Cataract Surgery by Intraoperative Surface Irrigation with 0.25% Povidone–Iodine
Abstract
:1. Introduction
2. Border Control
3. Increase of Multidrug-Resistant Bacteria and Fungi Causing Endophthalmitis
4. Factors for Emergence of Multidrug Resistant Bacteria
- (1)
- Long-term use of broad-spectrum antibiotics without identifying the causative organism
- (2)
- Long-term use of antibiotics after infectious disease has been cured
- (3)
- Preoperative administration of antibiotics, called infection prophylaxis or preoperative disinfection
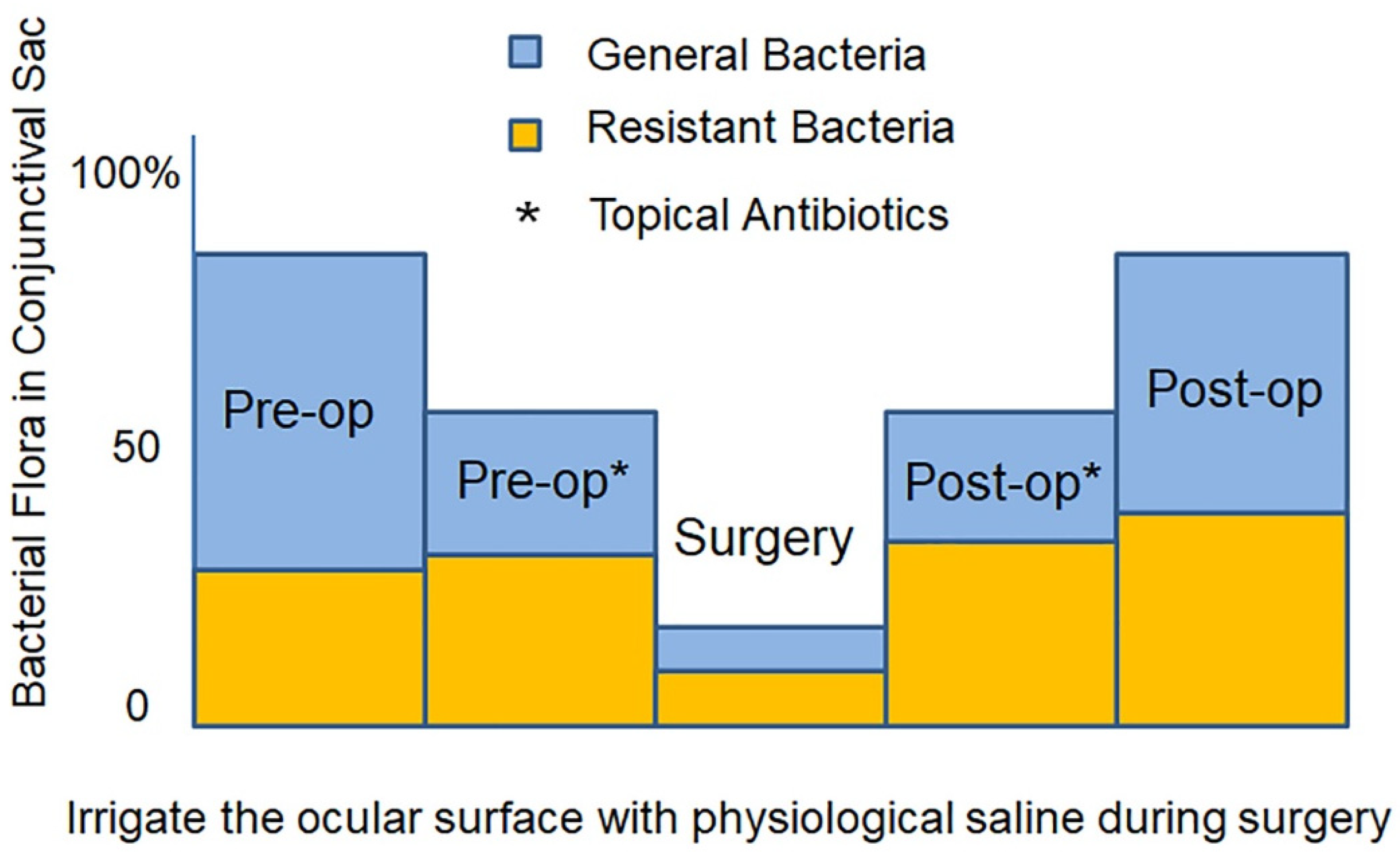
5. Intraocular Migration of Bacteria during Cataract Surgery
6. Prevention of Endophthalmitis during Cataract Surgery: Three-Step Approach
7. Povidone-Iodine as a “Border Control” Measure
8. Basic Properties of Povidone-Iodine
8.1. Effective Concentration
8.2. Safe Concentration for Irrigating Ocular Surface
8.3. Preparation, Color and Storage of 0.25% Povidone-Iodine
9. Clinical Application of 0.25% Povidone-Iodine
9.1. Preoperative Cleaning of Eyelid Skin
9.2. Irrigating the Ocular Surface during Cataract Surgery
9.3. Effectiveness in Cataract Surgery
9.4. Effectiveness in Intravitreal Injection
10. Clinical Application of 0.025–0.1% Povidone-Iodine
11. Pre-Cataract Surgery Povidone-Iodine Instillation/Irrigation and Endophthalmitis
12. Intracameral Antibiotics as Prophylaxis of Postoperative Endophthalmitis
13. Iodine Hypersensitivity
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Khairallah, M.; Kahloun, R.; Bourne, R.; Limburg, H.; Flaxman, S.R.; Jonas, J.B.; Keeffe, J.; Leasher, J.; Naidoo, K.; Pesudovs, K.; et al. Number of people blind or visually impaired by cataract worldwide and in world regions, 1990 to 2010. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6762–6769. [Google Scholar] [CrossRef] [Green Version]
- Kresloff, M.S.; Castellarin, A.A.; Zarbin, M.A. Endophthalmitis. Surv. Ophthalmol. 1998, 43, 193–224. [Google Scholar] [CrossRef]
- Speaker, M.G.; Milch, F.A.; Shah, M.K.; Eisner, W.; Kreiswirth, B.N. Role of external bacterial flora in the pathogenesis of acute postoperative endophthalmitis. Ophthalmology 1991, 98, 639–649. [Google Scholar] [CrossRef]
- Yannuzzi, N.A.; Si, N.; Relhan, N.; Kuriyan, A.E.; Albini, T.A.; Berrocal, A.M.; Davis, J.L.; Smiddy, W.E.; Townsend, J.; Miller, D.; et al. Endophthalmitis after clear corneal cataract surgery: Outcomes over two decades. Am. J. Ophthalmol. 2017, 174, 155–159. [Google Scholar] [CrossRef] [Green Version]
- Smith, T.C.; Benefield, R.J.; Kim, J.H. Risk of fungal endophthalmitis associated with cataract surgery: A mini-review. Mycopathologia 2015, 180, 291–297. [Google Scholar] [CrossRef]
- Toro, M.D.; Brézin, A.P.; Burdon, M.; Cummings, A.B.; Evren, K.O.; Malyugin, B.E.; Prieto, I.; Teus, M.A.; Tognetto, D.; Törnblom, R.; et al. Early impact of COVID-19 outbreak on eye care: Insights from EUROCOVCAT group. Eur. J. Ophthalmol. 2021, 31, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Tognetto, D.; Brézin, A.P.; Cummings, A.B.; Malyugin, B.E.; Evren, K.O.; Prieto, I.; Rejdak, R.; Teus, M.A.; Törnblom, R.; Toro, M.D.; et al. Rethinking elective cataract surgery diagnostics, assessments, and tools after the COVID-19 pandemic experience and beyond: Insights from the EUROCOVCAT group. Diagnostics 2020, 10, 1035. [Google Scholar] [CrossRef]
