Incidence of Severe Hypothermia and Its Impact on Postoperative Surgical Complications and Time Delay to Adjunct Treatments in Breast Surgery Cancer Patients: A Case-Controlled Study
Abstract
:1. Introduction
2. Methods
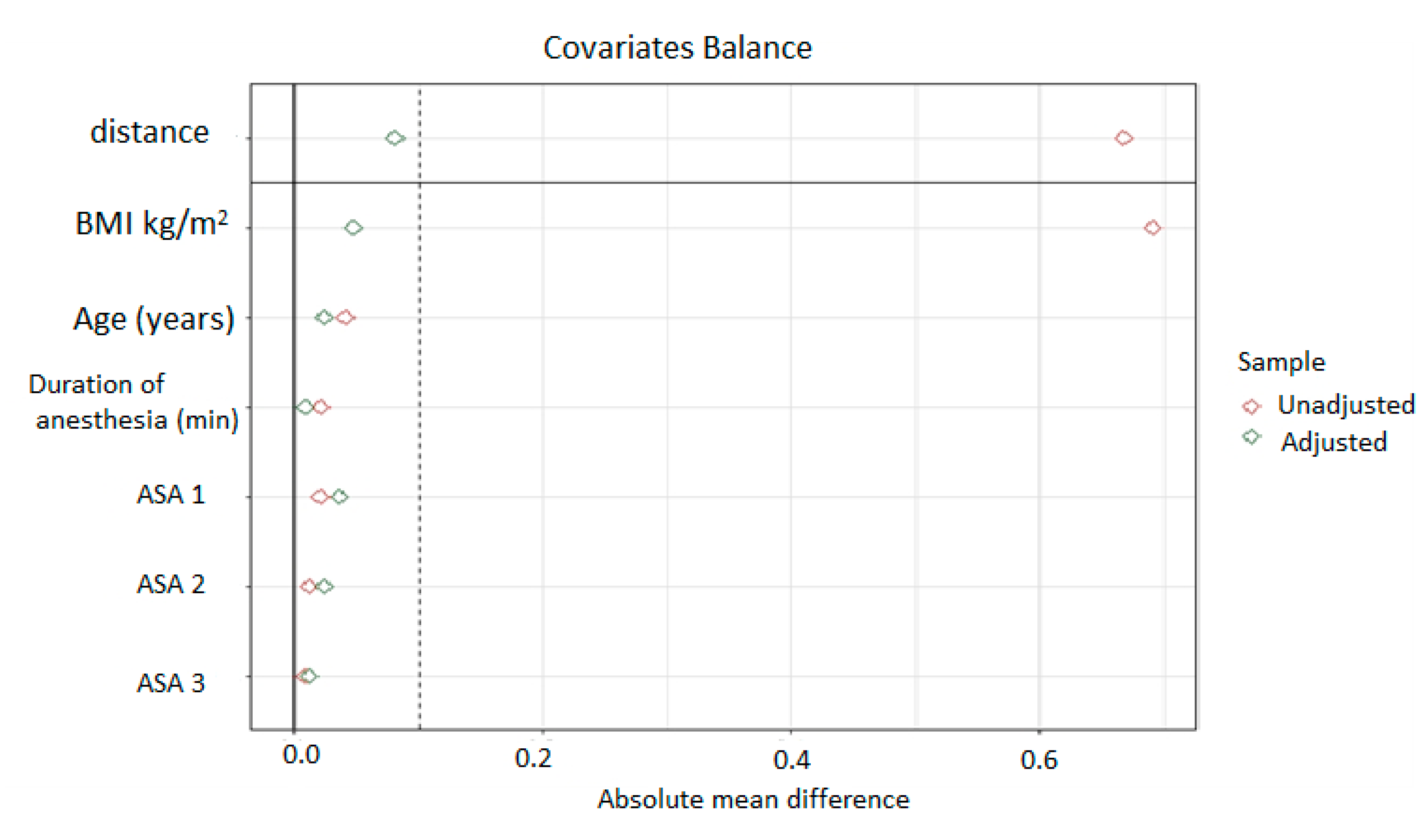
3. Statistical Analysis
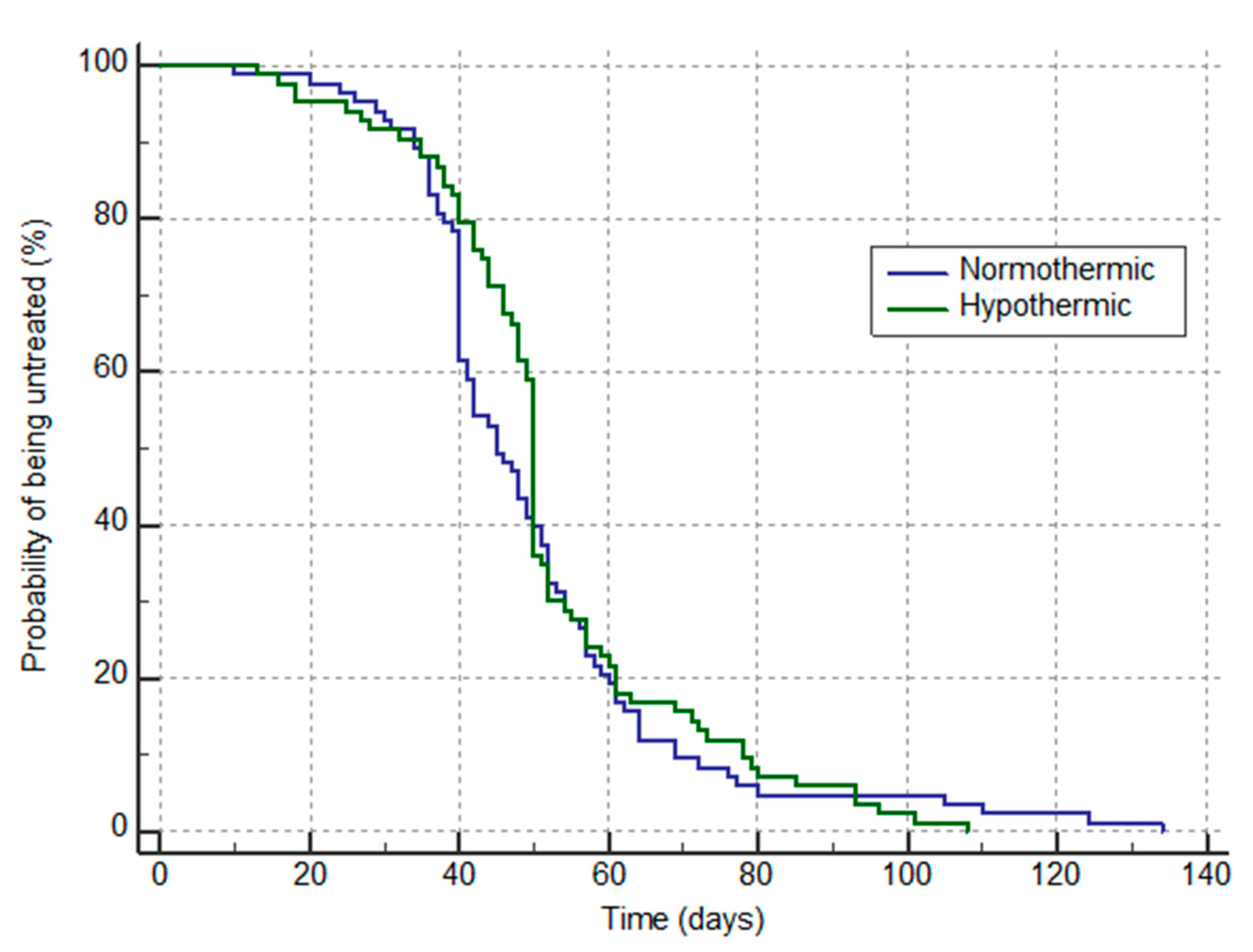
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frank, S.M.; Fleisher, L.A.; Breslow, M.J.; Higgins, M.S.; Olson, K.F.; Kelly, S.; Beattie, C. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events. A randomized clinical trial. JAMA 1997, 277, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Schmied, H.; Kurz, A.; Sessler, D.I.; Kozek, S.; Reiter, A. Mild hypothermia increases blood loss and transfusion requirements during total hip arthroplasty. Lancet 1996, 347, 289–292. [Google Scholar] [CrossRef]
- Kurz, A.; Sessler, D.I.; Christensen, R.; Clough, D.; Plattner, O.; Xiong, J. Thermoregulatory vasoconstriction and perianesthetic heat transfer. Acta Anaesthesiol. Scand. Suppl. 1996, 109, 30–33. [Google Scholar]
- Zheng, X.Q.; Huang, J.F.; Lin, J.L.; Chen, D.; Wu, A.M. Effects of preoperative warming on the occurrence of surgical site infection: A systematic review and meta-analysis. Int. J. Surg. 2020, 77, 40–47. [Google Scholar] [CrossRef]
- Becerra, A.; Valencia, L.; Ferrando, C.; Villar, J.; Rodriguez-Perez, A. Prospective observational study of the effectiveness of prewarming on perioperative hypothermia in surgical patients submitted to spinal anesthesia. Sci Rep. 2019, 9, 16477. [Google Scholar] [CrossRef]
- Becerra, A.; Valencia, L.; Villar, J.; Rodriguez-Perez, A. Short-periods of pre-warming in laparoscopic surgery. A non-randomized clinical trial evaluating current clinical practice. J. Clin. Med. 2021, 10, 1047. [Google Scholar] [CrossRef]
- Motamed, C.; Bourgain, J.L. Incidence and Distribution of Inadvertent Severe Intraoperative Hypothermia During Cancer Surgery: A Retrospective Single Center Study. Thrita 2014, 3, e14744. [Google Scholar] [CrossRef]
