Effects of Preoperative Intraocular Pressure Level on Surgical Results of Microhook Ab Interno Trabeculotomy
Abstract
1. Introduction
2. Materials and Methods
2.1. Methods
2.2. Surgical Procedure
2.3. Measurements
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tanito, M.; Tsutsui, A.; Manabe, K.; Mochiji, M. Comparison of outflow facility before and after the microhook ab interno trabeculotomy. Eye 2021, 1–6, Online ahead of print. [Google Scholar] [CrossRef]
- Chihara, E.; Nishida, A.; Kodo, M.; Yoshimura, N.; Matsumura, M.; Yamamoto, M.; Tsukada, T. Trabeculotomy ab externo: An alternative treatment in adult patients with primary open-angle glaucoma. Ophthalmic Surg. Lasers Imaging Retina 1993, 24, 735–739. [Google Scholar] [CrossRef]
- Kashiwagi, K.; Kogure, S.; Mabuchi, F.; Chiba, T.; Yamamoto, T.; Kuwayama, Y.; Araie, M. Change in visual acuity and associated risk factors after trabeculectomy with adjunctive mitomycin C. Acta Ophthalmol. 2016, 94, e561–e570. [Google Scholar] [CrossRef]
- Grover, D.S.; Godfrey, D.G.; Smith, O.; Feuer, W.J.; Montes de Oca, I.; Fellman, R.L. Gonioscopy-assisted transluminal trabeculotomy, ab interno trabeculotomy: Technique report and preliminary results. Ophthalmology 2014, 121, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Hirata, A.; Mizoguchi, T. Outcomes of 360 degrees suture trabeculotomy with deep sclerectomy combined with cataract surgery for primary open angle glaucoma and coexisting cataract. Clin. Ophthalmol. 2014, 8, 1301–1310. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tanito, M.; Sano, I.; Ikeda, Y.; Fujihara, E. Microhook ab interno trabeculotomy, a novel minimally invasive glaucoma surgery, in eyes with open-angle glaucoma with scleral thinning. Acta Ophthalmol. 2016, 94, e371–e372. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Sano, I.; Ikeda, Y.; Fujihara, E. Short-term results of microhook ab interno trabeculotomy, a novel minimally invasive glaucoma surgery in Japanese eyes: Initial case series. Acta Ophthalmol. 2017, 95, e354–e360. [Google Scholar] [CrossRef]
- Tanito, M.; Ikeda, Y.; Fujihara, E. Effectiveness and safety of combined cataract surgery and microhook ab interno trabeculotomy in Japanese eyes with glaucoma: Report of an initial case series. Jpn. J. Ophthalmol. 2017, 61, 457–464. [Google Scholar] [CrossRef]
