Longer Leukocytes Telomere Length Predicts a Significant Survival Advantage in the Elderly TRELONG Cohort, with Short Physical Performance Battery Score and Years of Education as Main Determinants for Telomere Elongation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Demographic, Physiological and Lifestyle Characteristics
2.3. Assessment of Physical Performance and Frailty Measures
2.4. Assessment of Chronic Disease and Other Covariates
2.5. Biochemical Measurements
2.6. DNA Extraction from Mononuclear Cells (PBMC)
2.7. APOE Genotyping
2.8. Leukocyte Telomere Length (LTL) Analysis
2.9. Statistical Analysis
2.9.1. Dependence of LTL on Age
2.9.2. Survival Analysis: Kaplan–Meier Curves
2.9.3. Survival Analysis: Cox Proportional Hazards Model
2.9.4. Characterization of Subjects with Elongated Telomeres
3. Results
3.1. Descriptive Results
3.1.1. Characteristic of Population at the Baseline and LTL Distribution
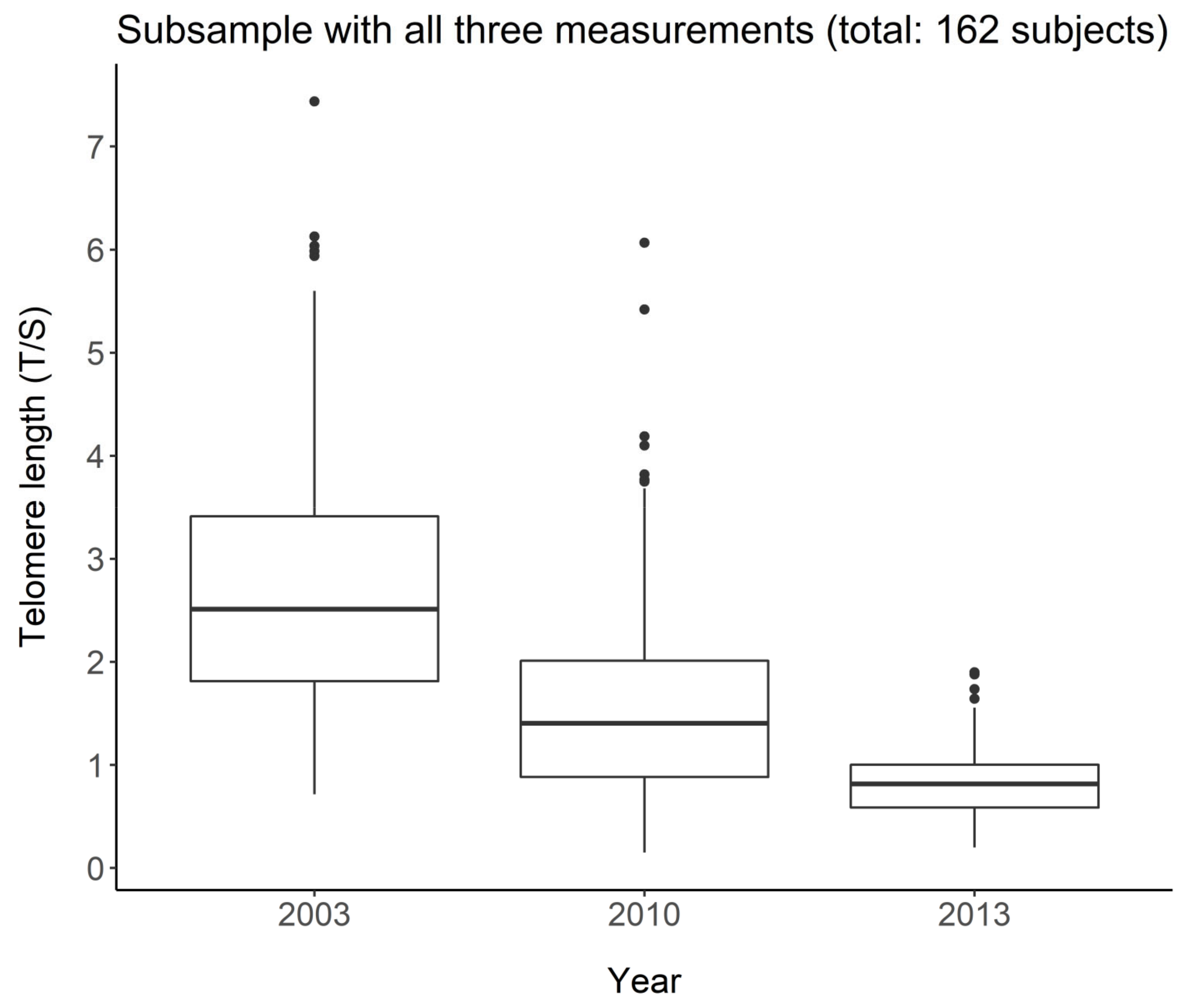
3.1.2. LTL and Age in the Longitudinal Study
3.1.3. LTL and Survival: Kaplan–Meier Curves
3.2. Outcome Results
3.2.1. Determinants of Survival
3.2.2. Determinants of LTL Elongation
4. Discussion
4.1. LTL and Survival
4.2. Determinants of Survival
4.3. LTL in the Longitudinal Study
4.4. Determinants of LTL Elongation
4.5. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Chang, A.Y.; Skirbekk, V.F.; Tyrovolas, S.; Kassebaum, N.J.; Dieleman, J.L. Measuring Population Ageing: An Analysis of the Global Burden of Disease Study 2017. Lancet Public Health 2019, 4, e159–e167. [Google Scholar] [CrossRef] [Green Version]
- Lipsi, R.M. Evoluzione Della Geografia Della Mortalità in Italia: Tavole Provinciali e Probabilità di Morte Per Causa. Anni: 1971–1973, 1981–1983, 1991–1993; Fonti e Strumenti: Rome, Italy, 2002. [Google Scholar]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [Green Version]
- Fulop, T.; Larbi, A.; Witkowski, J.M.; McElhaney, J.; Loeb, M.; Mitnitski, A.; Pawelec, G. Aging, Frailty and Age-Related Diseases. Biogerontology 2010, 11, 547–563. [Google Scholar] [CrossRef]
- Levine, M.E. Modeling the Rate of Senescence: Can Estimated Biological Age Predict Mortality More Accurately than Chronological Age? J. Gerontol. A. Biol. Sci. Med. Sci. 2013, 68, 667–674. [Google Scholar] [CrossRef] [Green Version]
- Peters, A.; Nawrot, T.S.; Baccarelli, A.A. Hallmarks of Environmental Insults. Cell 2021, 184, 1455–1468. [Google Scholar] [CrossRef]
- Blackburn, E.H.; Epel, E.S.; Lin, J. Human Telomere Biology: A Contributory and Interactive Factor in Aging, Disease Risks, and Protection. Science 2015, 350, 1193–1198. [Google Scholar] [CrossRef] [Green Version]
- Pavanello, S.; Angelici, L.; Hoxha, M.; Cantone, L.; Campisi, M.; Tirelli, A.S.; Vigna, L.; Pesatori, A.C.; Bollati, V. Sterol 27-Hydroxylase Polymorphism Significantly Associates With Shorter Telomere, Higher Cardiovascular and Type-2 Diabetes Risk in Obese Subjects. Front. Endocrinol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Pavanello, S.; Stendardo, M.; Mastrangelo, G.; Casillo, V.; Nardini, M.; Mutti, A.; Campisi, M.; Andreoli, R.; Boschetto, P. Higher Number of Night Shifts Associates with Good Perception of Work Capacity and Optimal Lung Function but Correlates with Increased Oxidative Damage and Telomere Attrition. BioMed Res. Int. 2019, 2019, e8327629. [Google Scholar] [CrossRef] [Green Version]