- Motamed, C.; Bourgain, J.L. An anaesthesia information management system as a tool for a quality assurance program: 10 Years of experience. Anaesth. Crit. Care Pain Med. 2016, 35, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Motamed, C.; Bourgain, J.L. Impact of a quality assurance program on the use of neuromuscular monitoring and reversal of muscle relaxants. Ann. Fr. Anesth. Reanim. 2009, 28, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Sessler, D.I. Temperature monitoring and perioperative thermoregulation. Anesthesiology 2008, 109, 318–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karalapillai, D.; Story, D.; Hart, G.K.; Bailey, M.; Pilcher, D.; Schneider, A.; Kaufman, M.; Cooper, D.J.; Bellomo, R. Postoperative hypothermia and patient outcomes after major elective non-cardiac surgery. Anaesthesia 2013, 68, 605–611. [Google Scholar] [CrossRef] [Green Version]
- Kurz, A.; Sessler, D.I.; Lenhardt, R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N. Engl. J. Med. 1996, 334, 1209–1215. [Google Scholar] [CrossRef]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef] [Green Version]
- Austin, P.C. Using the Standardized Difference to Compare the Prevalence of a Binary Variable Between Two Groups in Observational Research. Commun. Stat. Simul. Comput. 2009, 38, 1228–1234. [Google Scholar] [CrossRef]
- Alfonsi, P.; Bekka, S.; Aegerter, P.; SFAR Research Network Investigators. Prevalence of hypothermia on admission to recovery room remains high despite a large use of forced-air warming devices: Findings of a non-randomized observational multicenter and pragmatic study on perioperative hypothermia prevalence in France. PLoS ONE 2019, 14, e0226038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groene, P.; Zeuzem, C.; Baasner, S.; Hofmann-Kiefer, K. The influence of body mass index on temperature management during general anaesthesia-A prospective observational study. J. Eval. Clin. Pract. 2019, 25, 340–345. [Google Scholar] [CrossRef]
- Siddiqiui, T.; Pal, K.M.I.; Shaukat, F.; Mubashir, H.; Ali, A.A.; Malik, M.J.A.; Shahzad, N. Association between perioperative hypothermia and surgical site infection after elective abdominal surgery: A prospective cohort study. Cureus 2020, 12, e11145. [Google Scholar] [CrossRef]
- Beloeil, H.; Nouette-Gaulain, K. The perioperative period in cancer surgery: A critical moment! Is there a role for regional anesthesia in preventing cancer recurrence? Ann. Fr. Anesth. Reanim. 2012, 31, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Hanna, T.P.; Kingm, W.D.; Thibodeau, S.; Jalink, M.; Paulin, G.A.; Harvey-Jones, E.; O’Sullivan, D.E.; Booth, C.M.; Sullivan, R.; Aggarwal, A. Mortality due to cancer treatment delay: Systematic review and meta-analysis. BMJ. 2020, 371, m4087. [Google Scholar] [CrossRef]
