Rates, Indications, and Speech Perception Outcomes of Revision Cochlear Implantations
Abstract
:1. Introduction
2. Materials and Methods
- (a)
- Relevant history, including demographic characteristics and the time-course of symptoms that led to RCI (specifically the duration between Pri-CI and symptoms onset as well as between symptoms onset and RCI).
- (b)
- Reports of medical follow-up and surgical procedures, including imaging [computed tomography (CT), magnetic resonance imaging (MRI) or plain film Stenver’s view].
- (c)
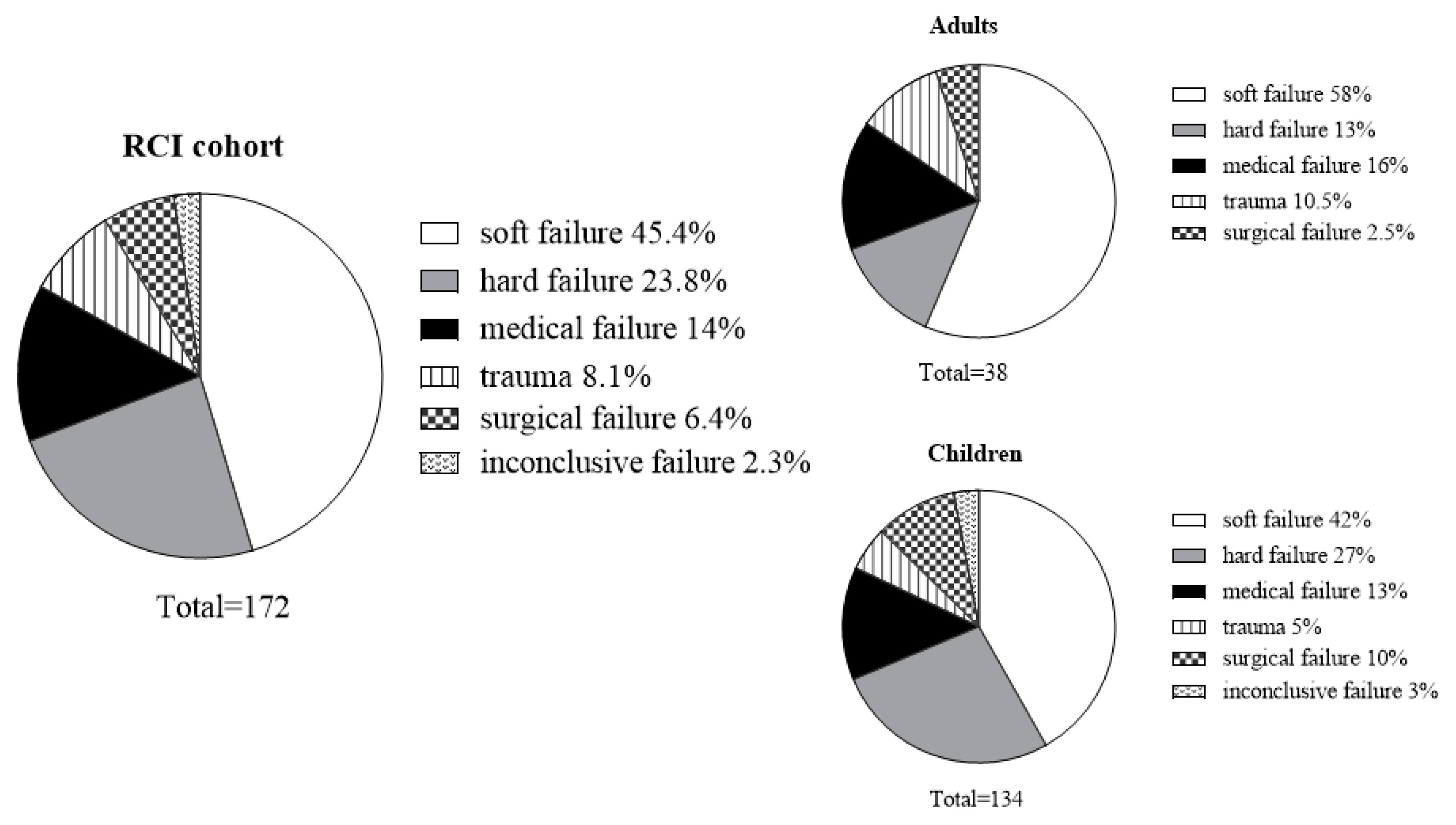
- RCI indication. A comprehensive case-by-case review by a panel of two senior audiologists and two neurotologists was performed to determine the following RCI indications: (a) Device-related indications—Soft failure was defined according to the 2005 consensus guidelines [13] and included cases with decreased or unexpected poor performance, non-auditory aversive symptoms, and intermittent function. In all of the soft failure cases, medical, imaging, programing, and hardware issues have been ruled out. Hard failure was determined according to the absence of communication between the internal and external hardware. (b) Non-device-related indications—Medical failure included cases with suspected biofilm infection, allergic response, neuralgia and chronic middle ear condition. Patients with severe congenital inner ear malformations (i.e., common cavity, hypoplastic nerve, others) were included in the medical indication group as well. Following a thorough case-by-case review, our assumption was that these patients’ medical status (reflected in severe inner ear anomalies) was the main reason for the symptoms (i.e., poor speech perception, extracochlear manifestations) that ultimately led to reimplantation. Surgical failure included malposition or inadequate electrode insertion, electrode migration or protrusion, and device extrusion. Trauma included implants that failed immediately after an event of head trauma.
- (d)
