Echocardiographic Evaluation of LV Function in Patients with Tachyarrhythmia and Reduced Left Ventricular Function in Response to Rhythm Restoration
Abstract
:1. Introduction
2. Methods
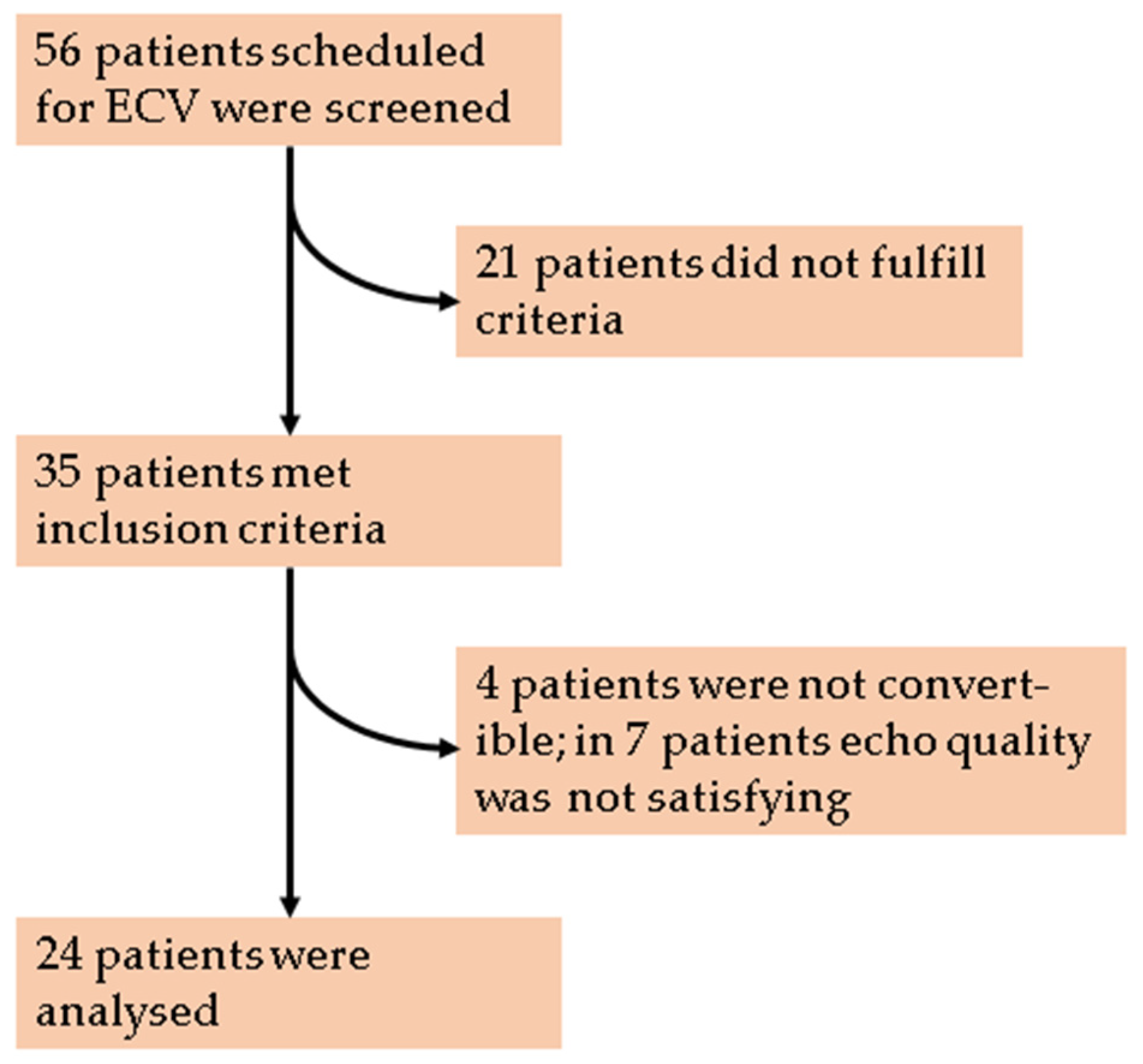
2.1. Study Population
2.2. Echocardiography and Data Analysis
2.3. Electrical Cardioversion and Anesthesia
2.4. Statistical Analysis
3. Results
3.1. Study Population and Baseline Characteristics
3.2. LVEF Correlates with GLS Pre and Post Electrical Cardioversion
3.3. Acute Effects of ECV on Geometric and Functional Parameters
3.4. Delta HR-Associated Increase of Ejection Fraction vs. Contractility
4. Discussion
5. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cha, Y.M.; Redfield, M.M.; Shen, W.K.; Gersh, B.J. Atrial fibrillation and ventricular dysfunction: A vicious electromechanical cycle. Circulation 2004, 109, 2839–2843. [Google Scholar] [CrossRef] [Green Version]
- Nattel, S.; Burstein, B.; Dobrev, D. Atrial remodeling and atrial fibrillation: Mechanisms and implications. Circ. Arrhythm. Electrophysiol. 2008, 1, 62–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirchhof, P.; Camm, A.J.; Goette, A.; Brandes, A.; Eckardt, L.; Elvan, A.; Fetsch, T.; van Gelder, I.C.; Haase, D.; Haegeli, L.M.; et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N. Engl. J. Med. 2020, 383, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Redfield, M.M.; Kay, G.N.; Jenkins, L.S.; Mianulli, M.; Jensen, D.N.; Ellenbogen, K.A. Tachycardia-related cardiomyopathy: A common cause of ventricular dysfunction in patients with atrial fibrillation referred for atrioventricular ablation. Mayo Clin. Proc. 2000, 75, 790–795. [Google Scholar] [CrossRef]
- Prabhu, S.; Taylor, A.J.; Costello, B.T.; Kaye, D.M.; McLellan, A.J.A.; Voskoboinik, A.; Sugumar, H.; Lockwood, S.M.; Stokes, M.B.; Pathik, B.; et al. Catheter Ablation Versus Medical Rate Control in Atrial Fibrillation and Systolic Dysfunction: The CAMERA-MRI Study. J. Am. Coll. Cardiol. 2017, 70, 1949–1961. [Google Scholar] [CrossRef] [PubMed]
- Marrouche, N.F.; Brachmann, J.; Andresen, D.; Siebels, J.; Boersma, L.; Jordaens, L.; Merkely, B.; Pokushalov, E.; Sanders, P.; Proff, J.; et al. Catheter Ablation for Atrial Fibrillation with Heart Failure. N. Engl. J. Med. 2018, 378, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Muller-Edenborn, B.; Minners, J.; Allgeier, J.; Burkhardt, T.; Lehrmann, H.; Ruile, P.; Merz, S.; Allgeier, M.; Neumann, F.J.; Arentz, T.; et al. Rapid improvement in left ventricular function after sinus rhythm restoration in patients with idiopathic cardiomyopathy and atrial fibrillation. Europace 2019, 21, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, K.; Daniak, S.M.; Salomone, K.; Kiely, T.; Patel, U.; Converso, K.; Manning, W.J.; Silverman, D.I. Effect of cardioversion of atrial fibrillation on improvement in left ventricular performance. Am. J. Cardiol. 2001, 88, 439–441. [Google Scholar] [CrossRef]