- Toro, M.; Choragiewicz, T.; Posarelli, C.; Figus, M.; Rejdak, R.; European COVID-19 Cataract Group (#EUROCOVCAT). Early impact of COVID-19 outbreak on the availability of cornea donors: Warnings and recommendations. Clin. Ophthalmol. 2020, 14, 2879–2882. [Google Scholar] [CrossRef]
- Dolar-Szczasny, J.; Toro, M.D.; Dworzańska, A.; Wójtowicz, T.; Korona-Glowniak, I.; Sawicki, R.; Boguszewska, A.; Polz-Dacewicz, M.; Tomasiewicz, K.; Załuska, W.; et al. Ocular Involvement of SARS-CoV-2 in a Polish cohort of COVID-19-Positive patients. Int. J. Environ. Res. Public Health 2021, 18, 2916. [Google Scholar] [CrossRef]
- Fahmy, J.A.; Moller, S.; Bentzon, M.W. Bacterial flora of the normal conjunctiva. I. Topographical distribution. Acta Ophthalmol. 1974, 52, 786–800. [Google Scholar] [CrossRef]
- McDermott, A.M. Antimicrobial compounds in tears. Exp. Eye Res. 2013, 117, 53–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hara, J.; Yasuda, F.; Higashitsutsumi, M. Preoperative disinfection of the conjunctival sac in cataract surgery. Ophthalmologica 1997, 211 (Suppl. S1), 62–67. [Google Scholar] [CrossRef]
- Capriotti, J.A.; Pelletier, J.S.; Shah, M.; Caivano, D.M.; Ritterband, D.C. Normal ocular flora in healthy eyes from a rural population in Sierra Leone. Int. Ophthalmol. 2009, 29, 81–84. [Google Scholar] [CrossRef]
- Ratnumnoi, R.; Keorochana, N.; Sontisombat, C. Normal flora of conjunctiva and lid margin, as well as its antibiotic sensitivity, in patients undergoing cataract surgery at Phramongkutklao Hospital. Clin. Ophthalmol. 2017, 11, 237–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yip, T.; Tse, K.C.; Ng, F.; Hung, I.; Lam, M.F.; Tang, S.; Lui, S.L.; Lai, K.N.; Chan, T.M.; Lo, W.K. Clinical course and outcomes of single-organism Enterococcus peritonitis in peritoneal dialysis patients. Clinical course and outcomes of single-organism Enterococcus peritonitis in peritoneal dialysis patients. Perit. Dial. Int. 2011, 31, 522–528. [Google Scholar] [CrossRef] [Green Version]
- Pietras-Baczewska, A.; Jasińska, E.; Toro, M.D.; Bonfiglio, V.; Reibaldi, M.; Avitabile, T.; Nowomiejska, K.; Rejdak, R. Urgent vitrectomy with vancomycin infusion, silicone oil endotamponade, and general antibiotic treatment in multiple cases of endophthalmitis from a single day of intravitreal injections-case series. J. Clin. Med. 2021, 10, 1059. [Google Scholar] [CrossRef]
- Sun, J.; Guo, Z.; Li, H.; Yang, B.; Wu, X. Acute infectious endophthalmitis after cataract surgery: Epidemiological characteristics, risk factors and incidence trends, 2008–2019. Infect. Drug Resist. 2021, 14, 1231–1238. [Google Scholar] [CrossRef]
- Haripriya, A.; Chang, D.F.; Namburar, S.; Smita, A.; Ravindran, R.D. Efficacy of intracameral moxifloxacin endophthalmitis prophylaxis at aravind eye hospital. Ophthalmology 2016, 123, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Lundstrom, M.; Wejde, G.; Stenevi, U.; Thorburn, W.; Montan, P. Endophthalmitis after cataract surgery: A nationwide prospective study evaluating incidence in relation to incision type and location. Ophthalmology 2007, 114, 866–870. [Google Scholar] [CrossRef]
- Pathengay, A.; Moreker, M.R.; Puthussery, R.; Ambatipudi, S.; Jalali, S.; Majji, A.B.; Mathai, A.; Husssain, N.; Dave, V.; Sharma, S.; et al. Clinical and microbiologic review of culture-proven endophthalmitis caused by multidrug-resistant bacteria in patients seen at a tertiary eye care center in southern India. Retina 2011, 31, 1806–1811. [Google Scholar] [CrossRef] [PubMed]