- Speech perception was evaluated by means of an open-set monosyllabic word recognition test [Hebrew Arthur Boothroyd [24]; HAB] scored for correct words and phonemes. When the HAB test could not be administered due to young age, poor cognitive-linguistic skills or limited speech perception ability, speech reception threshold (SRT) was used. Both tests were administered in a quiet listening condition. First, we compared between speech perception scores measured before RCI (i.e., most recent measurement prior to appearance of symptoms), to those obtained 6 months or more after RCI. The difference score was calculated and subsequently the performance in each case was classified as unchanged, declined, or improved. The clinically significant criterion was defined as a change of >10% in HAB phoneme scores. Phoneme score, rather than word score, was selected as it was found to be less dependent on linguistic abilities, showed reduced variability and was therefore considered a more valid measure [25,26,27]. Analysis of HAB results of 90 adult CI recipients from our CI program (not included in the current study) revealed that between-lists phoneme score variability did not exceed 10%. Accordingly, the selected criterion for post RCI change was set to >10%. When phoneme scores were unobtainable, a change of ≥10 dB in SRT was used.
3. Results
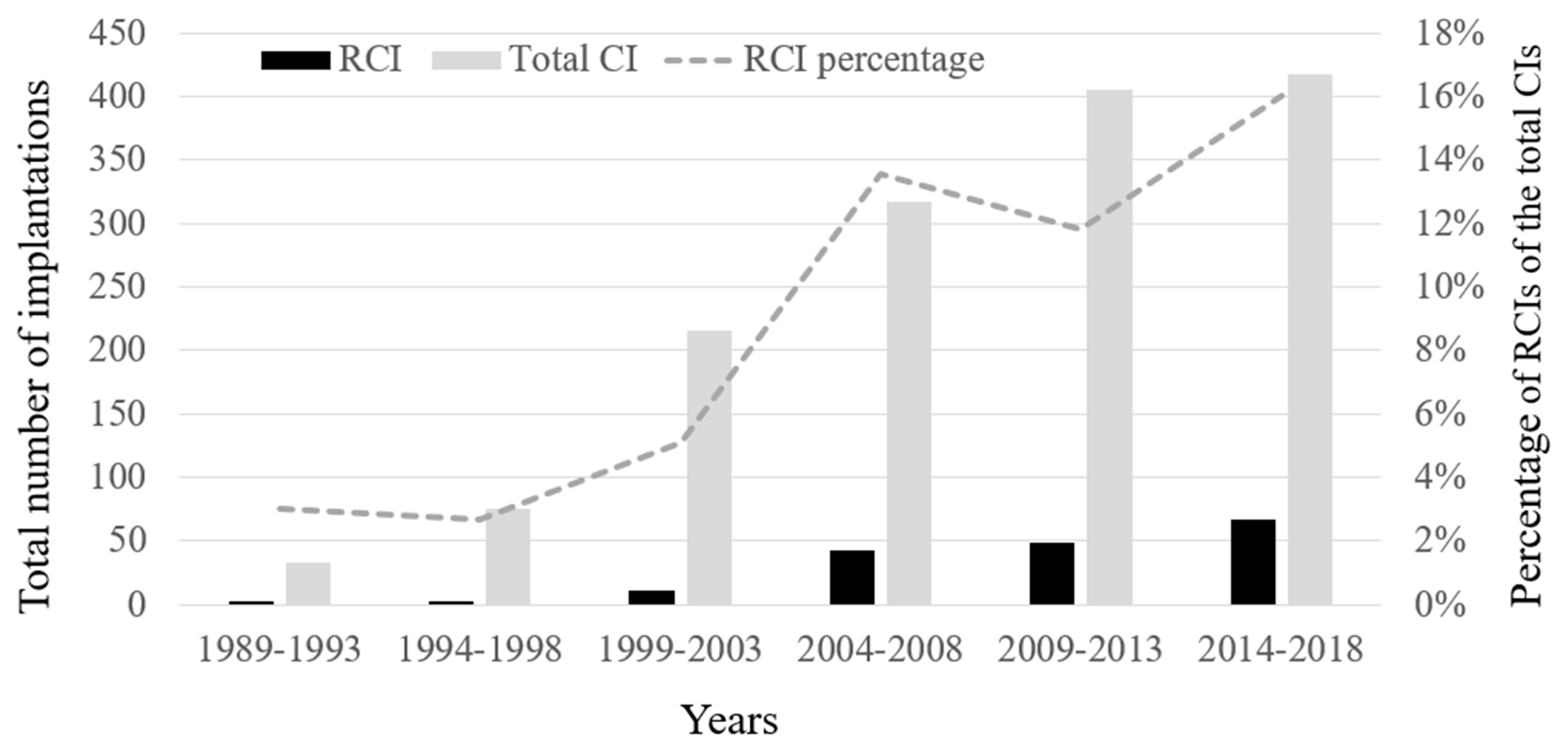
3.1. Demographics, RCI Rates and Time-Course
3.2. RCI Indications
3.3. Audiologic Outcomes
3.4. Surgical Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cullen, R.D.; Fayad, J.N.; Luxford, W.M.; Buchman, C.A. Revision cochlear implant surgery in children. Otol. Neurotol. 2008, 29, 214–220. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, G. European consensus statement on cochlear implant failures and explantations. Otol. Neurotol. 2005, 26, 1097–1099. [Google Scholar]
- Hochmair-Desoyer, I.; Burian, K. Reimplantation of a molded scala tympani electrode: Impact on psychophysical and speech discrimination abilities. Ann. Otol. Rhinol. Laryngol. 1985, 94, 65–70. [Google Scholar] [CrossRef]
- Wang, J.T.; Wang, A.Y.; Psarros, C.; Da Cruz, M. Rates of revision and device failure in cochlear implant surgery: A 30-year experience. Laryngoscope 2014, 124, 2393–2399. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Chen, Y.; Tan, P.; Chen, J.; Han, Y.; Gao, L.; Lu, Y.; Du, B. Complications and clinical analysis of 416 consecutive cochlear implantations. Int. J. Pediatr. Otorhinolaryngol. 2011, 75, 1143–1146. [Google Scholar] [CrossRef] [PubMed]
- Roby, B.; Ferrello, M.; Huang, T.; Rimell, F.; Levine, S. Symptom timeline preceding cochlear implant failure: An institutional experience. Otolaryngol. Head Neck Surg. 2012, 146, 782–787. [Google Scholar] [CrossRef]
