Prevalence and Clinical Implications of Ascites in Gastric Cancer Patients after Curative Surgery
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.D.; Chang, M.C.; Roh, H.R.; Chae, G.B.; Yang, D.H.; Choi, W.J. Factors influencing recurrence after curative resection for advanced gastric cancer. J. Korean Surg. Soc. 2003, 65, 301–308. [Google Scholar]
- Yoo, C.H.; Noh, S.H.; Shin, D.W.; Choi, S.H.; Min, J.S. Recurrence following curative resection for gastric carcinoma. Br. J. Surg. 2000, 87, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.Z.; Lang, N.P.; Thompson, C.; Osteen, P.K.; Westbrook, K.C. Peritoneal carcinomatosis in nongynecologic malignancy. A prospective study of prognostic factors. Cancer 1989, 63, 364–367. [Google Scholar]
- Sadeghi, B.; Arvieux, C.; Glehen, O.; Beaujard, A.C.; Rivoire, M.; Baulieux, J.; Fontaumard, E.; Brachet, A.; Caillot, J.L.; Faure, J.L.; et al. Peritoneal carcinomatosis from non-gynecologic malignancies: Results of the EVOCAPE 1 multicentric prospective study. Cancer 2000, 88, 358–363. [Google Scholar] [CrossRef]
- Raptopoulos, V.; Gourtsoyiannis, N. Peritoneal carcinomatosis. Eur. Radiol. 2001, 11, 2195–2206. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, H.H.; Kim, Y.H.; Hwang, S.H.; Lee, H.S.; Park, D.J.; Kim, S.Y.; Lee, K.H. Peritoneal metastasis: Detection with 16-or 64-detector row CT in patients undergoing surgery for gastric cancer. Radiology 2009, 253, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.C.; Sohotra, P.; Radecki, P.D. CT manifestations of peritoneal carcinomatosis. Am. J. Roentgenol. 1988, 150, 1035–1041. [Google Scholar]
- Lee, H.J.; Kim, M.J.; Lim, J.S.; Kim, K.W. Follow up CT findings of various types of recurrence after curative gastric surgery. J. Korean Radiol. Soc. 2007, 57, 553–562. [Google Scholar] [CrossRef]
- Bennett, J.J.; Gonen, M.; D’Angelica, M.; Jaques, D.P.; Brennan, M.F.; Coit, D.G. Is detection of asymptomatic recurrence after curative resection associated with improved survival in patients with gastric cancer? J. Am. Coll Surg. 2005, 201, 503–510. [Google Scholar] [CrossRef]
- Kim, J.H.; Jang, Y.J.; Park, S.S.; Park, S.H.; Mok, Y.J. Benefit of post-operative surveillance for recurrence after curative resection for gastric cancer. J. Gastrointest. Surg. 2010, 14, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lim, J.K.; Kim, M.G.; Kwon, S.J. The influence of post-operative surveillance on the prognosis after curative surgery for gastric cancer. Hepatogastroenterology 2014, 61, 2123–2132. [Google Scholar] [PubMed]
- Kim, S.H.; Choi, Y.H.; Kim, J.W.; Oh, S.; Lee, S.; Kim, B.G.; Lee, K.L. Clinical significance of computed tomography-detected ascites in gastric cancer patients with peritoneal metastases. Medicine 2018, 97, e9343. [Google Scholar] [CrossRef] [PubMed]