- Pavanello, S.; Stendardo, M.; Mastrangelo, G.; Bonci, M.; Bottazzi, B.; Campisi, M.; Nardini, M.; Leone, R.; Mantovani, A.; Boschetto, P. Inflammatory Long Pentraxin 3 Is Associated with Leukocyte Telomere Length in Night-Shift Workers. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Pavanello, S.; Hoxha, M.; Dioni, L.; Bertazzi, P.A.; Snenghi, R.; Nalesso, A.; Ferrara, S.D.; Montisci, M.; Baccarelli, A. Shortened Telomeres in Individuals with Abuse in Alcohol Consumption. Int. J. Cancer 2011, 129, 983–992. [Google Scholar] [CrossRef] [Green Version]
- Pavanello, S.; Pesatori, A.-C.; Dioni, L.; Hoxha, M.; Bollati, V.; Siwinska, E.; Mielzyńska, D.; Bolognesi, C.; Bertazzi, P.-A.; Baccarelli, A. Shorter Telomere Length in Peripheral Blood Lymphocytes of Workers Exposed to Polycyclic Aromatic Hydrocarbons. Carcinogenesis 2010, 31, 216–221. [Google Scholar] [CrossRef] [Green Version]
- Pavanello, S.; Carta, A.; Mastrangelo, G.; Campisi, M.; Arici, C.; Porru, S. Relationship between Telomere Length, Genetic Traits and Environmental/Occupational Exposures in Bladder Cancer Risk by Structural Equation Modelling. Int. J. Environ. Res. Public. Health 2018, 15, 5. [Google Scholar] [CrossRef] [Green Version]
- Pavanello, S.; Campisi, M.; Mastrangelo, G.; Hoxha, M.; Bollati, V. The Effects of Everyday-Life Exposure to Polycyclic Aromatic Hydrocarbons on Biological Age Indicators. Environ. Health 2020, 19, 1–13. [Google Scholar] [CrossRef]
- Gallucci, M.; Ongaro, F.; Bresolin, F.; Bernardi, U.; Salvato, C.; Minello, A.; Amici, G.P.; Barasciutti, E.; Mazzuco, S.; Gajo, G.B.; et al. The Treviso Longeva (Trelong) Study: A Biomedical, Demographic, Economic and Social Investigation on People 70 Years and over in a Typical Town of North-East of Italy. Arch. Gerontol. Geriatr. 2007, 44, 173–192. [Google Scholar] [CrossRef] [PubMed]
- Gallucci, M.; Antuono, P.; Ongaro, F.; Forloni, P.L.; Albani, D.; Amici, G.P.; Regini, C. Physical Activity, Socialization and Reading in the Elderly over the Age of Seventy: What Is the Relation with Cognitive Decline? Evidence from “The Treviso Longeva (TRELONG) Study”. Arch. Gerontol. Geriatr. 2009, 48, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Gallucci, M.; Ongaro, F.; Meggiolaro, S.; Antuono, P.; Gustafson, D.R.; Forloni, G.L.; Albani, D.; Gajo, G.B.; Durante, E.; Caberlotto, L.; et al. Factors Related to Disability: Evidence from the “Treviso Longeva (TRELONG) Study”. Arch. Gerontol. Geriatr. 2011, 52, 309–316. [Google Scholar] [CrossRef]
- Gallucci, M.; Mazzuco, S.; Ongaro, F.; Di Giorgi, E.; Mecocci, P.; Cesari, M.; Albani, D.; Forloni, G.L.; Durante, E.; Gajo, G.B.; et al. Body Mass Index, Lifestyles, Physical Performance and Cognitive Decline: The “Treviso Longeva (Trelong)” Study. J. Nutr. Health Aging 2013, 17, 378–384. [Google Scholar] [CrossRef]
- Albani, D.; Mazzuco, S.; Chierchia, A.; Fusco, F.; Boeri, L.; Martines, R.; Giorgi, E.D.; Frigato, A.; Durante, E.; Caberlotto, L.; et al. The SIRT1 Promoter Polymorphic Site Rs12778366 Increases IL-6 Related Human Mortality in the Prospective Study “Treviso Longeva (TRELONG)”. Int. J. Mol. Epidemiol. Genet. 2015, 6, 20–26. [Google Scholar]
- Gallucci, M.; Pallucca, C.; Di Battista, M.E.; Fougère, B.; Grossi, E. Artificial Neural Networks Help to Better Understand the Interplay Between Cognition, Mediterranean Diet, and Physical Performance: Clues from TRELONG Study. J. Alzheimers Dis. 2019, 71, 1321–1330. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A Short Physical Performance Battery Assessing Lower Extremity Function: Association with Self-Reported Disability and Prediction of Mortality and Nursing Home Admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Gill, T.M.; Baker, D.I.; Gottschalk, M.; Peduzzi, P.N.; Allore, H.; Byers, A. A Program to Prevent Functional Decline in Physically Frail, Elderly Persons Who Live at Home. Available online: https://www.nejm.org/doi/10.1056/NEJMoa020423 (accessed on 8 July 2021).