- O’Neill, G.; Tolley, N.S. Cochlear implant reliability: On the reporting of rates of revision surgery. Indian J. Otolaryngol. Head Neck Surg. 2020, 72, 257–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Chen, B.; Shi, Y.; Li, Y. A retrospective review of cochlear implant revision surgery: A 24-year experience in China. Eur. Arch. Oto Rhino Laryngol. 2021. [Google Scholar] [CrossRef]
- Brown, K.D.; Connell, S.S.; Balkany, T.J.; Eshraghi, A.E.; Telischi, F.F.; Angeli, S.A. Incidence and indications for revision cochlear implant surgery in adults and children. Laryngoscope 2009, 119, 152–157. [Google Scholar] [CrossRef]
- Battmer, R.-D.; Linz, B.; Lenarz, T. A review of device failure in more than 23 years of clinical experience of a cochlear implant program with more than 3,400 implantees. Otol. Neurotol. 2009, 30, 455–463. [Google Scholar] [CrossRef]
- Trotter, M.I.; Backhouse, S.; WAGSTAFF, S.; Hollow, R.; Briggs, R.J.S. Classification of cochlear implant failures and explantation: The Melbourne experience, 1982–2006. Cochlear Implants Int. 2009, 10 (Suppl. 1), 105–110. [Google Scholar] [CrossRef]
- Sorrentino, T.; Côté, M.; Eter, E.; Laborde, M.-L.; Cochard, N.; Deguine, O.; Fraysse, B. Cochlear reimplantations: Technical and surgical failures. Acta Otolaryngol. 2009, 129, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Balkany, T.J.; Hodges, A.V.; Buchman, C.A.; Luxford, W.M.; Pillsbury, C.H.; Roland, P.S.; Shallop, J.K.; Backous, D.D.; Franz, D.; Graham, J.M.; et al. Cochlear implant soft failures consensus development conference statement. Otol. Neurotol. 2005, 26. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, M.; Thierry, B.; Glynn, F.; De Lamaze, A.; Garabédian, E.N.; Loundon, N. Cochlear implant failure and revision surgery in pediatric population. Ann. Otol. Rhinol. Laryngol. 2015, 124, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Rivas, A.; Marlowe, A.L.; Chinnici, J.E.; Niparko, J.K.; Francis, H.W. Revision cochlear implantation surgery in adults: Indications and results. Otol. Neurotol. 2008, 29, 639–648. [Google Scholar] [CrossRef]
- Alexiades, G.; Roland, J.T., Jr.; Fishman, A.J.; Shapiro, W.; Waltzman, S.B.; Cohen, N.L. Cochlear reimplantation: Surgical techniques and functional results. Laryngoscope 2001, 111, 1608–1613. [Google Scholar] [CrossRef] [PubMed]