- Yajima, K.; Kanda, T.; Ohashi, M.; Wakai, T.; Nakagawa, S.; Sasamoto, R.; Hatakeyama, K. Clinical and diagnostic significance of preoperative computed tomography findings of ascites in patients with advanced gastric cancer. Am. J. Surg. 2006, 192, 185–190. [Google Scholar] [CrossRef]
- Chang, D.K.; Kim, J.W.; Kim, B.K.; Lee, K.L.; Song, C.S.; Han, J.K.; Song, I.S. Clinical significance of CT-defined minimal ascites in patients with gastric cancer. World J. Gastroenterol. 2005, 11, 6587–6592. [Google Scholar] [CrossRef]
- Healy, J.C.; Reznek, R.H. The peritoneum, mesenteries and omenta: Normal anatomy and pathological processes. Eur. Radiol. 1998, 8, 886–900. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Hayashi, N.; Maeda, E.; Matsuda, I.; Sasaki, H.; Ohtsu, H.; Ohtomo, K. Peritoneal fluid accumulation in healthy men and postmenopausal women: Evaluation on pelvic MRI. Am. J. Roentgenol. 2013, 200, 1181–1185. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Renaer, M.; Brosens, I.A. Origin of peritoneal fluid in women: An ovarian exudation product. Br. J. Obstet. Gynaecol. 1980, 87, 177–183. [Google Scholar] [CrossRef]
- Verger, C.; Luger, A.; Moore, H.L.; Nolph, K.D. Acute changes in peritoneal morphology and transport properties with infectious peritonitis and mechanical injury. Kidney Int. 1983, 23, 823–831. [Google Scholar] [CrossRef] [Green Version]
- Cheon, H.J.; Ryeom, H.K.; Bae, J.H.; Jang, Y.J.; Kim, G.C.; Kim, J.H.; Shin, K.M.; Chung, H.Y. Clinical significance of a small amount of isolated pelvic free fluid at multidetector CT in male patients after curative surgery for gastric carcinoma. J. Korean Soc. Radiol. 2014, 71, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Hwangbo, Y.; Jung, J.H.; Shim, J.; Kim, B.H.; Jung, S.H.; Lee, C.K.; Jang, J.Y.; Dong, S.H.; Kim, H.J.; Chang, Y.W.; et al. Etiologic and laboratory analyses of ascites in patients who underwent diagnostic paracentesis. Korean J. Hepatol. 2007, 13, 185–195. [Google Scholar] [PubMed]
- Runyon, B.A. Management of adult patients with ascites caused by cirrhosis. Hepatology 1998, 27, 264. [Google Scholar] [CrossRef]
- Sangisetty, S.L.; Miner, T.J. Malignant ascites: A review of prognostic factors, pathophysiology and therapeutic measures. World J. Gastrointest. Surg. 2012, 4, 87–95. [Google Scholar] [CrossRef]
- Press, O.W.; Press, N.O.; Kaufman, S.D. Evaluation and management of chylous ascites. Ann. Intern. Med. 1982, 96, 358. [Google Scholar] [CrossRef] [PubMed]
- Duarte, I.; Outerelo, C. Gastric cancer presenting as isolated ascites: A diagnostic challenge. Eur. J. Case Rep. Intern. Med. 2019, 6, 001141. [Google Scholar] [PubMed]
- Maeda, H.; Kobayashi, M.; Sakamoto, J. Evaluation and treatment of malignant ascites secondary to gastric cancer. World J. Gastroenterol. 2015, 21, 10936–10947. [Google Scholar] [CrossRef]