- Lawton, M.P.; Brody, E.M. Assessment of Older People: Self-Maintaining and Instrumental Activities of Daily Living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Colinet, B.; Jacot, W.; Bertrand, D.; Lacombe, S.; Bozonnat, M.-C.; Daurès, J.-P.; Pujol, J.-L. A New Simplified Comorbidity Score as a Prognostic Factor in Non-Small-Cell Lung Cancer Patients: Description and Comparison with the Charlson’s Index. Br. J. Cancer 2005, 93, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, D.R.; Mazzuco, S.; Ongaro, F.; Antuono, P.; Forloni, G.; Albani, D.; Gajo, G.B.; Durante, E.; Caberlotto, L.; Zanardo, A.; et al. Body Mass Index, Cognition, Disability, APOE Genotype, and Mortality: The “Treviso Longeva” Study. Am. J. Geriatr. Psychiatry 2012, 20, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Pavanello, S.; Campisi, M.; Fabozzo, A.; Cibin, G.; Tarzia, V.; Toscano, G.; Gerosa, G. The Biological Age of the Heart Is Consistently Younger than Chronological Age. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Cawthon, R.M. Telomere Length Measurement by a Novel Monochrome Multiplex Quantitative PCR Method. Nucleic Acids Res. 2009, 37, e21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing. 2014. Available online: http://www.R-project.org/ (accessed on 17 August 2021).
- Cawthon, R.M.; Smith, K.R.; O’Brien, E.; Sivatchenko, A.; Kerber, R.A. Association between Telomere Length in Blood and Mortality in People Aged 60 Years or Older. Lancet 2003, 361, 393–395. [Google Scholar] [CrossRef]
- Rode, L.; Nordestgaard, B.G.; Bojesen, S.E. Peripheral Blood Leukocyte Telomere Length and Mortality Among 64 637 Individuals From the General Population. JNCI J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef] [Green Version]
- Mons, U.; Müezzinler, A.; Schöttker, B.; Dieffenbach, A.K.; Butterbach, K.; Schick, M.; Peasey, A.; De Vivo, I.; Trichopoulou, A.; Boffetta, P.; et al. Leukocyte Telomere Length and All-Cause, Cardiovascular Disease, and Cancer Mortality: Results From Individual-Participant-Data Meta-Analysis of 2 Large Prospective Cohort Studies. Am. J. Epidemiol. 2017, 185, 1317–1326. [Google Scholar] [CrossRef] [Green Version]
- Arbeev, K.G.; Verhulst, S.; Steenstrup, T.; Kark, J.D.; Bagley, O.; Kooperberg, C.; Reiner, A.P.; Hwang, S.-J.; Levy, D.; Fitzpatrick, A.L.; et al. Association of Leukocyte Telomere Length With Mortality Among Adult Participants in 3 Longitudinal Studies. JAMA Netw. Open 2020, 3, e200023. [Google Scholar] [CrossRef] [Green Version]
- Martin-Ruiz, C.M.; Gussekloo, J.; van Heemst, D.; von Zglinicki, T.; Westendorp, R.G.J. Telomere Length in White Blood Cells Is Not Associated with Morbidity or Mortality in the Oldest Old: A Population-Based Study. Aging Cell 2005, 4, 287–290. [Google Scholar] [CrossRef]
- Bischoff, C.; Petersen, H.C.; Graakjaer, J.; Andersen-Ranberg, K.; Vaupel, J.W.; Bohr, V.A.; Kølvraa, S.; Christensen, K. No Association between Telomere Length and Survival among the Elderly and Oldest Old. Epidemiol. Camb. Mass 2006, 17, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Houben, J.M.J.; Giltay, E.J.; Rius-Ottenheim, N.; Hageman, G.J.; Kromhout, D. Telomere Length and Mortality in Elderly Men: The Zutphen Elderly Study. J. Gerontol. A. Biol. Sci. Med. Sci. 2011, 66, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Svensson, J.; Karlsson, M.K.; Ljunggren, Ö.; Tivesten, Å.; Mellström, D.; Movérare-Skrtic, S. Leukocyte Telomere Length Is Not Associated with Mortality in Older Men. Exp. Gerontol. 2014, 57, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Veeranki, S.P.; Magnussen, C.G.; Xi, B. Recommended Physical Activity and All Cause and Cause Specific Mortality in US Adults: Prospective Cohort Study. BMJ 2020, 370, m2031. [Google Scholar] [CrossRef] [PubMed]