- Tanito, M.; Sugihara, K.; Tsutsui, A.; Hara, K.; Manabe, K.; Matsuoka, Y. Midterm Results of Microhook ab Interno Trabeculotomy in Initial 560 Eyes with Glaucoma. J. Clin. Med. 2021, 10, 814. [Google Scholar] [CrossRef]
- Tanito, M.; Ohira, A.; Chihara, E. Factors leading to reduced intraocular pressure after combined trabeculotomy and cataract surgery. J. Glaucoma 2002, 11, 3–9. [Google Scholar] [CrossRef]
- Berdahl, J.P.; Gallardo, M.J.; ElMallah, M.K.; Williamson, B.K.; Kahook, M.Y.; Mahootchi, A.; Rappaport, L.A.; Lazcano-Gomez, G.S.; Díaz-Robles, D.; Dorairaj, S.K. Six-Month Outcomes of Goniotomy Performed with the Kahook Dual Blade as a Stand-Alone Glaucoma Procedure. Adv. Ther. 2018, 35, 2093–2102. [Google Scholar] [CrossRef] [PubMed]
- Porter, M.; Garza, A.; Gallardo, M. Excisional Goniotomy in Latino Patients with Open-Angle Glaucoma: Outcomes Through 24 Months. Clin. Ophthalmol. 2020, 14, 3619–3625. [Google Scholar] [CrossRef] [PubMed]
- Tojo, N.; Hayashi, A. The Outcomes of Trabectome Surgery in Patients with Low, Middle, and High Preoperative Intraocular Pressure. Clin. Ophthalmol. 2020, 14, 4099–4108. [Google Scholar] [CrossRef] [PubMed]
- Bussel, I.I.; Kaplowitz, K.; Schuman, J.S.; Loewen, N.A. Outcomes of ab interno trabeculectomy with the trabectome by degree of angle opening. Br. J. Ophthalmol. 2015, 99, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.F.; Bhatt, A.; Schmutz, M.; Mosaed, S. Trabectome outcomes across the spectrum of glaucoma disease severity. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 1703–1710. [Google Scholar] [CrossRef] [PubMed]
- Loewen, R.T.; Roy, P.; Parikh, H.A.; Dang, Y.; Schuman, J.S.; Loewen, N.A. Impact of a Glaucoma Severity Index on Results of Trabectome Surgery: Larger Pressure Reduction in More Severe Glaucoma. PLoS ONE 2016, 11, e0151926. [Google Scholar] [CrossRef]
- Dang, Y.; Roy, P.; Bussel, I.I.; Loewen, R.T.; Parikh, H.; Loewen, N.A. Combined analysis of trabectome and phaco-trabectome outcomes by glaucoma severity. F1000Research 2016, 5, 762. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Loewen, R.T.; Dang, Y.; Parikh, H.A.; Bussel, I.I.; Loewen, N.A. Stratification of phaco-trabectome surgery results using a glaucoma severity index in a retrospective analysis. BMC Ophthalmol. 2017, 17, 30. [Google Scholar] [CrossRef]