- Kim, K.W.; Choi, B.I.; Han, J.K.; Kim, T.K.; Kim, A.Y.; Lee, H.J.; Kim, Y.H.; Choi, J.; Do, K.; Kim, H.C.; et al. Postoperative anatomic and pathologic findings at CT following gastrectomy. Radiographics 2002, 22, 323–336. [Google Scholar] [CrossRef] [PubMed]

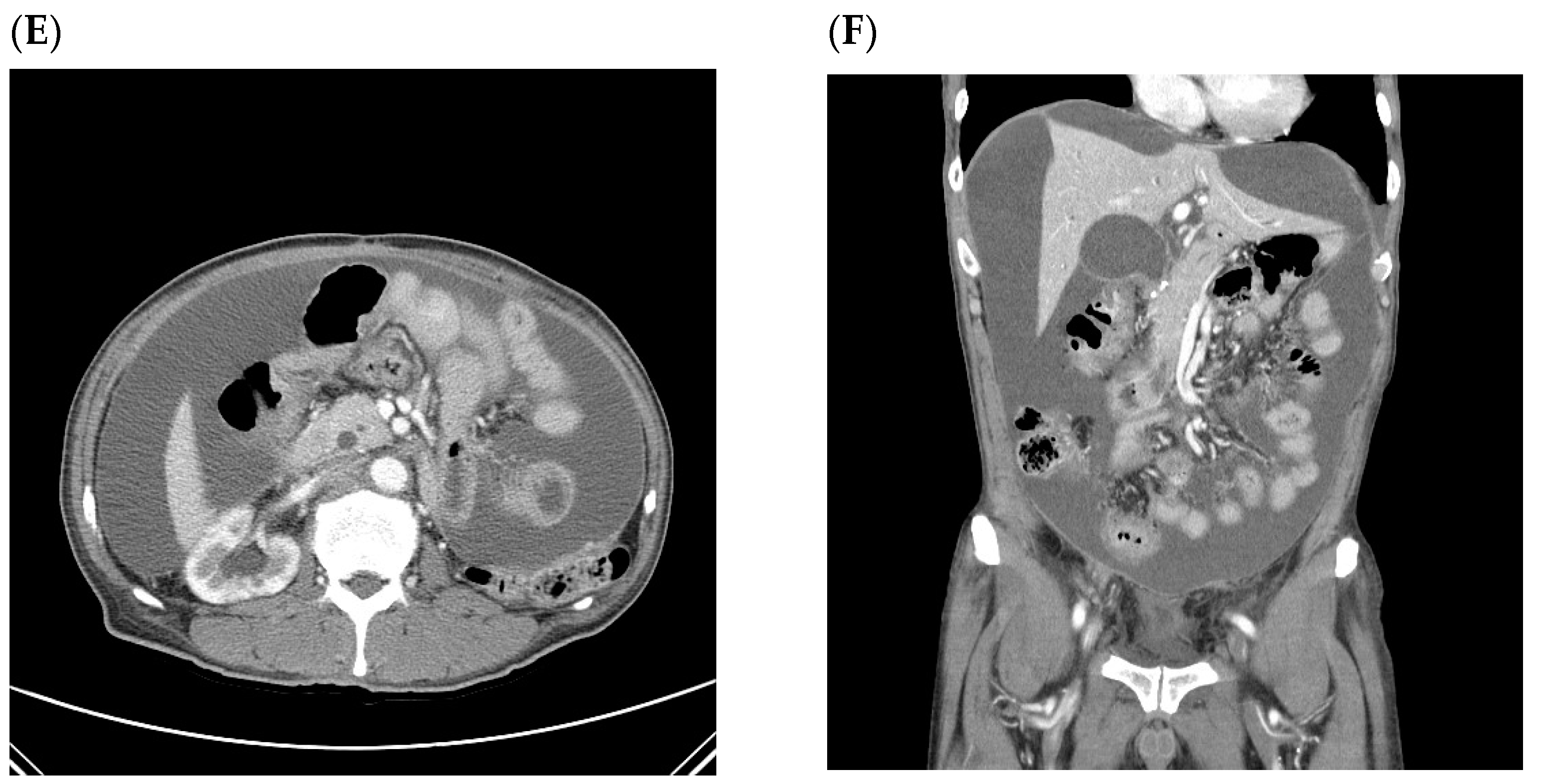


| Ascites without Recurrence | Ascites with Recurrence | p | |
|---|---|---|---|
| n (%) | n (%) | ||
| Sex | 0.869 | ||
| Male | 78 (60.3) | 31 (58.5) | |
| Female | 53 (39.7) | 22 (41.5) | |
| Age (years ± SD) | 55.9 ± 12.3 | 60.0 ± 12.1 | 0.046 |
| Type of resection | <0.001 | ||
| Partial gastrectomy | 105 (80.2) | 26 (49.1) | |
| Total gastrectomy | 26 (19.8) | 27 (50.9) | |
| Surgical approach | <0.001 | ||
| Open | 89 (67.9) | 52 (98.1) | |
| Laparoscopy | 42 (32.1) | 1 (1.9) | |
| pT stage | <0.001 | ||
| T1 | 80 (61.1) | 4 (7.5) | |
| T2 | 12 (9.2) | 4 (7.5) | |
| T3 | 18 (13.7) | 24 (45.3) | |
| T4 | 21 (16.0) | 21 (39.6) | |
| pN stage | <0.001 | ||
| N0 | 80 (61.1) | 8 (15.1) | |
| N1 | 23 (17.6) | 5 (9.4) | |
| N2 | 19 (14.5) | 7 (13.2) | |
| N3 | 9 (6.9) | 33 (62.3) | |
| Nutritional parameters | |||
| Albumin (g/dL ± SD) | 4.2 ± 0.4 | 4.0 ± 0.6 | <0.001 |
| Hemoglobin (g/dL ± SD) | 12.3 ± 1.4 | 11.4 ± 1.4 | <0.001 |
| Adjuvant chemotherapy | <0.001 | ||
| No | 80 (61.1) | 8 (15.1) | |
| Yes | 51 (38.9) | 45 (84.9) | |
| Location of ascites at first appearance | <0.001 | ||
| Pelvic cavity only | 128 (97.7) | 29 (54.7) | |
| Whole abdomen | 0 (0) | 16 (30.2) | |
| Other | 3 (2.3) | 8 (15.1) | |
| Volume of ascites at first appearance | <0.001 | ||
| Small | 130 (99.2) | 38 (71.7) | |
| Moderate | 1 (0.8) | 10 (18.9) | |
| Large | 0 (0) | 5 (9.4) | |
| Repeatability † | |||
| No | 52 (39.7) | ||
| Yes | 79 (60.3) | ||
| Timing of first appearance (months after surgery) | 9.0 (range of 3.0–108.0) | 11.5 (range of 3.0–71.0) |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Ascites (−), n (%) | Ascites (+), n (%) | p-Value | Hazard Ratio | 95% CI | p-Value | |
| Sex | 0.065 | |||||
| Male | 249 (75.9) | 79 (24.1) | 1 | |||
| Female | 110 (67.9) | 52 (32.1) | 1.436 | 0.926–2.227 | 0.106 | |
| Age (years) | <0.001 | |||||
| ≤45 | 9 (32.1) | 19 (67.9) | 6.465 | 2.803–14.915 | <0.001 | |
| >45 | 350 (75.8) | 112 (24.2) | 1 | |||
| Type of surgery | 0.211 | |||||
| Total gastrectomy | 306 (74.5) | 105 (25.5) | ||||
| Partial gastrectomy | 53 (67.1) | 26 (32.9) | ||||
| Surgical approach | 0.581 | |||||
| Open | 252 (74.0) | 89 (26.0) | ||||
| Laparoscopy | 106 (71.6) | 42 (28.4) | ||||
| Depth of invasion | 0.054 | |||||
| pT1–2 | 283 (75.5) | 92 (24.5) | 1 | |||
| pT3–4 | 76 (66.1) | 39 (33.9) | 0.889 | 0.435–1.819 | 0.748 | |
| Lymph node metastasis | ||||||
| pN0–1 | 310 (75.1) | 103 (24.9) | 0.049 | 1 | ||
| pN2–3 | 49 (63.6) | 28 (36.4) | 1.088 | 0.553–2.140 | 0.806 | |
| Adjuvant chemotherapy | 0.003 | |||||
| No | 268 (77) | 80 (23.0) | 1 | |||
| Yes | 91 (64.1) | 51 (35.9) | 2.098 | 0.985–4.470 | 0.055 | |