- Schimel, A.M.; Miller, D.; Flynn, H.W., Jr. Endophthalmitis isolates and antibiotic susceptibilities: A 10-year review of culture-proven cases. Am. J. Ophthalmol. 2013, 156, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Lalitha, P.; Mishra, C.; Parida, H.; Rameshkumar, G.; Kannan, N.B.; Ramasamy, K. Post-cataract Surgery Fungal Endophthalmitis: Management Outcomes and Prognostic Factors. Ocul. Immunol. Inflamm. 2020, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Asari, S. Post-cataract surgery endophthalmitis. Jpn. J. Ophthalmic Surg. 2006, 19, 365–370. (In Japanese) [Google Scholar]
- Behndig, A.; Cochener, B.; Güell, J.L.; Kodjikian, L.; Mencucci, R.; Nuijts, R.M.; Pleyer, U.; Rosen, P.; Szaflik, J.P.; Tassignon, M.J. Endophthalmitis prophylaxis in cataract surgery: Overview of current practice patterns in 9 European countries. J. Cataract. Refract. Surg. 2013, 39, 1421–1431. [Google Scholar] [CrossRef] [Green Version]
- Grzybowski, A.; Schwartz, S.G.; Matsuura, K.; Tone, S.O.; Arshinoff, S.; Ng, J.Q.; Meyer, J.J.; Liu, W.; Jacob, S.; Packer, M.; et al. Endophthalmitis prophylaxis in cataract surgery: Overview of current practice patterns around the world. Curr. Pharm. Des. 2017, 23, 565–573. [Google Scholar] [PubMed]
- Inoue, Y.; Usui, M.; Ohashi, Y.; Shiota, H.; Yamazaki, T. Preoperative Disinfection Study Group. Preoperative disinfection of the conjunctival sac with antibiotics and iodine compounds: A prospective randomized multicenter study. Jpn. J. Ophthalmol. 2008, 52, 151–161. [Google Scholar] [CrossRef]
- de Kaspar, H.M.; Kreutzer, T.C.; Aguirre-Romo, I.; Ta, C.N.; Dudichum, J.; Bayrhof, M.; Klauss, V.; Kampik, A. A prospective randomized study to determine the efficacy of preoperative topical levofloxacin in reducing conjunctival bacterial flora. Am. J. Ophthalmol. 2008, 145, 136–142. [Google Scholar] [CrossRef]
- Shimada, H.; Arai, S.; Nakashizuka, H.; Hattori, T.; Yuzawa, M. Reduction of anterior chamber contamination rate after cataract surgery by intraoperative irrigation with 0.25% povidone-iodine. Am. J. Ophthalmol. 2011, 151, 11–17. [Google Scholar] [CrossRef]
- Matsuura, K.; Miyazaki, D.; Sasaki, S.I.; Inoue, Y.; Sasaki, Y.; Shimizu, Y. Effectiveness of intraoperative iodine in cataract surgery: Cleanliness of the surgical field without preoperative topical antibiotics. Jpn. J. Ophthalmol. 2020, 64, 37–44. [Google Scholar] [CrossRef]
- Hsu, J.; Gerstenblith, A.T.; Garg, S.J.; Vander, J.F. Conjunctival flora antibiotic resistance patterns after serial intravitreal injections without postinjection topical antibiotics. Am. J. Ophthalmol. 2014, 157, 514–518.e1. [Google Scholar] [CrossRef]
- Storey, P.; Dollin, M.; Rayess, N.; Pitcher, J.; Reddy, S.; Vander, J.; Hsu, J.; Garg, S. Post-Injection Endophthalmitis Study Team. The effect of prophylactic topical antibiotics on bacterial resistance patterns in endophthalmitis following intravitreal injection. Graefes. Arch. Clin. Exp. Ophthalmol. 2016, 254, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Nejima, R.; Shimizu, K.; Ono, T.; Noguchi, Y.; Yagi, A.; Iwasaki, T.; Shoji, N.; Miyata, K. Effect of the administration period of perioperative topical levofloxacin on normal conjunctival bacterial flora. J. Cataract. Refract. Surg. 2017, 43, 42–48. [Google Scholar] [CrossRef]
