestations of gastroesophageal reflux disease (GERD): A clinical investigation of 225 patients using ambulatory 24-hour pH monitoring and an experimental investigation of the role of acid and pepsin in the development of laryngeal injury. Laryngoscope 1991, 101, 1–78. [Google Scholar] [PubMed]
- Postma, G.N.; Belafsky, P.C.; Tomek, M.S.; Koufman, J.A. Esophageal Motor Function in Laryngopharyngeal Reflux is Superior to That in Classic Gastroesophageal Reflux Disease. Ann. Otol. Rhinol. Laryngol. 2016, 110, 1114–1116. [Google Scholar] [CrossRef] [PubMed]
- Campagnolo, A.M.; Priston, J.; Thoen, R.H.; Medeiros, T.; Assunção, A.R. Laryngopharyngeal reflux: Diagnosis, treatment, and latest research. Int. Arch. Otorhinolaryngol. 2014, 18, 184–191. [Google Scholar] [PubMed] [Green Version]
- Belafsky, P.C.; Postma, G.N.; Koufman, J.A. The validity and reliability of the reflux finding score (RFS). Laryngoscope 2001, 111, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Belafsky, P.C.; Postma, G.N.; Koufman, J.A. Validity and Reliability of the Reflux Symptom Index (RSI). J. Voice 2002, 16, 274–277. [Google Scholar] [CrossRef]
- Horvath, L.; Hagmann, P.; Burri, E.; Kraft, M. A Novel Scoring System for Evaluating Laryngopharyngeal Reflux. Clin. Otolaryngol. 2021, 46, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Shaker, R. Unsedated trans-nasal pharyngoesophagogastroduodenoscopy (T-EGD): Technique. Gastrointest. Endosc. 1994, 40, 346–348. [Google Scholar] [CrossRef]
- Bush, C.M.; Postma, G.N. Transnasal esophagoscopy. Otolaryngol. Clin. North. Am. 2013, 46, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.; Dua, K.; Massey, B.; Berger, W.; Hogan, W.J.; Shaker, R. A comparative study of unsedated transnasal esophagogastroduodenoscopy and conventional EGD. Gastrointest. Endosc. 1996, 44, 422–424. [Google Scholar] [CrossRef]
- Jobe, B.A.; Hunter, J.G.; Chang, E.Y.; Kim, C.Y.; Eisen, G.M.; Robinson, J.D.; Diggs, B.S.; O’Rourke, R.W.; Rader, A.E.; Schipper, P.; et al. Office-Based Unsedated Small-Caliber Endoscopy Is Equivalent to Conventional Sedated Endoscopy in Screening and Surveillance for Barrett’s Esophagus: A Randomized and Blinded Comparison. Am. J. Gastroenterol. 2006, 101, 2693. [Google Scholar] [CrossRef] [PubMed]
- Horvath, L.; Hagmann, P.; Burri, E.; Kraft, M. Evaluation of Oropharyngeal pH-Monitoring in the Assessment of Laryngopharyngeal Reflux. J. Clin. Med. 2021, 10, 2409. [Google Scholar] [CrossRef] [PubMed]
- Genta, R.M.; Spechler, S.J.; Kielhorn, A.F. The Los Angeles and Savary-Miller systems for grading esophagitis: Utilization and correlation with histology. Dis. Esophagus. 2011, 24, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Andrus, J.G.; Dolan, R.W.; Anderson, T.D. Transnasal esophagoscopy: A high-yield diagnostic tool. Laryngoscope 2005, 115, 993–996. [Google Scholar] [CrossRef] [PubMed]
- Postma, G.N.; Cohen, J.T.; Belafsky, P.C.; Halum, S.L.; Gupta, S.K.; Bach, K.K.; Koufman, J.A. Transnasal esophagoscopy: Revisited (over 700 consecutive cases). Laryngoscope 2005, 115, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Rocke, J.; Ahmed, S. Transnasal Esophagoscopy-Our Experience. Int. Arch. Otorhinolaryngol. 2019, 23, 7–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Best, A.R.; Halum, S.L.; Parker, N.P. Current Indications for Transnasal Esophagoscopy: An American Broncho-Esophagological Association Survey. Ann. Otol. Rhinol. Laryngol. 2018, 127, 926–930. [Google Scholar] [CrossRef] [PubMed]
- Yagi, J.; Adachi, K.; Arima, N.; Tanaka, S.; Ose, T.; Azumi, T.; Sasaki, H.; Sato, M.; Kinoshita, Y. A prospective randomized comparative study on the safety and tolerability of transnasal esophagogastroduodenoscopy. Endoscopy 2005, 37, 1226–1231. [Google Scholar] [CrossRef] [PubMed]