- Tanito, M.; Ohira, A.; Chihara, E. Surgical outcome of combined trabeculotomy and cataract surgery. J. Glaucoma 2001, 10, 302–308. [Google Scholar] [CrossRef]
- Parikh, H.A.; Bussel, I.I.; Schuman, J.S.; Brown, E.N.; Loewen, N.A. Coarsened Exact Matching of Phaco-Trabectome to Trabectome in Phakic Patients: Lack of Additional Pressure Reduction from Phacoemulsification. PLoS ONE 2016, 11, e0149384. [Google Scholar] [CrossRef]
- Neiweem, A.E.; Bussel, I.I.; Schuman, J.S.; Brown, E.N.; Loewen, N.A. Glaucoma Surgery Calculator: Limited Additive Effect of Phacoemulsification on Intraocular Pressure in Ab Interno Trabeculectomy. PLoS ONE 2016, 11, e0153585. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Matsuo, M. Ab-interno trabeculotomy-related glaucoma surgeries. Taiwan J. Ophthalmol. 2019, 9, 67–71. [Google Scholar] [CrossRef]
- Tanito, M. Microhook ab interno trabeculotomy, a novel minimally invasive glaucoma surgery. Clin. Ophthalmol. 2018, 12, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Rosenquist, R.; Epstein, D.; Melamed, S.; Johnson, M.; Grant, W.M. Outflow resistance of enucleated human eyes at two different perfusion pressures and different extents of trabeculotomy. Curr. Eye Res. 1989, 8, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Gabelt, B.T.; Kaufman, P.L. Changes in aqueous humor dynamics with age and glaucoma. Prog. Retin. Eye Res. 2005, 24, 612–637. [Google Scholar] [CrossRef] [PubMed]
- Toris, C.B.; Yablonski, M.E.; Wang, Y.L.; Camras, C.B. Aqueous humor dynamics in the aging human eye. Am. J. Ophthalmol. 1999, 127, 407–412. [Google Scholar] [CrossRef]
- Dannheim, R.; Van der Zypen, E. Clinical, functional and electron microscopy studies on the regenerative ability of the iridocorneal angle region of primate eyes following trabeculotomy. Albrecht Von Graefes Arch. Clin. Exp. Ophthalmol. 1972, 184, 222–247. [Google Scholar] [CrossRef]
- Dannheim, R. The influence of healing on the long-term effect of trabeculotomy in primary open angle glaucoma (author’s transl). Klin. Monbl. Augenheilkd. 1978, 172, 27–38. [Google Scholar] [PubMed]
- Ito, S.; Nishikawa, M.; Tokura, T.; Yamane, A.; Yamagishi, K.; Miki, H. Histopathological study of trabecular meshwork after trabeculotomy in monkeys. Nippon Ganka Gakkai Zasshi 1994, 98, 811–819. [Google Scholar]