- Nakashizuka, H.; Wakatsuki, Y.; Machida, Y.; Okubo, Y.; Shinojima, A.; Hattori, T.; Shimada, H.; Yuzawa, M. Wet laboratory training using porcine eyes with eyelids. Can. J. Ophthalmol. 2017, 52, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, S.; Tasaka, Y.; Suzuki, T.; Zheng, X.; Shiraishi, A.; Uno, T.; Ohashi, Y. Influence of elevated intraocular pressure on the posterior chamber-anterior hyaloid membrane barrier during cataract operations. Arch. Ophthalmol. 2011, 129, 751–757. [Google Scholar] [CrossRef] [Green Version]
- Oshika, T.; Ohashi, Y. Endophthalmitis after cataract surgery: Effect of behind-the-lens washout. J. Cataract. Refract. Surg. 2017, 43, 1399–1405. [Google Scholar] [CrossRef]
- Shorstein, N.H.; Liu, L.; Carolan, J.A.; Herrinton, L. Endophthalmitis prophylaxis failures in patients injected with intracameral antibiotic during cataract surgery. Am. J. Ophthalmol. 2021, 227, 166–172. [Google Scholar] [CrossRef]
- Endophthalmitis Study Group, European Society of Cataract & Refractive Surgeons. Prophylaxis of postoperative endophthalmitis following cataract surgery: Results of the ESCRS multicenter study and identification of risk factors. J. Cataract. Refract. Surg. 2007, 33, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Bowen, R.C.; Zhou, A.X.; Bondalapati, S.; Lawyer, T.W.; Snow, K.B.; Evans, P.R.; Bardsley, T.; McFarland, M.; Kliethermes, M.; Shi, D.; et al. Comparative analysis of the safety and efficacy of intracameral cefuroxime, moxifloxacin and vancomycin at the end of cataract surgery: A meta-analysis. Br. J. Ophthalmol. 2018, 102, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Hariprasad, S.M.; Blinder, K.J.; Shah, G.K.; Apte, R.S.; Rosenblatt, B.; Holekamp, N.M.; Thomas, M.A.; Mieler, W.F.; Chi, J.; Prince, R.A. Penetration pharmacokinetics of topically administered 0.5% moxifloxacin ophthalmic solution in human aqueous and vitreous. Arch. Ophthalmol. 2005, 123, 39–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costello, P.; Bakri, S.J.; Beer, P.M.; Singh, R.J.; Falk, N.S.; Peters, G.B.; Melendez, J.A. Vitreous penetration of topical moxifloxacin and gatifloxacin in humans. Retina 2006, 26, 191–195. [Google Scholar] [CrossRef]
- Garner, J.S.; Favero, M.S. CDC guidelines for the prevention and control of nosocomial infections. Guideline for handwashing and hospital environmental control, 1985. Supersedes guideline for hospital environmental control published in 1981. Am. J. Infect. Control 1986, 14, 110–119. [Google Scholar] [CrossRef]
- Unal, M.; Yücel, I.; Akar, Y.; Oner, A.; Altin, M. Outbreak of toxic anterior segment syndrome associated with glutaraldehyde after cataract surgery. J. Cataract. Refract. Surg. 2006, 32, 1696–1701. [Google Scholar] [CrossRef]
- Slaughter, R.J.; Watts, M.; Vale, J.A.; Grieve, J.R.; Schep, L.J. The clinical toxicology of sodium hypochlorite. Clin. Toxicol. 2019, 57, 303–311. [Google Scholar] [CrossRef]
- Oh, J.Y.; Yu, J.M.; Ko, J.H. Analysis of ethanol effects on corneal epithelium. Invest. Ophthalmol. Vis. Sci. 2013, 54, 3852–3856. [Google Scholar] [CrossRef] [Green Version]
- Kashiwagi, K.; Saito, K.; Wang, Y.D.; Takahashi, H.; Ishijima, K.; Tsukahara, S. Safety of ozonated solution as an antiseptic of the ocular surface prior to ophthalmic surgery. Ophthalmologica 2001, 215, 351–356. [Google Scholar] [CrossRef]
- Paduch, R.; Urbanik-Sypniewska, T.; Kutkowska, J.; Chorągiewicz, T.; Matysik-Woźniak, A.; Zweifel, S.; Czarnek-Chudzik, A.; Załuska, W.; Rejdak, R.; Toro, M.D. Ozone-Based Eye Drops Activity on Ocular Epithelial Cells and Potential Pathogens Infecting the Front of the Eye. Antioxidants 2021, 10, 968. [Google Scholar] [CrossRef]