- Raymond, R.J.; Lee, A.J.; Messineo, F.C.; Manning, W.J.; Silverman, D.I. Cardiac performance early after cardioversion from atrial fibrillation. Am. Heart J. 1998, 136, 435–442. [Google Scholar] [CrossRef]
- Amundsen, B.H.; Helle-Valle, T.; Edvardsen, T.; Torp, H.; Crosby, J.; Lyseggen, E.; Stoylen, A.; Ihlen, H.; Lima, J.A.; Smiseth, O.A.; et al. Noninvasive myocardial strain measurement by speckle tracking echocardiography: Validation against sonomicrometry and tagged magnetic resonance imaging. J. Am. Coll. Cardiol. 2006, 47, 789–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korinek, J.; Kjaergaard, J.; Sengupta, P.P.; Yoshifuku, S.; McMahon, E.M.; Cha, S.S.; Khandheria, B.K.; Belohlavek, M. High spatial resolution speckle tracking improves accuracy of 2-dimensional strain measurements: An update on a new method in functional echocardiography. J. Am. Soc. Echocardiogr. 2007, 20, 165–170. [Google Scholar] [CrossRef]
- Onishi, T.; Saha, S.K.; Delgado-Montero, A.; Ludwig, D.R.; Onishi, T.; Schelbert, E.B.; Schwartzman, D.; Gorcsan, J., 3rd. Global longitudinal strain and global circumferential strain by speckle-tracking echocardiography and feature-tracking cardiac magnetic resonance imaging: Comparison with left ventricular ejection fraction. J. Am. Soc. Echocardiogr. 2015, 28, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Dogdus, M.; Yenercag, M.; Akhan, O.; Gok, G. Assessment of left atrial mechanics and left ventricular functions using 3D speckle-tracking echocardiography in patients with inappropriate sinus tachycardia. Int. J. Cardiovasc. Imaging 2020, 36, 1489–1495. [Google Scholar] [CrossRef] [PubMed]
- Modin, D.; Sengelov, M.; Jorgensen, P.G.; Bruun, N.E.; Olsen, F.J.; Dons, M.; Fritz Hansen, T.; Jensen, J.S.; Biering-Sorensen, T. Global longitudinal strain corrected by RR interval is a superior predictor of all-cause mortality in patients with systolic heart failure and atrial fibrillation. ESC Heart Fail. 2018, 5, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Lin, T.H.; Hsu, P.C.; Chu, C.Y.; Lee, W.H.; Su, H.M.; Voon, W.C.; Lai, W.T.; Sheu, S.H. Measuring left ventricular peak longitudinal systolic strain from a single beat in atrial fibrillation: Validation of the index beat method. J. Am. Soc. Echocardiogr. 2012, 25, 945–952. [Google Scholar] [CrossRef]
- Olsen, F.J.; Jørgensen, P.G.; Dons, M.; Svendsen, J.H.; Køber, L.; Jensen, J.S.; Biering-Sørensen, T. Echocardiographic quantification of systolic function during atrial fibrillation: Probing the ‘ten heart cycles’ rule. Future Cardiol. 2016, 12, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.S.; Lauridsen, K.G.; Torp, P.; Bach, L.F.; Rickers, H.; Lofgren, B. Maximum-fixed energy shocks for cardioverting atrial fibrillation. Eur. Heart J. 2020, 41, 626–631. [Google Scholar] [CrossRef]