- Mok, A.; Khaw, K.-T.; Luben, R.; Wareham, N.; Brage, S. Physical Activity Trajectories and Mortality: Population Based Cohort Study. BMJ 2019, 365, l2323. [Google Scholar] [CrossRef] [Green Version]
- Pahor, M.; Guralnik, J.M.; Ambrosius, W.T.; Blair, S.; Bonds, D.E.; Church, T.S.; Espeland, M.A.; Fielding, R.A.; Gill, T.M.; Groessl, E.J.; et al. Effect of Structured Physical Activity on Prevention of Major Mobility Disability in Older Adults: The LIFE Study Randomized Clinical Trial. JAMA 2014, 311, 2387–2396. [Google Scholar] [CrossRef]
- Savino, E.; Volpato, S.; Zuliani, G.; Guralnik, J.M. Assessment of Mobility Status and Risk of Mobility Disability in Older Persons. Curr. Pharm. Des. 2014, 20, 3099–3113. [Google Scholar] [CrossRef]
- Pavasini, R.; Guralnik, J.; Brown, J.C.; di Bari, M.; Cesari, M.; Landi, F.; Vaes, B.; Legrand, D.; Verghese, J.; Wang, C.; et al. Short Physical Performance Battery and All-Cause Mortality: Systematic Review and Meta-Analysis. BMC Med. 2016, 14, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Owens, I.P.F. Ecology and Evolution. Sex Differences in Mortality Rate. Science 2002, 297, 2008–2009. [Google Scholar] [CrossRef]
- Austad, S.N.; Fischer, K.E. Sex Differences in Lifespan. Cell Metab. 2016, 23, 1022–1033. [Google Scholar] [CrossRef] [Green Version]
- Radovanovic, D.; Seifert, B.; Urban, P.; Eberli, F.R.; Rickli, H.; Bertel, O.; Puhan, M.A.; Erne, P.; AMIS Plus Investigators. Validity of Charlson Comorbidity Index in Patients Hospitalised with Acute Coronary Syndrome. Insights from the Nationwide AMIS Plus Registry 2002–2012. Heart 2014, 100, 288–294. [Google Scholar] [CrossRef] [Green Version]
- Fraccaro, P.; Kontopantelis, E.; Sperrin, M.; Peek, N.; Mallen, C.; Urban, P.; Buchan, I.E.; Mamas, M.A. Predicting Mortality from Change-over-Time in the Charlson Comorbidity Index: A Retrospective Cohort Study in a Data-Intensive UK Health System. Medicine 2016, 95, e4973. [Google Scholar] [CrossRef]
- Ehrlenbach, S.; Willeit, P.; Kiechl, S.; Willeit, J.; Reindl, M.; Schanda, K.; Kronenberg, F.; Brandstätter, A. Influences on the Reduction of Relative Telomere Length over 10 Years in the Population-Based Bruneck Study: Introduction of a Well-Controlled High-Throughput Assay. Int. J. Epidemiol. 2009, 38, 1725–1734. [Google Scholar] [CrossRef] [Green Version]
- Crocco, P.; De Rango, F.; Dato, S.; Rose, G.; Passarino, G. Telomere Length as a Function of Age at Population Level Parallels Human Survival Curves. Aging 2021, 13, 204–218. [Google Scholar] [CrossRef]
- Barbi, E.; Lagona, F.; Marsili, M.; Vaupel, J.W.; Wachter, K.W. The Plateau of Human Mortality: Demography of Longevity Pioneers. Science 2018, 360, 1459–1461. [Google Scholar] [CrossRef] [Green Version]
- McCracken, J.; Baccarelli, A.; Hoxha, M.; Dioni, L.; Melly, S.; Coull, B.; Suh, H.; Vokonas, P.; Schwartz, J. Annual Ambient Black Carbon Associated with Shorter Telomeres in Elderly Men: Veterans Affairs Normative Aging Study. Environ. Health Perspect. 2010, 118, 1564–1570. [Google Scholar] [CrossRef] [Green Version]