- Suzuki, H.; Sato, S.; Murano, N.; Matsui, H.; Oharazawa, H.; Takahashi, H. Morphological observations of rat corneal endothelial cells after exposure to ozonated solution. Jpn. J. Ophthalmol. 2009, 53, 151–158. [Google Scholar] [CrossRef]
- Zamora, J.L. Chemical and microbiologic characteristics and toxicity of povidone-iodine solutions. Am. J. Surg. 1986, 151, 400–406. [Google Scholar] [CrossRef]
- Rikimaru, T.; Kondo, M.; Kajimura, K.; Hashimoto, K.; Oyamada, K.; Miyazaki, S.; Sagawa, K.; Aizawa, H.; Oizumi, K. Efficacy of common antiseptics against multidrug-resistant mycobacterium tuberculosis. Int. J. Tuberc. Lung. Dis. 2002, 6, 763–770. [Google Scholar] [PubMed]
- Bonowitz, A.; Schaller, M.; Laude, J.; Reimer, K.; Korting, H.C. Comparative therapeutic and toxic effects of different povidone iodine (PVP-I) formulations in a model of oral candidiasis based on in vitro reconstituted epithelium. J. Drug. Target 2001, 9, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Clement, C.; Capriotti, J.A.; Kumar, M.; Reimer, K.; Korting, H.C. Clinical and antiviral efficacy of an ophthalmic formulation of dexamethasone povidone-iodine in a rabbit model of adenoviral keratoconjunctivitis. Invest. Ophthalmol. Vis. Sci. 2011, 52, 339–344. [Google Scholar] [CrossRef]
- Kobayashi, T.; Gibbon, L.; Mito, T.; Shiraishi, A.; Uno, T.; Ohashi, Y. Efficacy of commercial soft contact lens disinfectant solutions against Acanthamoeba. Jpn. J. Ophthalmol. 2011, 55, 547–557. [Google Scholar] [CrossRef]
- Oduwole, K.O.; Glynn, A.A.; Molony, D.C.; Murray, D.; Rowe, S.; Holland, L.M.; McCormack, D.J.; O’Gara, J.P. Anti-biofilm activity of sub-inhibitory povidone-iodine concentrations against Staphylococcus epidermidis and Staphylococcus aureus. J. Orthop. Res. 2010, 28, 1252–1256. [Google Scholar] [CrossRef]
- Ciulla, T.A.; Starr, M.B.; Masket, S. Bacterial endophthalmitis prophylaxis for cataract surgery: An evidence-based update. Ophthalmology 2002, 109, 13–24. [Google Scholar] [CrossRef]
- Merani, R.; McPherson, Z.E.; Luckie, A.P.; Gilhotra, J.S.; Runciman, J.; Durkin, S.; Muecke, J.; Donaldson, M.; Aralar, A.; Rao, A.; et al. Aqueous chlorhexidine for intravitreal injection Antisepsis: A case series and review of the literature. Ophthalmology 2016, 123, 2588–2594. [Google Scholar] [CrossRef] [Green Version]
- Ali, F.S.; Jenkins, T.L.; Boparai, R.S.; Obeid, A.; Ryan, M.E.; Wibblesman, T.D.; Chiang, A.; Garg, S.J.; Post-Injection Endophthalmitis Study Group. Aqueous chlorhexidine compared with povidone-iodine as ocular antisepsis before intravitreal injection: A randomized clinical trial. Ophthalmol. Retina 2021, 5, 788–796. [Google Scholar] [CrossRef]
- Nakazawa, Y.; Tamura, T.; Hori, S. The minimum killing concentrations of chlorhexidine gluconate and olanexidine gluconate on clinically isolated S. aureus strains. Jpn. J. Ecol. 2018, 33, 52–55, (Abstract: In English). [Google Scholar]
- Hooper, D.C. Mechanisms of action and resistance of older and newer fluoroquinolones. Clin. Infect. Dis. 2000, 31 (Suppl. S2), S24–S28. [Google Scholar] [CrossRef]
- Callegan, M.C.; Novosad, B.D.; Ramadan, R.T.; Wiskur, B.; Moyer, A.L. Rate of bacterial eradication by ophthalmic solutions of fourth-generation fluoroquinolones. Adv. Ther. 2009, 26, 447–454. [Google Scholar] [CrossRef]
