Conductive Hearing Loss with Age—A Histologic and Audiometric Evaluation
Abstract
:1. Introduction
2. Materials and Methods
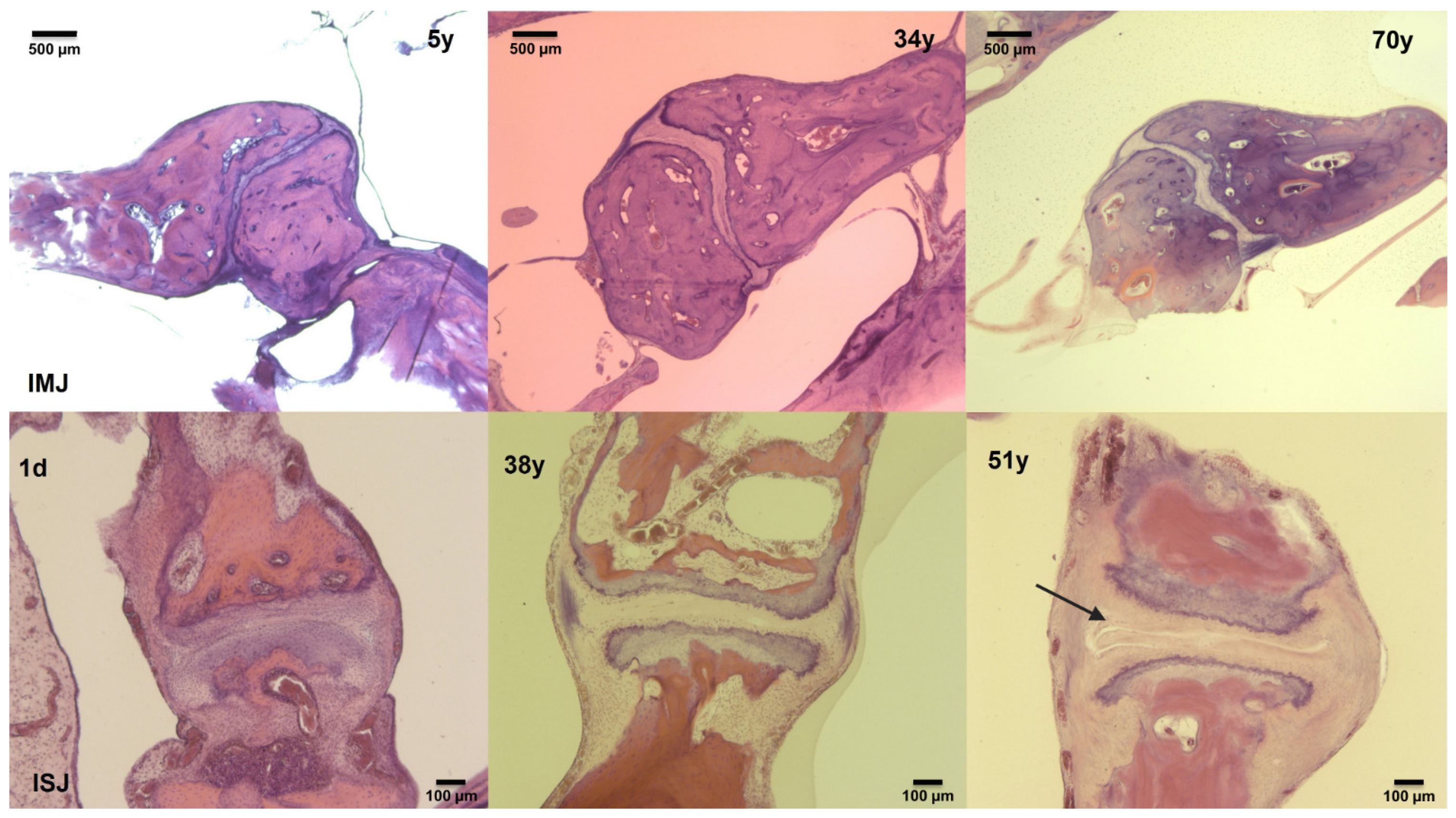
2.1. Histological Analysis of Middle-Ear Joints
- (1)
- B-line (Bone-line)—distance between the osseous portions of the corresponding ossicles.