- Trufelli, D.C.; Matos, L.L.; Santi, P.X.; Del Giglio, A. Adjuvant treatment delay in breast cancer patients. Rev. Assoc. Med. Bras. 2015, 61, 411–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Barbera, L.; Brouwers, M.; Browman, G.; Mackillop, W.J. Does delay in starting treatment affect the outcomes of radiotherapy? A systematic review. J. Clin. Oncol. 2003, 21, 555–563. [Google Scholar] [CrossRef]
- Cefaro, G.A.; Genovesi, D.; Marchese, R.; Di Tommaso, M.; Di Febo, F.; Ballone, E.; Di Nicola, M. The effect of delaying adjuvant radiation treatment after conservative surgery for early breast cancer. Breast J. 2007, 13, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Emmert, A.; Gries, G.; Wand, S.; Buentzel, J.; Brauer, A.; Quintel, M.; Brandes, I.F. Association between perioperative hypothermia and patient outcomes after thoracic surgery: A single center retrospective analysis. Medicine 2018, 97, e0528. [Google Scholar] [CrossRef] [PubMed]
- Camus, Y.; Delva, E.; Sessler, D.I.; Lienhart, A. Pre-induction skin-surface warming minimizes intraoperative core hypothermia. J. Clin. Anesth. 1995, 7, 384–388. [Google Scholar] [CrossRef]
- Torossian, A.; Brauer, A.; Hocker, J.; Bein, B.; Wulf, H.; Horn, E.P. Preventing inadvertent perioperative hypothermia. Dtsch. Arztebl. Int. 2015, 112, 166–172. [Google Scholar] [CrossRef] [Green Version]
- Calvo Vecino, J.M.; Casans Frances, R.; Ripolles Melchor, J.; Marin Zaldivar, C.; Gomez Rios, M.A.; Perez Ferrer, A.; Zaballos Bustingorri, J.M.; Abad Gurumeta, A.; Grupo de trabajo de la GPC de Hipotermia Perioperatoria No Intencionada de la SEDAR. Clinical practice guideline. Unintentional perioperative hypothermia. Rev. Esp. Anestesiol. Reanim. (Engl. Ed.) 2018, 65, 564–588. [Google Scholar] [CrossRef] [PubMed]
- Camus, Y.; Delva, E.; Bossard, A.E.; Chandon, M.; Lienhart, A. Prevention of hypothermia by cutaneous warming with new electric blankets during abdominal surgery. Br. J. Anaesth. 1997, 79, 796–797. [Google Scholar] [CrossRef]
| Group Control n = 80 | Group Hypothermia n = 80 | p Value | |
|---|---|---|---|
| Age (year), mean (SD) | 58.95 (11.56) | 59.25 (12.87) | 0.87 |
| Sex ratio (F/M) % | 83/17 | 82/18 | 1 |
| BMI (kg/m2), mean ± SD | 24.34 ± 4.01 | 21.4 ± 6.1 | 0.756 |
| ASA (1/2/3/) (n) | 16/60/7 | 13/62/8 | 0.81 |