- Reis, M.; Boisvert, I.; Looi, V.; da Cruz, M. Speech recognition outcomes after cochlear reimplantation surgery. Trends Hear. 2017, 21, 2331216517706398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahtani, S.; Glynn, F.; Mawman, D.J.; O’Driscoll, M.P.; Green, K.; Bruce, I.; Freeman, S.R.M.; Lloyd, S.K.W. Outcomes of cochlear reimplantation in adults. Otol. Neurotol. 2014, 35, 1366–1372. [Google Scholar] [CrossRef]
- Côté, M.; Ferron, P.; Bergeron, F.; Bussières, R. Cochlear reimplantation: Causes of failure, outcomes, and audiologic performance. Laryngoscope 2007, 117, 1225–1235. [Google Scholar] [CrossRef]
- Dillon, M.T.; Adunka, O.F.; Anderson, M.L.; Adunka, M.C.; King, E.R.; Buchman, C.A.; Pillsbury, H.C. Influence of age at revision cochlear implantation on speech perception outcomes. JAMA Otolaryngol. Neck Surg. 2015, 141, 219–224. [Google Scholar] [CrossRef] [Green Version]
- Lassig, A.-A.D.; Zwolan, T.A.; Telian, S.A. Cochlear implant failures and revision. Otol. Neurotol. 2005, 26, 624–634. [Google Scholar] [CrossRef]
- Manrique-Huarte, R.; Huarte, A.; Manrique, M.J. Surgical findings and auditory performance after cochlear implant revision surgery. Eur. Arch. Oto Rhino Laryngol. 2016, 273, 621–629. [Google Scholar] [CrossRef]
- Chung, D.; Kim, A.H.; Parisier, S.; Linstrom, C.; Alexiades, G.; Hoffman, R.; Kohan, D. Revision cochlear implant surgery in patients with suspected soft failures. Otol. Neurotol. 2010, 31, 1194–1198. [Google Scholar] [CrossRef] [PubMed]
- Boothroyd, A. Statistical theory of speech descrimination score. J. Acoust. Soc. Am. 1968, 43, 362–367. [Google Scholar] [CrossRef]
- Boothroyd, A. The performance/intensity function: An underused resource. Ear Hear. 2008, 29, 479–491. [Google Scholar] [CrossRef]
- Leigh, J.R.; Moran, M.; Hollow, R.; Dowell, R.C. Evidence-based guidelines for recommending cochlear implantation for postlingually deafened adults. Int. J. Audiol. 2016, 55 (Suppl. 2), S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Billings, C.J.; Penman, T.M.; Ellis, E.M.; Baltzell, L.S.; McMillan, G.P. Phoneme and Word Scoring in Speech-in-Noise Audiometry. Am. J. Audiol. 2016, 25, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Kronenberg, J.; Baumgartner, W.; Migirov, L.; Dagan, T.; Hildesheimer, M. The Suprameatal approach: An alternative surgical approach to cochlear implantation. Otol. Neurotol. 2004, 25, 41–45. [Google Scholar] [CrossRef] [PubMed]