- Heuer, D.K.; Barton, K.; Grehn, F.; Shaaraway, T.; Sherwood, M. Consensus on Definitions of Success. In Guidelines on Design and Reporting of Surgical Trials; Shaarawy, T.M., Sherwood, M.B., Grehn, F., Eds.; Kugler Publications: Amsterdam, The Netherlands, 2009; pp. 15–24. [Google Scholar]
- Hara, K.; Takai, Y.; Tanito, M. Outcomes after Combined Deep Sclerectomy and Trabeculotomy to Treat Primary Open-Angle Glaucoma and Exfoliation Glaucoma. Shimane J. Med. Sci. 2019, 35, 43–52. [Google Scholar]



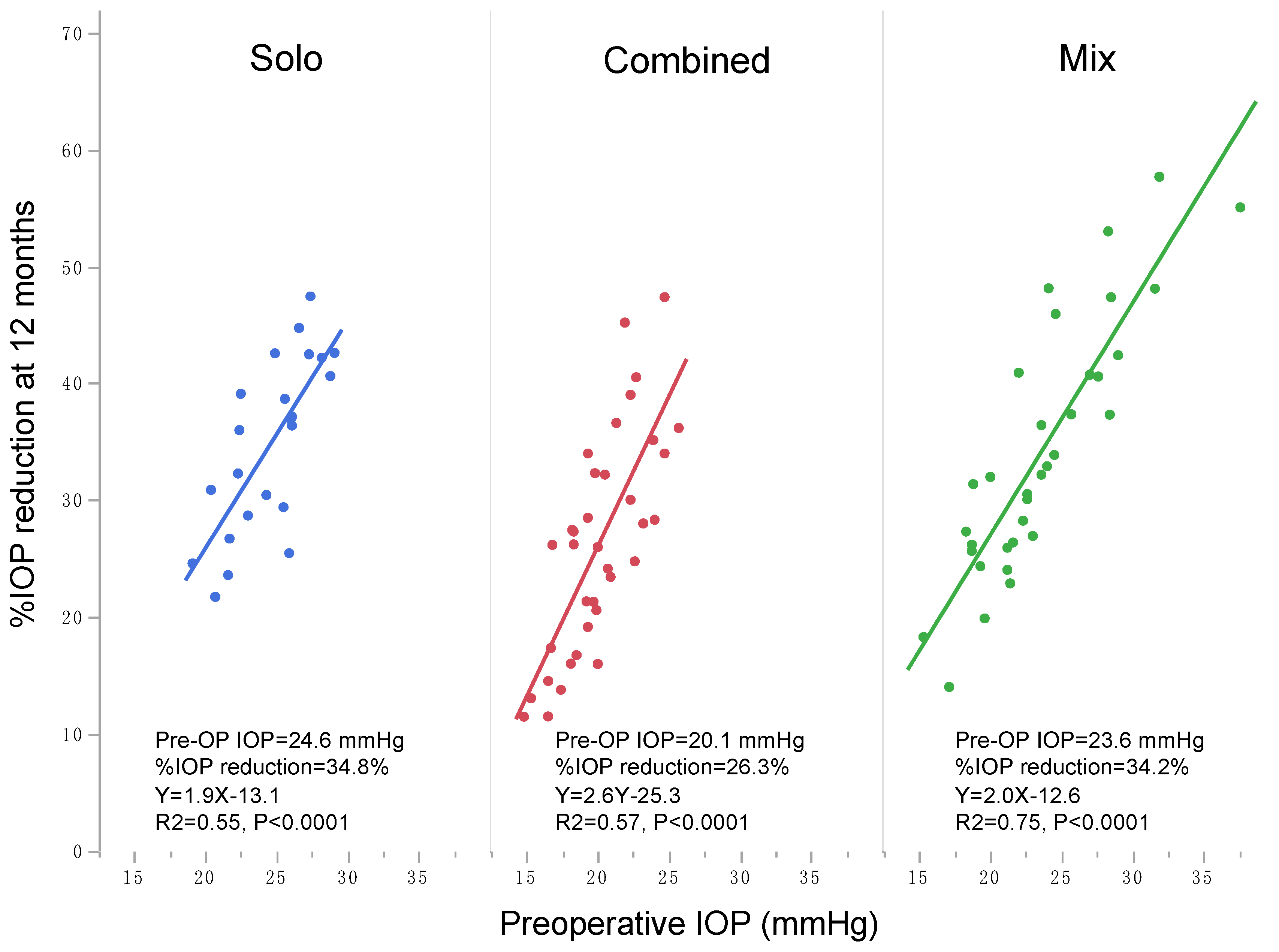
| Parameters | Total | Q1 | Q2 | Q3 | Q4 | p Value † |
|---|---|---|---|---|---|---|
| Preoperative IOP | ≤15 mmHg | >15 mmHg and ≤18 mmHg | >18 mmHg and ≤21 mmHg | >21 mmHg | ||
| Eyes/Subjects | 126/126 | 38/38 | 30/30 | 36/36 | 22/22 | |
| Age, years | 70.5 ± 11.4 (33, 88) | 71.2 ± 12.0 (33, 88) | 70.7 ± 12.3 (34, 87) | 67.6 ± 11.6 (38, 86) | 74.0 ± 8.0 (56, 85) | 0.2046 |
| Sex, subjects (%) | ||||||
| Male | 60 (48) | 14 (37) | 17 (57) | 14 (39) | 15 (68) | 0.0998 |
| Female | 66 (52) | 24 (63) | 13 (43) | 22 (61) | 7 (32) | |
| Glaucoma type, eyes | ||||||
| POAG | 90 (71) | 34 (89) | 26 (87) | 22 (61) | 8 (36) | <0.0001 ** |
| EXG | 36 (29) | 4 (11) | 4 (13) | 14 (39) | 14 (64) | |
| Lens status, eyes | ||||||
| Phakia | 118 (94) | 36 (95) | 29 (97) | 33 (92) | 20 (91) | 0.7312 |
| Pseudophakia | 8 (6) | 2 (5) | 1 (3) | 3 (8) | 2 (9) | |
| Surgical procedure, eyes | ||||||
| µLOT alone | 25 (20) | 6 (16) | 7 (23) | 8 (22) | 4 (18) | 0.4315 |
| µLOT + cataract surgery | 101 (80) | 32 (84) | 23 (77) | 28 (78) | 18 (22) | |
| Trabeculotomy site, eyes | ||||||
| Nasal and temporal | 111 (88) | 32 (84) | 25 (83) | 33 (92) | 21 (95) | |
| Nasal only | 4 (3) | 2 (5) | 0 (0) | 1 (3) | 1 (5) | |
| Temporal only | 11 (9) | 4 (11) | 5 (17) | 2 (6) | 0 (0) | |
| Extent of trabeculotomies, clock hours | ||||||
| Nasal and temporal | 6.9 ± 0.9 (5, 9) | 6.8 ± 0.9 (5, 8) | 7.0 ± 1.0 (5, 9) | 6.8 ± 0.8 (5, 8) | 6.9 ± 0.9 (5, 9) | |
| Nasal only | 3.8 ± 0.5 (3, 4) | 3.5 ± 0.7 (3, 4) | 4 | 4 | ||
| Temporal only | 3.6 ± 0.7 (3, 5) | 3.8 ± 0.5 (3, 4) | 3.2 ± 0.4 (3, 4) | 4.5 ± 0.7 (4, 5) |
| Parameters | Total | Q1 | Q2 | Q3 | Q4 | p Value † |
|---|---|---|---|---|---|---|
| IOP | ||||||