- (2)
- Discus—distance between the ossicles.
- (3)
- C tot—total cartilage of the ossicle.
- (4)
- cC—calcified cartilage of the ossicle.
- (5)
- hC—hyaline cartilage of the ossicle, equal to C tot minus cC.
2.2. Air-Bone Gap (ABG) Data
2.3. Statistical Analysis
3. Results
3.1. Histological Analysis of Middle-Ear Joints
3.2. ABG Patient Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Zelaya, C.E.; Lucas, J.W.; Hoffman, H.J. Self-Reported Hearing Trouble in Adults Aged 18 and Over: United States, 2014; National Center for Health Statistics: Hyattsville, MD, USA, 2015; pp. 1–8. [Google Scholar]
- Saumil, N.M.; Joseph, B.N., Jr. Disorders of aging. In Schuknecht’s Pathology of the Ear, 3rd ed.; Saumil, N.M., Joseph, B.N., Jr., Eds.; People’s Medical Publisher House: Shelton, CT, USA, 2010; pp. 431–476. [Google Scholar]
- Mazelová, J.; Popelar, J.; Syka, J. Auditory function in presbycusis: Peripheral vs. central changes. Exp. Gerontol. 2003, 38, 87–94. [Google Scholar] [CrossRef]
- Glorig, A.; Davis, H. Age, noise and hearing loss. Ann. Otol. Rhinol. Laryngol. 1961, 70, 556–571. [Google Scholar] [CrossRef]
- Nixon, J.C.; Glorig, A.; High, W.S. Changes in air and bone conduction thresholds as a function of age. J. Laryngol. Otol. 1962, 76, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Nondahl, D.M.; Tweed, T.S.; Cruickshanks, K.J.; Wiley, T.L.; Dalton, D.S. Aging and the 4-kHz air-bone gap. J. Speech Lang. Hear. Res. 2012, 55, 1128–1134. [Google Scholar] [CrossRef] [Green Version]
- Willi, U.B.; Ferrazzini, M.A.; Huber, A.M. The incudo-malleolar joint and sound transmission losses. Hear. Res. 2002, 174, 32–44. [Google Scholar] [CrossRef]
- Goetzinger, C.P.; Proud, G.O.; Dirks, D.; Embrey, J. A study of hearing in advanced age. Arch. Otolaryngol. 1961, 73, 662–674. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J.; Welsh, O.L.; Luterman, D.M. Auditory responses in selected elderly men. J. Gerontol. 1963, 18, 267–270. [Google Scholar] [CrossRef]
- Sataloff, J.; Vassallo, L.; Menduke, H. Presbycusis: Air and bone conduction thresholds. Laryngoscope 1965, 75, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Marshall, L.; Martinez, S.A.; Schlaman, M.E. Reassessment of high-frequency air-bone gaps in older adults. Arch. Otolaryngol. 1983, 109, 601–606. [Google Scholar] [CrossRef]
- Margolis, R.H.; Eikelboom, R.H.; Johnson, C.; Ginter, S.M.; de Swanepoel, W.; Moore, B.C. False air-bone gaps at 4 kHz in listeners with normal hearing and sensorineural hearing loss. Int. J. Audiol. 2013, 52, 526–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerig, R.; Ihrle, S.; Röösli, C.; Dalbert, A.; Dobrev, I.; Pfiffner, F.; Eiber, A.; Huber, A.M.; Sim, J.H. Contribution of the incudo-malleolar joint to middle-ear sound transmission. Hear. Res. 2015, 327, 218–226. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.; Gan, R.Z. Dynamic properties of human incudostapedial joint-Experimental measurement and finite element modeling. Med. Eng. Phys. 2018, 54, 14–21. [Google Scholar] [CrossRef]