| Tumorectomy +/− Lymph nodes (n) | 29 | 27 | |
| Mastectomy +/− lymph nodes resection (excluding axillary) (n) | 19 | 20 | |
| Axillary nodes resection/tumorectomy redux (n) | 20 | 18 | |
| Tumor resection + axillary nodes + oncoplasty/Mastectomy and axillary nodes (n) | 15 | 18 | 0.923 |
| Intraoperative temperature monitoring (n) | 23 (27.7) | 11 (13.3) | 0.03 |
| Active warming (n) | 27 (32.5) | 11 (13.2) | 0.006 |
| Simple blanket (n) Duration of surgery (mean SD) | 7 (9) 135(58) | 7 (8) 134(57) | 1 0.9 |
| Temperature at arrival PACU (mean SD) | 36.81 (0.29) | 35.01 (0.2) | <0.001 |
| Duration of PACU min mean (SD) | 89 (31) | 91 (41) | 0.7 |
| Group Control n = 80 | Group Hypothermia n = 80 | Risk | p Value | |
|---|---|---|---|---|
| Infection (n; %) | 6; 7.5 | 7; 8 | 1.16 (0.41–3.31) | 0.77 |
| Cutaneous Necrosis (n; %) | 0; 0 | 4; 4 | 9 (0.49–164) | 0.14 |
| Hemorrhage (n; %) | 7; 6.6 | 8; 10 | 1.14 (0.43–3) | 0.78 |
| Lymphocele (n; %) | 20; 25 | 18; 22 | 0.9 (0.51–1.56) | 0.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motamed, C.; Weil, G.; Dridi, C.; Bourgain, J.L. Incidence of Severe Hypothermia and Its Impact on Postoperative Surgical Complications and Time Delay to Adjunct Treatments in Breast Surgery Cancer Patients: A Case-Controlled Study. J. Clin. Med. 2021, 10, 3702. https://doi.org/10.3390/jcm10163702
Motamed C, Weil G, Dridi C, Bourgain JL. Incidence of Severe Hypothermia and Its Impact on Postoperative Surgical Complications and Time Delay to Adjunct Treatments in Breast Surgery Cancer Patients: A Case-Controlled Study. Journal of Clinical Medicine. 2021; 10(16):3702. https://doi.org/10.3390/jcm10163702
Chicago/Turabian StyleMotamed, Cyrus, Gregoire Weil, Chaima Dridi, and Jean Louis Bourgain. 2021. "Incidence of Severe Hypothermia and Its Impact on Postoperative Surgical Complications and Time Delay to Adjunct Treatments in Breast Surgery Cancer Patients: A Case-Controlled Study" Journal of Clinical Medicine 10, no. 16: 3702. https://doi.org/10.3390/jcm10163702
APA StyleMotamed, C., Weil, G., Dridi, C., & Bourgain, J. L. (2021). Incidence of Severe Hypothermia and Its Impact on Postoperative Surgical Complications and Time Delay to Adjunct Treatments in Breast Surgery Cancer Patients: A Case-Controlled Study. Journal of Clinical Medicine, 10(16), 3702. https://doi.org/10.3390/jcm10163702