| Pre-op, mmHg | 18.8 ± 6.0 (11, 63) | 14.0 ± 1.3 (11, 15) | 17.2 ± 0.9 (16, 18) | 19.8 ± 0.9 (19, 21) | 27.5 ± 9.3 (22, 65) | <0.0001 ** |
| p-value ‡, vs. ≤15 mmHg group | 0.0092 ** | <0.0001 ** | <0.0001 ** | |||
| p-value ‡, vs. >15 and ≤18 mmHg group | 0.0366 * | <0.0001 ** | ||||
| p-value ‡, vs. >18 and ≤21 mmHg group | <0.0001 ** | |||||
| 12 M post-op, mmHg | 12.9 ± 3.9 (4, 38) | 11.6 ± 2.9 (4, 16) | 12.2 ± 2.7 (7, 16) | 14.2 ± 2.8 (7, 21) | 14.3 ± 6.5 (7, 38) | 0.0048 ** |
| p-value ‡, vs. ≤15 mmHg group | 0.8920 | 0.0146 * | 0.0320 * | |||
| p-value ‡, vs. >15 and ≤18 mmHg group | 0.1379 | 0.1833 | ||||
| p-value ‡, vs. >18 and ≤21 mmHg group | 0.9993 | |||||
| Difference, mmHg | 5.9 | 2.4 | 5.0 | 5.6 | 13.2 | |
| p value #, pre- vs. post-op | <0.0001 ** | <0.0001 ** | <0.0001 ** | <0.0001 ** | <0.0001 ** | |
| %IOP reduction, % | 28.4 ± 19.5 (−10.5, 76.3) | 17.7 ± 19.2 (−9.1, +69.2) | 28.8 ± 15.5 (0, 61.1) | 28.2 ± 14.9 (−10.5, 65) | 46.7 ± 19.0 (8.7, 76.3) | <0.0001 ** |
| p-value ‡, vs. ≤15 mmHg group | 0.0469 * | 0.0493 * | <0.0001 ** | |||
| p-value ‡, vs. >15 and ≤18 mmHg group | 0.9990 | 0.0017 ** | ||||
| p-value ‡, vs. >18 and ≤21 mmHg group | 0.0007 ** | |||||
| p value #, pre- vs. post-op | <0.0001 ** | |||||
| Medication | ||||||
| Pre-op | 2.7 ± 1.2 (0, 5) | 2.6 ± 1.2 (1, 5) | 2.4 ± 1.2 (0, 4) | 2.9 ± 1.1 (1, 5) | 2.7 ± 1.1 (1, 4) | 0.2499 |
| 12 M post-op | 2.3 ± 1.0 (0, 4) | 2.3 ± 1.0 (0, 4) | 2.1 ± 1.1 (0, 4) | 2.6 ± 0.9 (1, 4) | 2.2 ± 0.9 (0, 3) | 0.1966 |
| Difference | 0.4 | 0.3 | 0.3 | 0.3 | 0.5 | |
| p value #, pre- vs. post-op | <0.0001 ** | 0.0392 * | 0.0180 * | 0.0318 * | 0.0774 | |
| %Medication reduction, % | 3.0 ± 47.7 (−200, +100) | 0.8 ± 48.2 (−200, 100) | 9.0 ± 22.0 (−50, 66.7) | 0.7 ± 53.0 (−200, 66.7) | 3.0 ± 60.7 (−200, 100) | 0.9063 |
| Complications, n (%) | Interventions, n (%) | ||
|---|---|---|---|
| Intraoperative | Intraoperative | ||
| Iris prolapse, IFIS | 4 (3) | CTR implantation | 2 (2) |
| Angle recession | 1 (<1) | Goniocynechialysis | 1 (<1) |
| Any complication | 5 (4) | Any intervention | 3 (2) |
| Postoperative | Postoperative | ||
| Layered hyphema | 42 (33) | Hyphema washout | 9 (7) |
| Transient IOP elevation >30 mmHg | 6 (5) | Posterior synechialysis, pupiloplasty | 2 (2) |