- Ruah, C.B.; Schachern, P.A.; Zelterman, D.; Paparella, M.M.; Yoon, T.H. Age-related morphologic changes in the human tympanic membrane. A light and electron microscopic study. Arch. Otolaryngol. Head Neck Surg. 1991, 117, 627–634. [Google Scholar] [CrossRef]
- Harty, M. Elastic tissue in the middle-ear cavity. J. Laryngol. Otol. 1953, 67, 723–729. [Google Scholar] [CrossRef]
- Belal, A. Prebycusis: Physiological or pathological. J. Laryngol. Otol. 1975, 89, 1011–1025. [Google Scholar] [CrossRef] [PubMed]
- Etholm, B.; Belal, A. Senile changes in the middle ear joints. Ann. Otol. Rhinol. Laryngol. 1974, 83, 49–54. [Google Scholar] [CrossRef]
- Wiley, T.L.; Nondahl, D.M.; Cruickshanks, K.J.; Tweed, T.S. Five-year changes in middle ear function for older adults. J. Am. Acad. Audiol. 2005, 16, 129–139. [Google Scholar] [CrossRef] [Green Version]
- Feeney, M.P.; Sanford, C.A. Age effects in the human middle ear: Wideband acoustical measures. J. Acoust. Soc. Am. 2004, 116, 3546–3558. [Google Scholar] [CrossRef] [PubMed]
- Gussen, R. The human incudomalleal joint. Chondroid articular cartilage and degenerative arthritis. Arthritis Rheum. 1971, 14, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Savić, D.; Djerić, D. Histopathological characteristics of degenerative modifications of the incudomalleolar joint. Ann. Otolaryngol. Chir. Cervicofac. 1988, 105, 203–206. [Google Scholar]
- Saumil, N.M.; Joseph, B.N., Jr. Methods of removal, preparation and study. In Schuknecht’s Pathology of the Ear, 3rd ed.; Saumil, N.M., Joseph, B.N., Jr., Eds.; PMPH: Shelton, CT, USA, 2010; pp. 3–51. [Google Scholar]
- Fausch, C.; Röösli, C. The incudomalleolar articulation in Down syndrome (trisomy 21): A temporal bone study. Otol. Neurotol. 2015, 36, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Ogando, P.B.; Röösli, C.; Karmody, C.S.; Northrop, C.C. The incudostapedial articulation in Down’s syndrome (trisomy 21): A temporal bone study. Otol. Neurotol. 2013, 34, 1489–1495. [Google Scholar] [CrossRef]
- International Organization for Standardization. International Standard ISO 7029:2000(E), Acoustics—Statistical Distribution of Hearing Thresholds as a Function of Age; International Organization for Standardization: Geneva, Switzerland, 2000; pp. 1–9. [Google Scholar]
- Nychka, D. Bayesian confidence intervals for smoothing splines. J. Am. Stat. Assoc. 1988, 83, 1134–1143. [Google Scholar] [CrossRef]
- Stenklev, N.C.; Vik, O.; Laukli, E. The aging ear: An otomicroscopic and tympanometric study. Acta Otolaryngol. 2004, 124, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Mazlan, R.; Kei, J.; Ya, C.L.; Yusof, W.N.; Saim, L.; Zhao, F. Age and Gender Effects on Wideband Absorbance in Adults With Normal Outer and Middle Ear Function. J. Speech Lang. Hear. Res. 2015, 58, 1377–1386. [Google Scholar] [CrossRef]