| Age/Sex | Type of Operation | TN Stage | Characteristic of Ascites | Timing of Malignant Ascites | Site of First Recurrence | Interval between Surgery and Recurrence (Month) | Interval between First Appearance of Benign Ascites and Recurrence (Month) | |
|---|---|---|---|---|---|---|---|---|
| Patients with ascites presumed to be benign before confirmation of recurrence (n = 8) | ||||||||
| Pt 1 | 65/M | PG | T3N3a | small pelvic cavity | Simultaneous with recurrence | T colon (increased ascites in the pelvic cavity) | 44.4 | 8.1 |
| Pt 2 | 53/F | TG | T4bN3b | small pelvic cavity | Simultaneous with recurrence | Peritoneum (increased ascites, peritoneal thickening, bowel obstruction, and Krukenberg tumors) | 18.2 | 13.2 |
| Pt 3 | 40/F | TG | T4aN3a | small pelvic cavity | Simultaneous with recurrence | Peritoneum (nodularity and increased ascites) | 73.2 | 70.3 |
| Pt 4 | 59/F | TG | T4aN3a | small pelvic cavity | Simultaneous with recurrence | Peritoneum (increased ascites and peritoneal thickening) | 25.2 | 21.7 |
| Pt 5 | 39/F | TG | T3N3b | small pelvic cavity | Simultaneous with recurrence | Peritoneum (Krukenberg tumors, nodularity, and increased ascites) | 12.1 | 9 |
| Pt 6 | 57/F | TG | T3N3a | small pelvic cavity | Simultaneous with recurrence | Peritoneum (bowel obstruction and increased ascites) | 9.8 | 4.4 |
| Pt 7 | 61/F | TG | T3N1 | small pelvic cavity | Simultaneous with recurrence | Peritoneum (increased ascites, T colon, and mesentery LNs) | 29.6 | 26 |
| Pt 8 | 68/F | PG | T4aN2 | small pelvic cavity | Simultaneous with recurrence | Peritoneum (increased ascites and peritoneal thickening) | 11.7 | 5.3 |
| A patient who first recurred with malignant ascites without other CT findings related to peritoneal seeding (later confirmed by cytology) (n = 1) | ||||||||
| Pt 9 | 49/F | TG | T3N3a | small pelvic cavity | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Kwon, S.-J.; Kim, M.; Kang, B.-K. Prevalence and Clinical Implications of Ascites in Gastric Cancer Patients after Curative Surgery. J. Clin. Med. 2021, 10, 3557. https://doi.org/10.3390/jcm10163557
Lee J-H, Kwon S-J, Kim M, Kang B-K. Prevalence and Clinical Implications of Ascites in Gastric Cancer Patients after Curative Surgery. Journal of Clinical Medicine. 2021; 10(16):3557. https://doi.org/10.3390/jcm10163557
Chicago/Turabian StyleLee, Ju-Hee, Sung-Joon Kwon, Mimi Kim, and Bo-Kyeong Kang. 2021. "Prevalence and Clinical Implications of Ascites in Gastric Cancer Patients after Curative Surgery" Journal of Clinical Medicine 10, no. 16: 3557. https://doi.org/10.3390/jcm10163557
APA StyleLee, J.-H., Kwon, S.-J., Kim, M., & Kang, B.-K. (2021). Prevalence and Clinical Implications of Ascites in Gastric Cancer Patients after Curative Surgery. Journal of Clinical Medicine, 10(16), 3557. https://doi.org/10.3390/jcm10163557