| Macular edema | 5 (4) | Pars-plana vitrectomy | 2 (2) |
| Fibrin formation in anterior chamber | 3 (2) | Anterior chamber injection of tPA | 1 (<1) |
| Posterior synechia, corectopia | 2 (2) | Sub-Tenon triamcinolone injection | 1 (<1) |
| Vitreous hemorrhage | 2 (<1) | Intravitreal anti-VEGF injection | 1 (<1) |
| Cataract | 1 (<1) | Nd:YAG laser capsulotomy | 1 (<1) |
| Persistent hypotony | 1 (<1) | Anterior chamber OVD injection | 1 (<1) |
| Iritis | 1 (<1) | Incision of CCC edge by Nd:YAG laser | 1 (<1) |
| After cataract | 1 (<1) | ||
| Contraction of CCC edge | 1 (<1) | ||
| Age-related macular degeneration | 1 (<1) | ||
| Any complication | 57 (45) | Any intervention | 16 (13) |
| Parameters | r (95% CI Range) | Standard β | p Value |
|---|---|---|---|
| Age (/year) | −0.08 (−0.14, −0.01) | −0.22 | 0.0283 * |
| Female (/male) | 0.15 (−0.46, 0.76) | 0.04 | 0.6268 |
| EXG (/POAG) | −0.17 (−0.90, 0.56) | −0.04 | 0.6444 |
| µLOT alone (/combined µLOT + cataract surgery) | 0.17 (−0.90, 1.23) | 0.03 | 0.7566 |
| Phakic eye (/pseudophakic eye) | −0.68 (−2.32, 0.96) | −0.09 | 0.4099 |
| Extent of trabeculotomy (/clock hours) | 0.18 (−0.32, 0.69) | 0.06 | 0.4695 |
| Preoperative IOP (/mmHg) | 0.33 (0.22, 0.44) | 0.51 | <0.0001 ** |
| Preoperative number of medications (/medication) | 0.09 (−0.47, 0.64) | 0.03 | 0.7611 |
| Parameters | Total | Q1 | Q2 | Q3 | Q4 | p Value † |
|---|---|---|---|---|---|---|
| IOP ≤18 mmHg | ||||||
| Success, n (%) | 119 (94) | 38 (100) | 30 (100) | 34 (94) | 17 (77) | 0.0003 ** |
| Failure, n (%) | 7 (6) | 0 (0) | 0 (0) | 2 (6) | 5 (23) | |
| IOP ≤15 mmHg | ||||||
| Success, n (%) | 105 (83) | 37 (97) | 27 (90) | 24 (67) | 17 (77) | 0.0025 ** |
| Failure, n (%) | 21 (17) | 1 (3) | 3 (10) | 12 (33) | 5 (23) | |
| IOP ≤ 12 mmHg | ||||||
| Success, n (%) | 55 (44) | 20 (53) | 15 (50) | 10 (28) | 10 (45) | 0.1869 |
| Failure, n (%) | 71 (56) | 18 (47) | 15 (50) | 26 (72) | 12 (55) | |
| IOP reduction ≥ 20% | ||||||
| Success, n (%) | 77 (61) | 15 (39) | 17 (57) | 27 (75) | 18 (82) | 0.0002 ** |
| Failure, n (%) | 49 (39) | 23 (61) | 13 (43) | 9 (25) | 4 (18) | |
| IOP reduction ≥ 0% | ||||||
| Success, n (%) | 116 (92) | 34 (89) | 29 (97) | 34 (94) | 19 (86) | 0.8393 |
| Failure, n (%) | 10 (8) | 4 (11) | 1 (3) | 2 (6) | 3 (14) | |
| IOP ≤ 18 mmHg and IOP reduction ≥ 20% | ||||||