- Sobaci, G.; Tuncer, K.; Taş, A.; Ozyurt, M.; Bayer, A.; Kutlu, U. The effect of intraoperative antibiotics in irrigating solutions on aqueous humor contamination and endophthalmitis after phacoemulsification surgery. Eur. J. Ophthalmol. 2003, 13, 773–778. [Google Scholar] [CrossRef]
- Gritz, D.C.; Cevallos, A.V.; Smolin, G.; Whitcher, J.P., Jr. Antibiotic supplementation of intraocular irrigating solutions. An in vitro model of antibacterial action. Ophthalmology 1996, 103, 1204–1208. [Google Scholar] [CrossRef]
- Shelanski, H.A.; Shelanski, M.V. PVP-iodine: History, toxicity and therapeutic uses. J. Int. Coll. Surg. 1956, 25, 727–734. [Google Scholar] [PubMed]
- Van den Broek, P.J.; Buys, L.F.; Van Furth, R. Interaction of povidone-iodine compounds, phagocytic cells, and microorganisms. Antimicrob. Agents Chemother. 1982, 22, 593–597. [Google Scholar] [CrossRef] [Green Version]
- Berkelman, R.L.; Holland, B.W.; Anderson, R.L. Increased bactericidal activity of dilute preparations of povidone-iodine solutions. J. Clin. Microbiol. 1982, 15, 635–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, J.; Wu, M.; Shen, T. The toxic effect of different concentrations of povidone iodine on the rabbit’s cornea. Cutan. Ocul. Toxicol. 2009, 28, 119–124. [Google Scholar] [CrossRef]
- Shimada, H.; Kato, K.; Ishida, K.; Yamaguchi, T.; Shinoda, K. Evaluation of retinal function and pathology after intravitreal injection of povidone-iodine and polyvinyl alcohol-iodine in rabbits. Transl. Vis. Sci. Technol. 2020, 9, 5. [Google Scholar] [CrossRef] [Green Version]
- Trost, L.W.; Kivilcim, M.; Peyman, G.A.; Aydin, E.; Kazi, A.A. The effect of intravitreally injected povidone-iodine on Staphylococcus epidermidis in rabbit eyes. J. Ocul. Pharmacol. Ther. 2007, 23, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Kim, B.K.; Mun, S.J.; Choi, H.T.; Chung, Y.T. Reversible cataract after exposure to distilled water: A case report. BMC. Ophthalmol. 2018, 18, 180. [Google Scholar] [CrossRef]
- Shimada, H.; Nakashizuka, H.; Grzybowski, A.R. Prevention and treatment of postoperative endophthalmitis using povidone-iodine. Curr. Pharm. Des. 2017, 23, 574–585. [Google Scholar]
- Shimada, H. Basic setup and disinfection. Dev. Ophthalmol. 2014, 54, 63–70. [Google Scholar]
- Shimada, H.; Arai, S.; Kawamata, T.; Nakashizuka, H.; Hattori, T.; Yuzawa, M. Frequency, source, and prevention of cotton fibers in the anterior chamber during cataract surgery. J. Cataract. Refract. Surg. 2008, 34, 1389–1392. [Google Scholar] [CrossRef]
- Matsuura, K.; Mori, T.; Miyamoto, T.; Suto, C.; Saeki, Y.; Tanaka, S.; Kawamura, H.; Ohkubo, S.; Tanito, M.; Inoue, Y. Survey of Japanese ophthalmic surgeons regarding perioperative disinfection and antibiotic prophylaxis in cataract surgery. Clin. Ophthalmol. 2014, 8, 2013–2018. [Google Scholar] [CrossRef] [Green Version]
- Shimada, H.; Nakashizuka, H.; Hattori, T.; Kitagawa, Y.; Manabe, A.; Otani, K.; Yuzawa, M. Reducing bacterial contamination inside fluid catch bag in 25-gauge vitrectomy by 0.25% povidone-iodine ocular surface irrigation. Int. Ophthalmol. 2013, 33, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Donnenfeld, E.D.; Perry, H.D.; Snyder, R.W.; Moadel, R.; Elsky, M.; Jones, H. Intracorneal, aqueous humor, and vitreous humor penetration of topical and oral ofloxacin. Arch. Ophthalmol. 1997, 115, 173–176. [Google Scholar] [CrossRef]
- Yalvac, I.S.; Basci, N.E.; Bozkurt, A.; Duman, S. Penetration of topically applied ciprofloxacin and ofloxacin into the aqueous humor and vitreous. J. Cataract. Refract Surg. 2003, 29, 487–491. [Google Scholar] [CrossRef]