- Kirchhof, P.; Eckardt, L.; Loh, P.; Weber, K.; Fischer, R.J.; Seidl, K.H.; Bocker, D.; Breithardt, G.; Haverkamp, W.; Borggrefe, M. Anterior-posterior versus anterior-lateral electrode positions for external cardioversion of atrial fibrillation: A randomised trial. Lancet 2002, 360, 1275–1279. [Google Scholar] [CrossRef]
- Hsu, L.F.; Jais, P.; Sanders, P.; Garrigue, S.; Hocini, M.; Sacher, F.; Takahashi, Y.; Rotter, M.; Pasquie, J.L.; Scavee, C.; et al. Catheter ablation for atrial fibrillation in congestive heart failure. N. Engl. J. Med. 2004, 351, 2373–2383. [Google Scholar] [CrossRef]
- Eiringhaus, J.; Hamer, O.W.; Hollemann, D.; Brochhausen, C.; Vollmann, D.; Maier, L.S.; Sossalla, S. The detrimental potential of arrhythmia-induced cardiomyopathy. ESC Heart Fail. 2018, 5, 960–964. [Google Scholar] [CrossRef]
- Watanabe, H.; Okamura, K.; Chinushi, M.; Furushima, H.; Tanabe, Y.; Kodama, M.; Aizawa, Y. Clinical characteristics, treatment, and outcome of tachycardia induced cardiomyopathy. Int. Heart J. 2008, 49, 39–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gosselink, A.T.; Blanksma, P.K.; Crijns, H.J.; Van Gelder, I.C.; de Kam, P.J.; Hillege, H.L.; Niemeijer, M.G.; Lie, K.I.; Meijler, F.L. Left ventricular beat-to-beat performance in atrial fibrillation: Contribution of Frank-Starling mechanism after short rather than long RR intervals. J. Am. Coll. Cardiol. 1995, 26, 1516–1521. [Google Scholar] [CrossRef] [Green Version]
- Luis, S.A.; Yamada, A.; Khandheria, B.K.; Speranza, V.; Benjamin, A.; Ischenko, M.; Platts, D.G.; Hamilton-Craig, C.R.; Haseler, L.; Burstow, D.; et al. Use of three-dimensional speckle-tracking echocardiography for quantitative assessment of global left ventricular function: A comparative study to three-dimensional echocardiography. J. Am. Soc. Echocardiogr. 2014, 27, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Konstam, M.A.; Abboud, F.M. Ejection Fraction: Misunderstood and Overrated (Changing the Paradigm in Categorizing Heart Failure). Circulation 2017, 135, 717–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marwick, T.H. Measurement of strain and strain rate by echocardiography: Ready for prime time? J. Am. Coll. Cardiol. 2006, 47, 1313–1327. [Google Scholar] [CrossRef] [Green Version]
- Weidemann, F.; Jamal, F.; Sutherland, G.R.; Claus, P.; Kowalski, M.; Hatle, L.; De Scheerder, I.; Bijnens, B.; Rademakers, F.E. Myocardial function defined by strain rate and strain during alterations in inotropic states and heart rate. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H792–H799. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, A.J.; Bossard, M.; Aeschbacher, S.; Schoen, T.; Voellmin, G.; Suter, Y.; Lehmann, A.; Hochgruber, T.; Pumpol, K.; Sticherling, C.; et al. Effects of sinus rhythm maintenance on left heart function after electrical cardioversion of atrial fibrillation: Implications for tachycardia-induced cardiomyopathy. Can. J. Cardiol. 2015, 31, 36–43. [Google Scholar] [CrossRef]




| Clinical Characteristic | n = 24 |
|---|---|