- Verdun, R.E.; Karlseder, J. Replication and Protection of Telomeres. Nature 2007, 447, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.S.M.; Thomasini, R.L.; Lustosa, L.P.; Pereira, D.S.; Pereira, L.S.M.; Kassab, G.B.I.; Silva, T.J.; Guerra, R.O.; Parentoni, A.N. Is the Leukocyte Telomere Length Associated with Decreased Physical Functional Capacity in the Elderly? Rejuvenation Res. 2020, 23, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Sibille, K.T.; Chen, H.; Bartley, E.J.; Riley, J.; Glover, T.L.; King, C.D.; Zhang, H.; Cruz-Almeida, Y.; Goodin, B.R.; Sotolongo, A.; et al. Accelerated Aging in Adults with Knee Osteoarthritis Pain: Consideration for Frequency, Intensity, Time, and Total Pain Sites. Pain Rep. 2017, 2, e591. [Google Scholar] [CrossRef] [PubMed]
- Arsenis, N.C.; You, T.; Ogawa, E.F.; Tinsley, G.M.; Zuo, L. Physical Activity and Telomere Length: Impact of Aging and Potential Mechanisms of Action. Oncotarget 2017, 8, 45008–45019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werner, C.; Fürster, T.; Widmann, T.; Pöss, J.; Roggia, C.; Hanhoun, M.; Scharhag, J.; Büchner, N.; Meyer, T.; Kindermann, W.; et al. Physical Exercise Prevents Cellular Senescence in Circulating Leukocytes and in the Vessel Wall. Circulation 2009, 120, 2438–2447. [Google Scholar] [CrossRef]
- Puterman, E.; Lin, J.; Blackburn, E.; O’Donovan, A.; Adler, N.; Epel, E. The Power of Exercise: Buffering the Effect of Chronic Stress on Telomere Length. PLoS ONE 2010, 5, e10837. [Google Scholar] [CrossRef]
- Steptoe, A.; Hamer, M.; Butcher, L.; Lin, J.; Brydon, L.; Kivimäki, M.; Marmot, M.; Blackburn, E.; Erusalimsky, J.D. Educational Attainment but Not Measures of Current Socioeconomic Circumstances Are Associated with Leukocyte Telomere Length in Healthy Older Men and Women. Brain. Behav. Immun. 2011, 25, 1292–1298. [Google Scholar] [CrossRef]
- Surtees, P.G.; Wainwright, N.W.J.; Pooley, K.A.; Luben, R.N.; Khaw, K.-T.; Easton, D.F.; Dunning, A.M. Educational Attainment and Mean Leukocyte Telomere Length in Women in the European Prospective Investigation into Cancer (EPIC)-Norfolk Population Study. Brain. Behav. Immun. 2012, 26, 414–418. [Google Scholar] [CrossRef]
- Adler, N.; Pantell, M.S.; O’Donovan, A.; Blackburn, E.; Cawthon, R.; Koster, A.; Opresko, P.; Newman, A.; Harris, T.B.; Epel, E. Educational Attainment and Late Life Telomere Length in the Health, Aging and Body Composition Study. Brain. Behav. Immun. 2013, 27, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Gardner, M.; Bann, D.; Wiley, L.; Cooper, R.; Hardy, R.; Nitsch, D.; Martin-Ruiz, C.; Shiels, P.; Sayer, A.A.; Barbieri, M.; et al. Gender and Telomere Length: Systematic Review and Meta-Analysis. Exp. Gerontol. 2014, 51, 15–27. [Google Scholar] [CrossRef]
- Mayer, S.; Brüderlein, S.; Perner, S.; Waibel, I.; Holdenried, A.; Ciloglu, N.; Hasel, C.; Mattfeldt, T.; Nielsen, K.V.; Möller, P. Sex-Specific Telomere Length Profiles and Age-Dependent Erosion Dynamics of Individual Chromosome Arms in Humans. Cytogenet. Genome Res. 2006, 112, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, T.S.; Staessen, J.A.; Gardner, J.P.; Aviv, A. Telomere Length and Possible Link to X Chromosome. Lancet Lond. Engl. 2004, 363, 507–510. [Google Scholar] [CrossRef]
- Aviv, A. Telomeres, Sex, Reactive Oxygen Species, and Human Cardiovascular Aging. J. Mol. Med. Berl. Ger. 2002, 80, 689–695. [Google Scholar] [CrossRef]
- Aviv, A.; Shay, J.W. Reflections on Telomere Dynamics and Ageing-Related Diseases in Humans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20160436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Ezquerro, J.D.; Rodríguez-Castañeda, A.; Ortiz-Ramírez, M.; Sánchez-García, S.; Rosas-Vargas, H.; Sánchez-Arenas, R.; García-de la Torre, P. Oxidative Stress, Telomere Length, and Frailty in an Old Age Population. Rev. Investig. Clin. Organo Hosp. Enferm. Nutr. 2019, 71, 393–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sazzini, M.; Abondio, P.; Sarno, S.; Gnecchi-Ruscone, G.A.; Ragno, M.; Giuliani, C.; De Fanti, S.; Ojeda-Granados, C.; Boattini, A.; Marquis, J.; et al. Genomic History of the Italian Population Recapitulates Key Evolutionary Dynamics of Both Continental and Southern Europeans. BMC Biol. 2020, 18, 1–19. [Google Scholar] [CrossRef] [PubMed]