- O’Connor, K.N.; Tam, M.; Blevins, N.H.; Puria, S. Tympanic membrane collagen fibers: A key to high-frequency sound conduction. Laryngoscope 2008, 118, 483–490. [Google Scholar] [CrossRef]
- O’Connor, K.N.; Cai, H.; Puria, S. The effects of varying tympanic-membrane material properties on human middle-ear sound transmission in a three-dimensional finite-element model. J. Acoust. Soc. Am. 2017, 142, 2836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cisneros Gimeno, A.I.; Whyte Orozco, J.R.; Obón Nogues, J.A.; Yus Gotor, C.; De La Torre, M.A.C.; Whyte Orozco, A. Contribution to morphological knowledge of the development of the human incudo-mallear joint. Acta Otolaryngol. 2009, 129, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Whyte Orozco, J.R.; Cisneros Gimeno, A.I.; Yus Gotor, C.; Obón Nogues, J.A.; Pérez Sanz, R.; Gañet Solé, J.F.; Fraile Rodrigo, J.J. Ontogenic development of the incudostapedial joint. Acta Otorrinolaringol. Esp. 2008, 59, 384–389. [Google Scholar] [CrossRef]
- Olszewski, J. The morphometry of the ear ossicles in humans during development. Anat. Anz. 1990, 171, 187–191. [Google Scholar]




| Age (Year) | Total (Ears) | Gender | Side | ||
|---|---|---|---|---|---|
| Male | Female | Left | Right | ||
| 20–29 | 60 | 38 | 22 | 31 | 29 |
| 30–39 | 343 | 161 | 182 | 177 | 166 |
| 40–49 | 269 | 153 | 116 | 136 | 133 |
| 50–59 | 220 | 124 | 96 | 114 | 106 |
| 60–69 | 476 | 217 | 259 | 244 | 232 |
| 70–80 | 392 | 126 | 266 | 200 | 192 |
| Total | 1760 | 819 | 941 | 902 | 858 |
| IMJ | ||||
|---|---|---|---|---|
| Parameter | Mean ± SD | Median (Range) | p-Value | Age-Related Change |
| Centerline Discus | 89 ± 43 | 85 (5 to 234) | <0.001 | Widening |
| Peripheral Inc C tot | 66 ± 23 | 61 (28 to 162) | <0.001 | Decrease |
| Peripheral Inc cC | 42 ± 19 | 40 (13 to 128) | 0.006 | Decrease |
| Peripheral Inc hC | 24 ± 9 | 22 (9 to 50) | <0.001 | Decrease |
| Peripheral Discus | 60 ± 31 | 58 (4 to 167) | <0.001 | Widening |
| ISJ | ||||
| Midline Stap C tot | 83 ± 29 | 83 (25 to 166) | <0.001 | Increase |
| Midline Stap cC | 59 ± 25 | 58 (15 to 133) | 0.002 | Increase |
| Midline Inc hC | 34 ± 12 | 32 (9 to 77) | 0.001 | Decrease |
| Postline Discus | 63 ± 43 | 58 (4 to 223) | 0.009 | Widening |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobrev, I.; Dillinger, D.; Meier, L.; Veraguth, D.; Pfiffner, F.; Probst, R.; Röösli, C. Conductive Hearing Loss with Age—A Histologic and Audiometric Evaluation. J. Clin. Med. 2021, 10, 2341. https://doi.org/10.3390/jcm10112341
Dobrev I, Dillinger D, Meier L, Veraguth D, Pfiffner F, Probst R, Röösli C. Conductive Hearing Loss with Age—A Histologic and Audiometric Evaluation. Journal of Clinical Medicine. 2021; 10(11):2341. https://doi.org/10.3390/jcm10112341
Chicago/Turabian StyleDobrev, Ivo, Daniel Dillinger, Letizia Meier, Dorothe Veraguth, Flurin Pfiffner, Rudolf Probst, and Christof Röösli. 2021. "Conductive Hearing Loss with Age—A Histologic and Audiometric Evaluation" Journal of Clinical Medicine 10, no. 11: 2341. https://doi.org/10.3390/jcm10112341