- De Raeve, L. Cochlear implants in Belgium: Prevalence in paediatric and adult cochlear implantation. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2016, 133, S57–S60. [Google Scholar] [CrossRef] [PubMed]
- Deep, N.L.; Dowling, E.M.; Jethanamest, D.; Carlson, M.L. Cochlear implantation: An overview. J. Neurol. Surg. B. Skull Base 2019, 80, 169–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wijaya, C.; Simões-Franklin, C.; Glynn, F.; Walshe, P.; Reilly, R.; Viani, L. Revision cochlear implantation: The Irish experience. Cochlear Implants Int. 2019, 20, 281–287. [Google Scholar] [PubMed]
- Layfield, E.; Hwa, T.P.; Naples, J.; Maina, I.; Brant, J.A.; Eliades, S.J.; Bigelow, D.C.; Ruckenstein, M.J. Failure and Revision Surgery after Cochlear Implantation in the Adult Population: A 10-Year Single-Institution Retrospective and Systematic Review of the Literature. Otol. Neurotol. 2021, 42, 408–413. [Google Scholar] [CrossRef]
- Kou, Y.-F.; Hunter, J.B.; Kutz, J.W.; Isaacson, B.; Lee, K.H. Revision pediatric cochlear implantation in a large tertiary center since 1986. Cochlear Implants Int. 2020, 21, 353–357. [Google Scholar] [CrossRef]
- Lane, C.; Zimmerman, K.; Agrawal, S.; Parnes, L. Cochlear implant failures and reimplantation: A 30-year analysis and literature review. Laryngoscope 2020, 130, 782–789. [Google Scholar] [CrossRef]
- Distinguin, L.; Blanchard, M.; Rouillon, I.; Parodi, M.; Loundon, N. Pediatric cochlear reimplantation: Decision-tree efficacy. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2018, 135, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Ulanovski, D.; Attias, J.; Sokolov, M.; Greenstein, T.; Raveh, E. Pediatric Cochlear implant soft failure. Am. J. Otolaryngol. 2018, 39, 107–110. [Google Scholar] [CrossRef]
- Kimura, K.S.; O’Connell, B.P.; Nassiri, A.M.; Dedmon, M.M.; Haynes, D.S.; Bennett, M.L. Outcomes of Revision Cochlear Implantation. Otol. Neurotol. 2020, 41, e705–e711. [Google Scholar] [CrossRef] [PubMed]
- Elgandy, M.; Mobashir, M.; Sheikh, E.; Hansen, M.; Tyler, R.; Dunn, C.; Gantz, B. Revision cochlear implant surgery. Int. Tinnitus J. 2018, 22, 188–197. [Google Scholar] [CrossRef] [Green Version]



| Total | Children | Adults | |