- Koerner, J.C.; George, M.J.; Meyer, D.R.; Rosco, M.G.; Habib, M.M. Povidone-iodine concentration and dosing in cataract surgery. Surv. Ophthalmol. 2018, 63, 862–868. [Google Scholar] [CrossRef]
- Nakashizuka, H.; Shoji, J.; Shimada, H.; Yuzawa, M. Experimental visualization and quantification of vitreous contamination following intravitreal injections. Retina 2016, 36, 1882–1887. [Google Scholar] [CrossRef]
- Reibaldi, M.; Avitabile, T.; Bandello, F.; Longo, A.; Bonfiglio, V.; Russo, A.; Castellino, N.; Rejdak, R.; Nowomiejska, K.; Toro, M.; et al. The effectiveness of 0.6% povidone iodine eye drops in reducing the conjunctival bacterial load and needle contamination in patients undergoing anti-VEGF Intravitreal Injection: A prospective, randomized study. J. Clin. Med. 2019, 8, 1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musumeci, R.; Bandello, F.; Martinelli, M.; Calaresu, E.; Cocuzza, C.E. In vitro bactericidal activity of 0.6% povidone-iodine eye drops formulation. Eur. J. Ophthalmol. 2019, 29, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Pinna, A.; Donadu, M.G.; Usai, D.; Dore, S.; D’Amico-Ricci, G.; Boscia, F.; Zanetti, S. In vitro antimicrobial activity of a new ophthalmic solution containing povidone-iodine 0.6% (IODIM®). Acta Ophthalmol. 2020, 98, e178–e180. [Google Scholar] [CrossRef]
- de Kaspar, H.M.; Chang, R.T.; Singh, K.; Egbert, P.R.; Blumenkranz, M.S.; Ta, C.N. Prospective randomized comparison of 2 different methods of 5% povidone-iodine applications for anterior segment intraocular surgery. Arch. Ophthalmol. 2005, 123, 161–165. [Google Scholar] [CrossRef]
- Tanaka, K.; Shimada, H.; Mori, R.; Nakashizuka, H.; Hattori, T.; Okubo, Y. No increase in incidence of post-intravitreal injection endophthalmitis without topical antibiotics: A prospective study. Jpn. J. Ophthalmol. 2019, 63, 396–401. [Google Scholar] [CrossRef]
- Nowomiejska, K.; Lukasik, P.; Brzozowska, A.; Toro, M.D.; Sedzikowska, A.; Bartosik, K.; Rejdak, R. Prevalence of ocular demodicosis and ocular surface conditions in patients selected for cataract surgery. J. Clin. Med. 2020, 9, 3069. [Google Scholar] [CrossRef]
- Nakashizuka, H.; Shimada, H.; Hattori, T.; Noguchi, T.; Kokubo, N.; Yuzawa, M. Vitrectomy using 0.025% povidone-iodine in Balanced Salt Solution PLUS for the treatment of postoperative endophthalmitis. Retina 2015, 35, 1087–1094. [Google Scholar] [CrossRef]
- Shimada, H.; Arai, S.; Nakashizuka, H.; Hattori, T.; Yuzawa, M. Reduced anterior chamber contamination by frequent surface irrigation with diluted iodine solutions during cataract surgery. Acta Ophthalmol. 2017, 95, e373–e378. [Google Scholar] [CrossRef] [Green Version]
- Fan, F.; Zhao, Z.; Zhao, X.; Ma, Q.; Li, K.; Fu, W.; Jia, Z. Reduction of ocular surface damage and bacterial survival using 0.05% povidone-iodine ocular surface irrigation before cataract surgery. Ophthalmic Res. 2019, 62, 166–172. [Google Scholar] [CrossRef]
- Musumeci, R.; Troiano, P.; Martinelli, M.; Piovella, M.; Carbonara, C.; Rossi, S.; Alessio, G.; Molteni, L.; Molteni, C.G.; Saderi, L.; et al. Effectiveness of 0.66% Povidone-Iodine Eye Drops on Ocular Surface Flora before Cataract Surgery: A Nationwide Microbiological Study. J. Clin. Med.. 2021, 10, 2198. [Google Scholar] [CrossRef] [PubMed]