| Variable | Mean ± Standard Deviation (or Number (%)) | Number of Missing Data | |
|---|---|---|---|
| Demographic, physiological variables and lifestyles | |||
| Gender (n (%)) | Female | 331 (54.00%) | 0 |
| Male | 282 (46.00%) | ||
| Age (years) | 83.99 ± 8.06 | 0 | |
| Marital status (n (%)) | Unmarried | 60 (9.80%) | 1 |
| Married | 266 (43.46%) | ||
| Divorced | 5 (0.82%) | ||
| Widower | 281 (45.92) | ||
| Education (years) | 6.98 ± 4.82 | 3 | |
| Institutionalization (n (%)) | 86 (14.03%) | 0 | |
| Body Mass Index (BMI) (kg/m2) | 24.85 ± 4.11 | 50 | |
| Hypovisus (n (%)) | 207 (34.97%) | 21 | |
| Hearing loss (n (%)) | 210 (37.17%) | 48 | |
| Smoking (n (%)) | 247 (40.29%) | 0 | |
| Wine intake * (n (%)) | 363 (59.22%) | 0 | |
| Physical activity ** (n (%)) | 430 (70.15%) | 0 | |
| Biochemical measurements | |||
| Total cholesterol (mg/dL) | 214.4 ± 43.57 | 59 | |
| Low-density lipoprotein (LDL) cholesterol (mg/dL) | 135.93 ± 37.44 | 59 | |
| High-density lipoprotein (HDL) cholesterol (mg/dL) | 55.99 ± 14.84 | 50 | |
| Erythrocyte sedimentation rate (ESR) (mm/h) | 35.58 ± 21.44 | 50 | |
| Fibrinogen (mg/dL) | 349.24 ± 75.94 | 52 | |
| Albumin (g/dL) | 4.18 ± 0.36 | 59 | |
| Creatinine (mg/dL) | 1.06 ± 0.48 | 49 | |
| Hemoglobin (g/dL) | 13.64 ± 1.64 | 61 | |
| Platelets (n × 103/microL) | 237.3 ± 75.28 | 50 | |
| White cells (n × 103/microL) | 6.46 ± 1.7 | 50 | |
| Red cells (n × 106/microL) | 4.44 ± 0.53 | 50 | |
| High sensitivity C-reactive protein(hs-CRP) (mg/L) | 0.61 ± 1.43 | 60 | |
| Interleukin-6 (IL-6) (pg/mL) | 1.68 ± 2.09 | 94 | |
| Lg IL-6 | −0.07 ± 1.09 | 94 | |
| Insulin-like growth factor-1 (IGF-1) (ng/mL) | 178.74 ± 77.93 | 53 | |
| Assessment of chronic diseases and other covariates | |||
| Hypertension (n (%)) | 368 (60.03%) | 0 | |
| Sistolic blood pressure (mm/hg) | 144.88 ± 20.09 | 29 | |
| Diastolic blood pressure (mm/hg) | 81.22 ± 11.38 | 29 | |
| Disease Count Index (DCI) | 2.22 ± 1.62 | 0 | |
| Charlson Comorbidity Index (CCI) | 5.69 ± 2.01 | 0 | |
| Ischemic heart disease (n (%)) | 108 (17.62%) | 0 | |
| Heart failure (n (%)) | 50 (8.16%) | 0 | |
| Malignant neoplasm (n (%)) | 100 (16.31%) | 0 | |
| Diabetes (n (%)) | 101 (16.48%) | 0 | |
| Ictus (n (%)) | 59 (9.62%) | 0 | |
| Chronic obstructive pulmonary disease (n (%)) | 31 (5.06%) | 0 | |
| Mini Mental State Examination (MMSE) | 23.89 ± 6.65 | 0 | |
| Geriatric depression scale (GDS) | 3.86 ± 3.7 | 30 | |
| Physical performance and frailty measures | |||
| Frailty (n (%)) | Absent | 131 (21.37%) | 0 |
| Moderate | 247 (40.29%) | ||
| Severe | 235 (38.34%) | ||
| Activities of daily living (ADL) score | 5.03 ± 1.83 | 17 | |
| Instrumental activities of daily living (IADL)score | 5.28 ± 3 | 26 | |
| Short physical performance battery (SPPB) | 5.45 ± 3.78 | 0 | |
| Handgrip (kg) | 12.17 ± 9.79 | 1 | |
| Lift 2 kg (n (%)) *** | 394 (67.35%) | 28 | |
| Hands behind the head (able) (n (%)) | 506 (83.91%) | 10 | |
| Hands behind the shoulders (able) (n (%)) | 502 (83.25%) | 10 | |
| LTL (T/S) | 2.36 ± 1.14 | 41 | |
| APOE Genotyping | 89 (15.92%) | 54 | |
| Variables (Wave 2003) | LTL (T/S) | p-Value (Mann–Whitney Test or Kruskal–Wallis Test) | |
|---|---|---|---|
| Demographic, physiological variables and lifestyles | |||