| Horvath Score | Severity | Pathologic RSI | Pathologic RFS | Pathologic PHM | Positive Ryan | Pathologic TNE | Total |
|---|---|---|---|---|---|---|---|
| 4–5 | Severe LPR | 84 (80%) | 63 (60%) | 105 (100%) | 98 (93%) | 103 (98%) | 105 (53%) |
| 2–3 | Nonsevere LPR | 55 (67%) | 17 (21%) | 79 (96%) | 7 (9%) | 76 (93%) | 82 (41%) |
| Moderate | 21 (60%) | 7 (20%) | 33 (94%) | 6 (17%) | 31 (89%) | 35 (18%) | |
| Mild | 17 (65%) | 3 (12%) | 26 (100%) | 1 (4%) | 24 (92%) | 26 (13%) | |
| Neutral | 10 (91%) | 4 (36%) | 10 (91%) | 0 (0%) | 11 (100%) | 11 (6%) | |
| Alkaline | 7 (70%) | 3 (30%) | 10 (100%) | 0 (0%) | 10 (100%) | 10 (5%) | |
| 0–1 | No LPR | 6 (46%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (15%) | 13 (7%) |
| TNE | Severe LPR | Moderate LPR | Mild LPR | Neutral LPR | Alkaline LPR | No LPR | Total |
|---|---|---|---|---|---|---|---|
| Pathologic findings | 103 (98%) | 31 (89%) | 24 (92%) | 11 (100%) | 10 (100%) | 2 (15%) | 181 (91%) |
| Insufficient cardia | 79 (75%) | 24 (69%) | 19 (73%) | 10 (91%) | 8 (80%) | 0 (0%) | 140 (70%) |
| Hiatal hernia | 72 (69%) | 20 (57%) | 16 (62%) | 9 (82%) | 8 (80%) | 0 (0%) | 125 (63%) |
| Lymphoid follicles | 48 (46%) | 19 (54%) | 13 (50%) | 8 (73%) | 6 (60%) | 1 (8%) | 95 (48%) |
| Visible reflux | 49 (47%) | 19 (54%) | 12 (46%) | 6 (55%) | 5 (50%) | 1 (8%) | 92 (46%) |
| Peptic esophagitis | 31 (30%) | 8 (23%) | 5 (19%) | 7 (64%) | 3 (30%) | 1 (8%) | 55 (28%) |
| Tertiary contraction | 30 (29%) | 13 (37%) | 8 (31%) | 6 (55%) | 5 (50%) | 1 (8%) | 63 (32%) |
| Insufficient UES | 20 (19%) | 10 (29%) | 3 (12%) | 5 (45%) | 1 (10%) | 0 (0%) | 39 (20%) |
| Barrett esophagus | 3 (3%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (2%) |
| Ectopic mucosa | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1%) |
| Normal findings | 2 (2%) | 4 (11%) | 2 (8%) | 0 (0%) | 0 (0%) | 11 (85%) | 19 (9%) |
| Method | True Positive | False Positive | False Negative | True Negative | Total |
|---|---|---|---|---|---|
| RSI | 138 | 6 | 49 | 7 | 200 |
| RFS | 73 | 4 | 69 | 54 | 200 |
| PHM | 98 | 6 | 7 | 89 | 200 |
| TNE | 179 | 2 | 8 | 11 | 200 |
| Method | Sensitivity | Specificity | Accuracy | Pos. Pred. Value | Neg. Pred. Value |
|---|---|---|---|---|---|
| RSI | 74% † (p = 0.000) | 54% (p = 0.101) | 73% † (p = 0.000) | 96% (p = 0.080) | 13% † (p = 0.000) |
| RFS | 51% † (p = 0.000) | 93% (p = 0.231) | 64% † (p = 0.000) | 95% (p = 0.067) | 44% (p = 0.186) |
| PHM | 93% (p = 0.290) | 94% (p = 0.193) | 94% (p = 0.334) | 94% * (p = 0.029) | 93% † (p = 0.000) |
| TNE | 96% | 85% | 95% | 99% | 58% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horvath, L.; Fostiropoulos, K.; Burri, E.; Kraft, M. Value of Transnasal Esophagoscopy in the Workup of Laryngo-Pharyngeal Reflux. J. Clin. Med. 2021, 10, 3188. https://doi.org/10.3390/jcm10143188
Horvath L, Fostiropoulos K, Burri E, Kraft M. Value of Transnasal Esophagoscopy in the Workup of Laryngo-Pharyngeal Reflux. Journal of Clinical Medicine. 2021; 10(14):3188. https://doi.org/10.3390/jcm10143188
Chicago/Turabian StyleHorvath, Lukas, Karolos Fostiropoulos, Emanuel Burri, and Marcel Kraft. 2021. "Value of Transnasal Esophagoscopy in the Workup of Laryngo-Pharyngeal Reflux" Journal of Clinical Medicine 10, no. 14: 3188. https://doi.org/10.3390/jcm10143188
APA StyleHorvath, L., Fostiropoulos, K., Burri, E., & Kraft, M. (2021). Value of Transnasal Esophagoscopy in the Workup of Laryngo-Pharyngeal Reflux. Journal of Clinical Medicine, 10(14), 3188. https://doi.org/10.3390/jcm10143188