|---|---|---|---|
| Number of (%) | |||
| RCIs | 172 | 134 (76%) | 38 (24%) |
| patients | 145 | 115 (79%) | 30 (21%) |
| Gender (% †) | |||
| female | 69 (48%) | 50 (43.5%) | 19 (63%) |
| male | 76 (52%) | 65 (56.5%) | 11 (37%) |
| Side (% ‡) | |||
| right | 80 (46.5%) | 56 (42%) | 24 (63%) |
| left | 92 (53.5%) | 78 (58%) | 14 (37%) |
| Age at primary CI (years) | |||
| M ± SD | 12.5 ± 16.9 | 4.7 ± 3.8 | 39.9 ± 16.6 |
| median (range) | 4.5 (9 months-76.8) | 3.3 (9 months-17.8) | 34.7 (18.5–76.8) |
| Approach of primary CI (% ‡) | |||
| SMA | 115 (67%) | 89 (66.4%) | 26 (68.4%) |
| PTA | 57 (33%) | 45 (33.6%) | 12 (32.6%) |
| Hearing loss etiology (% †) | |||
| Genetic | 57 (39.3%) | 53 (46.1%) | 4 (13.3%) |
| unknown | 48 (33.1%) | 35 (30.4%) | 13 (43.3%) |
| inner ear malformation | 10 (6.9%) | 8 (7%) | 2 (6.7%) |
| intrauterine infection | 8 (7%) | 1 (3.3%) | |
| neonatal complication | 7 (4.8%) | 6 (5.2%) | 1 (3.3%) |
| meningitis | 3 (2.1%) | 3 (2.6%) | 0 (0%) |
| other | 11 (7.6%) | 2 (1.7%) | 9 (30%) |
| Time-Course (Months) | Total | Children | Adults | Statistical Analysis Mann–Whitney Test |
|---|---|---|---|---|
| Pri-CI to RCI | ||||
| M ± SD | 80 ± 70.5 | 80 ± 65.4 | 83 ± 86.8 | U(172) = 2396 p-value = 0.58 |
| range | 1–248 | 0.5–346 | ||
| Pri-CI to onset of symptoms | ||||
| M ± SD | 54 ± 62.1 | 56.5 ± 60.2 | 45 ± 68.2 | U(170) = 1923 p-value = 0.03 * effect size r = −0.17 |
| range | 0–219 | 0–285 | ||
| Onset of symptoms to RCI | ||||
| M ± SD | 26 ± 40.7 | 23.5 ± 35.8 | 38 ± 53.7 | U(170) = 2000 p-value = 0.06 |
| range | 0.5–240 | 0.5–234 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagiv, D.; Yaar-Soffer, Y.; Yakir, Z.; Henkin, Y.; Shapira, Y. Rates, Indications, and Speech Perception Outcomes of Revision Cochlear Implantations. J. Clin. Med. 2021, 10, 3215. https://doi.org/10.3390/jcm10153215
Sagiv D, Yaar-Soffer Y, Yakir Z, Henkin Y, Shapira Y. Rates, Indications, and Speech Perception Outcomes of Revision Cochlear Implantations. Journal of Clinical Medicine. 2021; 10(15):3215. https://doi.org/10.3390/jcm10153215
Chicago/Turabian StyleSagiv, Doron, Yifat Yaar-Soffer, Ziva Yakir, Yael Henkin, and Yisgav Shapira. 2021. "Rates, Indications, and Speech Perception Outcomes of Revision Cochlear Implantations" Journal of Clinical Medicine 10, no. 15: 3215. https://doi.org/10.3390/jcm10153215