| Gender | Male | 2.35 | 0.9729 |
| Female | 2.361 | ||
| Age (Medianyears) | <83 | 2.614 | <0.0001 |
| ≥83 | 2.109 | ||
| Marital status | Unmarried | 2.304 | 0.6236 |
| Married | 2.403 | ||
| Divorced/Separated | 2.716 | ||
| Widower | 2.308 | ||
| Education (years) | <5 | 2.37 | 0.8859 |
| ≥5 | 2.357 | ||
| Institutionalization | No | 2.41 | 0.0079 |
| Yes | 2.045 | ||
| Body Mass Index (BMI) (kg/m2) | Underweight (<18.5) | 1.993 | 0.2774 |
| Normal (18.5–24.9) | 2.307 | ||
| Overweight | 2.512 | ||
| (25.0–29.9) | |||
| Obese (30.0–39.8) | 2.364 | ||
| Ipovisus | No | 2.349 | 0.8921 |
| Yes | 2.385 | ||
| Hearing loss | No | 2.45 | 0.0002 |
| Yes | 2.174 | ||
| Smoking | No | 2.301 | 0.3118 |
| Yes | 2.434 | ||
| Wine intake | 0 | 2.342 | 0.6453 |
| 2 glasses/day | 2.351 | ||
| +2 glasses/day | 2.409 | ||
| Physical activity | Able (yes) | 2.457 | 0.0016 |
| Unable (no) | 2.13 | ||
| Biochemical measurements | |||
| Total cholesterol (mg/dL) | <214 | 2.355 | 0.8047 |
| ≥214 | 2.386 | ||
| Low-density lipoprotein (LDL) cholesterol (mg/dL) | <134 | 2.381 | 0.7521 |
| ≥134 | 2.36 | ||
| High-density lipoprotein (HDL) cholesterol (mg/dL) | <54 | 2.288 | 0.507 |
| ≥54 | 2.387 | ||
| Erythrocyte sedimentation rate (ESR) (mm/h) | <32 | 2.323 | 0.7566 |
| ≥32 | 2.36 | ||
| Fibrinogen (mg/dL) | <339 | 2.362 | 0.9624 |
| ≥339 | 2.329 | ||
| Albumin (g/dL) | <4.2 | 2.249 | 0.0236 |
| ≥4.2 | 2.457 | ||
| Creatinine (mg/dL) | <1 | 2.343 | 0.8339 |
| ≥1 | 2.341 | ||
| Hemoglobin (g/dL) | <13.8 | 2.292 | 0.0132 |
| ≥13.8 | 2.44 | ||
| Platelets (n × 103/microL) | <232 | 2.37 | 0.8637 |
| ≥232 | 2.314 | ||
| White cells (n × 103/microL) | <6.35 | 2.332 | 0.7527 |
| ≥6.35 | 2.351 | ||
| Red cells (n × 106/microL) | <4.49 | 2.289 | 0.1161 |
| ≥4.49 | 2.394 | ||
| High sensitivity C-reactive protein (hs-CRP) (mg/L) | <0.21 | 2.393 | 0.8004 |
| ≥0.21 | 2.351 | ||
| Interleukin-6 (IL-6) (pg/mL) | <0.9 | 2.395 | 0.4656 |
| ≥0.9 | 2.374 | ||
| Insulin-like growth factor-1 (IGF-1) (ng/mL) | <167 | 2.268 | 0.0423 |
| ≥167 | 2.442 | ||
| Assessment of chronic diseases and other covariates | |||
| Hypertension | No | 2.398 | 0.4552 |
| Yes | 2.329 | ||
| Systolic blood pressure (mm/hg) | <144.6 | 2.393 | 0.6309 |
| ≥144.6 | 2.346 | ||
| Diastolic blood pressure (mm/hg) | <80.5 | 2.337 | 0.3803 |
| ≥80.5 | 2.402 | ||
| DiseaseCount Index (DCI) | 0–2 | 2.415 | 0.0296 |
| 3–10 | 2.262 | ||
| CharlsonComorbidity Index (CCI) | 3–5 | 2.472 | 0.0091 |
| 6–14 | 2.25 | ||
| Ischemicheartdisease | No | 2.379 | 0.269 |
| Yes | 2.252 | ||
| Heartfailure | No | 2.373 | 0.4305 |
| Yes | 2.172 | ||
| Malignant neoplasm | No | 2.337 | 0.8135 |
| Yes | 2.455 | ||
| Diabetes | No | 2.328 | 0.3831 |
| Yes | 2.494 | ||
| Ictus | No | 2.372 | 0.3477 |
| Yes | 2.218 | ||
| Chronic obstructive pulmonary disease | No | 2.369 | 0.3647 |
| Yes | 2.113 | ||
| Mini Mental State Examination (MMSE) | ≤26 | 2.253 | 0.0119 |
| >26 | 2.454 | ||
| Geriatric depression scale (GDS) | ≤3 | 2.409 | 0.272 |
| >3 | 2.323 | ||
| Physical performance and frailty measures | |||
| Frailty | Absent | 2.526 | 0.0015 |
| Moderate | 2.455 | ||
| Severe | 2.173 | ||
| Activities of daily living (ADL) score | Able = 6 | 2.466 | 0.0014 |
| Unable ≤ 5 | 2.079 | ||
| Instrumental activities of daily living (IADL)score | Able = 8 | 2.611. | <0.0001 |
| Unable ≤ 8 | 2.2 | ||
| Short physical performance battery (SPPB) | <6 | 2.24 | 0.014 |
| ≥6 | 2.466 | ||
| Handgrip (kg) | <12.2 | 2.21 | 0.0013 |
| ≥12.2 | 2.524 | ||
| Lift 2 Kg | Able | 2.462 | 0.0094 |
| Unable | 2.216 | ||
| Hands behind the head | Able | 2.398 | 0.0727 |
| Unable | 2.158 | ||