- Nentwich, M.M.; Ta, C.N.; Kreutzer, T.C.; Li, B.; Schwarzbach, F.; Yactayo-Miranda, Y.M.; Kampik, A.; de Kaspar, H.M. Incidence of postoperative endophthalmitis from 1990 to 2009 using povidone-iodine but no intracameral antibiotics at a single academic institution. J. Cataract. Refract. Surg. 2015, 41, 58–66. [Google Scholar] [CrossRef]
- Witkin, A.J.; Shah, A.R.; Engstrom, R.E.; Kron-Gray, M.M.; Baumal, C.R.; Johnson, M.W.; Witkin, D.I.; Leung, J.; Albini, T.A.; Moshfeghi, A.A.; et al. Postoperative hemorrhagic occlusive retinal vasculitis: Expanding the clinical spectrum and possible association with vancomycin. Ophthalmology 2015, 122, 1438–1451. [Google Scholar] [CrossRef] [PubMed]
- Mesnard, C.; Beral, L.; Hage, R.; Merle, H.; Farès, S.; David, T. Endophthalmitis after cataract surgery despite intracameral antibiotic prophylaxis with licensed cefuroxime. J. Cataract. Refract. Surg. 2016, 42, 1318–1323. [Google Scholar] [CrossRef] [PubMed]
- Grzybowski, A.; Kanclerz, P.; Myers, W.G. The use of povidone-iodine in ophthalmology. Curr. Opin. Ophthalmol. 2018, 29, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, C.; Costa, H.; Oliveira, G.; Romariz, J.; Praça, F. Anaphylaxis to povidone in a child. Pediatr. Allergy. Immunol. 2005, 16, 361–362. [Google Scholar] [CrossRef] [PubMed]
- Adachi, A.; Fukunaga, A.; Hayashi, K.; Kunisada, M.; Horikawa, T. Anaphylaxis to polyvinylpyrrolidone after vaginal application of povidone-iodine. Contact Dermat. 2003, 48, 133–136. [Google Scholar] [CrossRef] [PubMed]









| Mechanism | Selectivity | Tolerance | Contact Time | |
|---|---|---|---|---|
| Antimicrobial Agent | Cell wall/protein/DNA synthesis inhibition | + | + | 15–140 min |
| Povidone-Iodine | Membrane protein denaturation | − | − | 15–180 s |
| Povidone-Iodine 2.5–10% | Povidone-Iodine 0.05–0.5% | |
|---|---|---|
| Free iodine concentration (ppm) | 3–10 | 15–24 |
| Exposure time (s) | 30–180 | 15 |
| Microbicidal duration | long | short |
| Site of use | Skin cleansing | Ocular surface irrigation |
| Method of use | Once cleaning | Repeatedly irrigated |
| Method of Ocular Surface Irrigation (No.) | Microbial Contamination Rate (%) | Corneal Endothelial Cell Density (/mm2) | ||
|---|---|---|---|---|
| Start of Surgery Ocular Surface Fluid | End of Surgery Anterior Chamber Fluid | Preop | Day 7 Postop | |
| Physiological saline (n = 200) | 11/200 (5.5%) * | 10/200 (5.0%) ** | 2614 ± 233 + | 2463 ± 269 ++ |
| CNS (7) Micrococcus sp. (1) Enterococcus faecalis (1) Staphylococcus aureus (1) Corynebacterium spp. (1) | CNS (6) Enterococcus sp. (1) Enterococcus faecalis (1) Staphylococcus aureus (1) Klebsiella pneumoniae (1) | |||
| 0.25% povidone-iodine (n = 200) | 12/200 (6.0%) * | 0/200 (0%) ** | 2534 ± 173 + | 2338 ± 204 ++ |
| CNS (8) Staphylococcus aureus (2) Micrococcus sp. (1) Klebsiella spp. (1) | ||||
| p | >0.99 * | 0.0017 ** | 0.2254 + | 0.4044 ++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimada, H.; Nakashizuka, H. Cataract Surgery by Intraoperative Surface Irrigation with 0.25% Povidone–Iodine. J. Clin. Med. 2021, 10, 3611. https://doi.org/10.3390/jcm10163611
Shimada H, Nakashizuka H. Cataract Surgery by Intraoperative Surface Irrigation with 0.25% Povidone–Iodine. Journal of Clinical Medicine. 2021; 10(16):3611. https://doi.org/10.3390/jcm10163611
Chicago/Turabian StyleShimada, Hiroyuki, and Hiroyuki Nakashizuka. 2021. "Cataract Surgery by Intraoperative Surface Irrigation with 0.25% Povidone–Iodine" Journal of Clinical Medicine 10, no. 16: 3611. https://doi.org/10.3390/jcm10163611