| Age | 65.6 (9.0) |
| Gender, male | 17 (70.8) |
| Weight (kg) | 86.5 (18.6) |
| Height (cm) | 171.0 (8.9) |
| BMI (kg/m2) | 29.4 (5.3) |
| BSA (m2) | 2.0 (0.3) |
| Diabetes | 6 (25.0) |
| Arterial hypertension | 17 (70.8) |
| Renal insufficiency | 5 (20.8) |
| CHA2DS2-VASc-Score | 3.1 (1.4) |
| Smoker status | 6 (25.0) |
| Hyperlipidemia | 13 (54.2) |
| NYHA class | 2.6 (0.7) |
| Coronary artery disease (%) | 11 (45.8) |
| Myocardial revascularisation by CABG | 3 (12.5) |
| Aortic valve replacement | 2 (8.3) |
| Betablocker | 15 (62.5) |
| ACEI | 14 (58.3) |
| Mineralocorticoid antagonist | 4 (16.7) |
| Calcium antagonist | 12 (50.0) |
| Diuretics | 11 (45.8) |
| Digoxin | 1 (4.2) |
| Amiodarone | 2 (8.3) |
| OAC | 18 (75.0) |
| Echocardiographic Parameter (n = 24) | Pre | Post | p-Value |
|---|---|---|---|
| HR (1/min) | 117.4 (21.6) | 76.9 (11.5) | <0.001 |
| R-R Interval (ms) | 531 (120) | 796 (110) | <0.001 |
| LVEF, Simpson (%) | 32.5 (9.1) | 37.9 (9.8) | <0.001 |
| LVEF, 3D (%) | 31.2 (8.2) | 37.1 (10.3) | <0.001 |
| LVEF, Teichholz (%) | 30.1 (9.1) | 36.6 (10.7) | 0.004 |
| LVEDD (mm) | 50.7 (8.0) | 52.6 (7.2) | <0.001 |
| LVESD (mm) | 43.5 (7.9) | 43.2 (6.8) | 0.698 |
| LVEDV (mL) | 143.9 (49.4) | 154.3 (48.1) | <0.001 |
| LVESV (mL) | 101.1 (42.5) | 99.4 (41.5) | 0.127 |
| LVEDVi (mL/m2) | 70.5 (21.2) | 75.8 (20.3) | <0.001 |
| LVESDVi (mL/m2) | 49.3 (18.0) | 48.6 (17.8) | 0.220 |
| LA, area (cm2) | 27.3 (3.4) | 28.1 (4.4) | 0.215 |
| LAVi (mL/m2) | 51.2 (10.2) | 52.9 (12.2) | 0.181 |
| GLS (%) | −8.9 (3.8) | −10.5 (3.9) | <0.001 |
| GLSc (%/s(1/2)) | −12.5 (5.5) | −11.9 (4.5) | 0.057 |
| SR (%) | −0.62 (0.19) | −0.67 (0.19) | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schach, C.; Körtl, T.; Wachter, R.; Maier, L.S.; Sossalla, S. Echocardiographic Evaluation of LV Function in Patients with Tachyarrhythmia and Reduced Left Ventricular Function in Response to Rhythm Restoration. J. Clin. Med. 2021, 10, 3706. https://doi.org/10.3390/jcm10163706
Schach C, Körtl T, Wachter R, Maier LS, Sossalla S. Echocardiographic Evaluation of LV Function in Patients with Tachyarrhythmia and Reduced Left Ventricular Function in Response to Rhythm Restoration. Journal of Clinical Medicine. 2021; 10(16):3706. https://doi.org/10.3390/jcm10163706
Chicago/Turabian StyleSchach, Christian, Thomas Körtl, Rolf Wachter, Lars S. Maier, and Samuel Sossalla. 2021. "Echocardiographic Evaluation of LV Function in Patients with Tachyarrhythmia and Reduced Left Ventricular Function in Response to Rhythm Restoration" Journal of Clinical Medicine 10, no. 16: 3706. https://doi.org/10.3390/jcm10163706
APA StyleSchach, C., Körtl, T., Wachter, R., Maier, L. S., & Sossalla, S. (2021). Echocardiographic Evaluation of LV Function in Patients with Tachyarrhythmia and Reduced Left Ventricular Function in Response to Rhythm Restoration. Journal of Clinical Medicine, 10(16), 3706. https://doi.org/10.3390/jcm10163706