| Hands behind the shoulders | Able | 2.402 | 0.0477 |
| Unable | 2.149 | ||
| APOE Genotyping | ApoE-4 No | 2.359 | 0.5314 |
| ApoE-4Yes | 2.277 | ||
| Estimates | Standard Error | 95% Confidence Interval | T Value | p-Value | |
|---|---|---|---|---|---|
| Intercecpt | 6.9129 | 0.3602 | 6.2068; 7.6189 | 19.1891 | <0.001 |
| Age | −0.0796 | 0.0043 | −0.0881; 0.0711 | −18.4265 | <0.001 |
| Estimates | Standard Error | Hazard Ratio | 95% Confidence Interval Limits | Z Value | p-Value | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Age | 0.0746 | 0.0077 | 1.0775 | 1.0613 | 1.0938 | 9.6721 | <0.001 |
| LTL | −0.1862 | 0.0486 | 0.8301 | 0.7547 | 0.9131 | −3.8285 | <0.001 |
| Male | 0.549 | 0.0939 | 1.7316 | 1.4405 | 2.0814 | 5.8469 | <0.001 |
| CCI | 0.0921 | 0.0259 | 1.0965 | 1.0422 | 1.1536 | 3.5549 | <0.001 |
| SPPB | −0.1055 | 0.0155 | 0.8999 | 0.8729 | 0.9276 | −6.7971 | <0.001 |
| Estimates | Standard Error | Hazard Ratio | 95% Confidence Interval Limits | Z Value | p-Value | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Age | 0.0816 | 0.0084 | 1.085 | 1.0673 | 1.1030 | 9.7344 | <0.001 |
| LTL | −0.2538 | 0.0519 | 0.7759 | 0.7008 | 0.8589 | −4.886 | <0.001 |
| Male | 0.5595 | 0.1044 | 1.7499 | 1.4260 | 2.1471 | 5.3614 | <0.001 |
| CCI | 0.1533 | 0.0284 | 1.1657 | 1.1026 | 1.2324 | 5.401 | <0.001 |
| SPPB | −0.0919 | 0.0165 | 0.9122 | 0.8832 | 0.9422 | −5.5876 | <0.001 |
| Estimates | Standard Error | Odd Ratio | 95% Confidence Interval Limits | Z Value | p-Value | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Intercept | −2.4259 | 0.5663 | 0.0884 | 0.0291 | 0.2682 | −4.2834 | <0.0001 |
| Male | −0.8237 | 0.3363 | 0.4388 | 0.2270 | 0.8483 | −2.4495 | 0.0143 |
| SPPB | 0.1434 | 0.0528 | 1.1542 | 1.0407 | 1.2800 | 2.7172 | 0.0066 |
| Education | 0.0915 | 0.0336 | 1.0958 | 1.0260 | 1.1704 | 2.7237 | 0.0065 |
| DCI | −0.3696 | 0.1361 | 0.6912 | 0.5292 | 0.9023 | −2.7151 | 0.0066 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavanello, S.; Campisi, M.; Grassi, A.; Mastrangelo, G.; Durante, E.; Veronesi, A.; Gallucci, M. Longer Leukocytes Telomere Length Predicts a Significant Survival Advantage in the Elderly TRELONG Cohort, with Short Physical Performance Battery Score and Years of Education as Main Determinants for Telomere Elongation. J. Clin. Med. 2021, 10, 3700. https://doi.org/10.3390/jcm10163700
Pavanello S, Campisi M, Grassi A, Mastrangelo G, Durante E, Veronesi A, Gallucci M. Longer Leukocytes Telomere Length Predicts a Significant Survival Advantage in the Elderly TRELONG Cohort, with Short Physical Performance Battery Score and Years of Education as Main Determinants for Telomere Elongation. Journal of Clinical Medicine. 2021; 10(16):3700. https://doi.org/10.3390/jcm10163700
Chicago/Turabian StylePavanello, Sofia, Manuela Campisi, Alberto Grassi, Giuseppe Mastrangelo, Elisabetta Durante, Arianna Veronesi, and Maurizio Gallucci. 2021. "Longer Leukocytes Telomere Length Predicts a Significant Survival Advantage in the Elderly TRELONG Cohort, with Short Physical Performance Battery Score and Years of Education as Main Determinants for Telomere Elongation" Journal of Clinical Medicine 10, no. 16: 3700. https://doi.org/10.3390/jcm10163700
APA StylePavanello, S., Campisi, M., Grassi, A., Mastrangelo, G., Durante, E., Veronesi, A., & Gallucci, M. (2021). Longer Leukocytes Telomere Length Predicts a Significant Survival Advantage in the Elderly TRELONG Cohort, with Short Physical Performance Battery Score and Years of Education as Main Determinants for Telomere Elongation. Journal of Clinical Medicine, 10(16), 3700. https://doi.org/10.3390/jcm10163700