| Success, n (%) | 76 (60) | 15 (39) | 17 (57) | 27 (75) | 17 (77) | 0.0005 ** |
| Failure, n (%) | 50 (40) | 23 (61) | 13 (43) | 9 (25) | 5 (23) | |
| IOP ≤ 15 mmHg and IOP reduction ≥ 20% | ||||||
| Success, n (%) | 73 (58) | 15 (39) | 17 (57) | 24 (67) | 17 (77) | 0.0020 ** |
| Failure, n (%) | 53 (42) | 23 (61) | 13 (43) | 12 (33) | 5 (23) | |
| IOP ≤ 12 mmHg and IOP reduction ≥ 20% | ||||||
| Success, n (%) | 50 (40) | 15 (39) | 15 (50) | 10 (28) | 10 (45) | 0.8026 |
| Failure, n (%) | 76 (60) | 23 (61) | 15 (50) | 26 (72) | 12 (55) | |
| IOP ≤ 18 mmHg and IOP reduction ≥ 0% | ||||||
| Success, n (%) | 110 (87) | 34 (89) | 29 (97) | 32 (89) | 15 (68) | 0.0324 * |
| Failure, n (%) | 16 (13) | 4 (11) | 1 (3) | 4 (11) | 7 (32) | |
| IOP ≤ 15 mmHg and IOP reduction ≥ 0% | ||||||
| Success, n (%) | 98 (78) | 34 (89) | 26 (87) | 23 (64) | 15 (68) | 0.0069 ** |
| Failure, n (%) | 28 (22) | 4 (11) | 4 (13) | 13 (36) | 7 (32) | |
| IOP ≤ 12 mmHg and IOP reduction ≥ 0% | ||||||
| Success, n (%) | 52 (41) | 19 (50) | 15 (50) | 9 (25) | 9 (41) | 0.1192 |
| Failure, n (%) | 74 (59) | 19 (50) | 15 (50) | 27 (75) | 13 (59) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanito, M.; Sugihara, K.; Tsutsui, A.; Hara, K.; Manabe, K.; Matsuoka, Y. Effects of Preoperative Intraocular Pressure Level on Surgical Results of Microhook Ab Interno Trabeculotomy. J. Clin. Med. 2021, 10, 3327. https://doi.org/10.3390/jcm10153327
Tanito M, Sugihara K, Tsutsui A, Hara K, Manabe K, Matsuoka Y. Effects of Preoperative Intraocular Pressure Level on Surgical Results of Microhook Ab Interno Trabeculotomy. Journal of Clinical Medicine. 2021; 10(15):3327. https://doi.org/10.3390/jcm10153327
Chicago/Turabian StyleTanito, Masaki, Kazunobu Sugihara, Aika Tsutsui, Katsunori Hara, Kaoru Manabe, and Yotaro Matsuoka. 2021. "Effects of Preoperative Intraocular Pressure Level on Surgical Results of Microhook Ab Interno Trabeculotomy" Journal of Clinical Medicine 10, no. 15: 3327. https://doi.org/10.3390/jcm10153327
APA StyleTanito, M., Sugihara, K., Tsutsui, A., Hara, K., Manabe, K., & Matsuoka, Y. (2021). Effects of Preoperative Intraocular Pressure Level on Surgical Results of Microhook Ab Interno Trabeculotomy. Journal of Clinical Medicine, 10(15), 3327. https://doi.org/10.3390/jcm10153327







