The Defect in Regulatory T Cells in Psoriasis and Therapeutic Approaches
Abstract
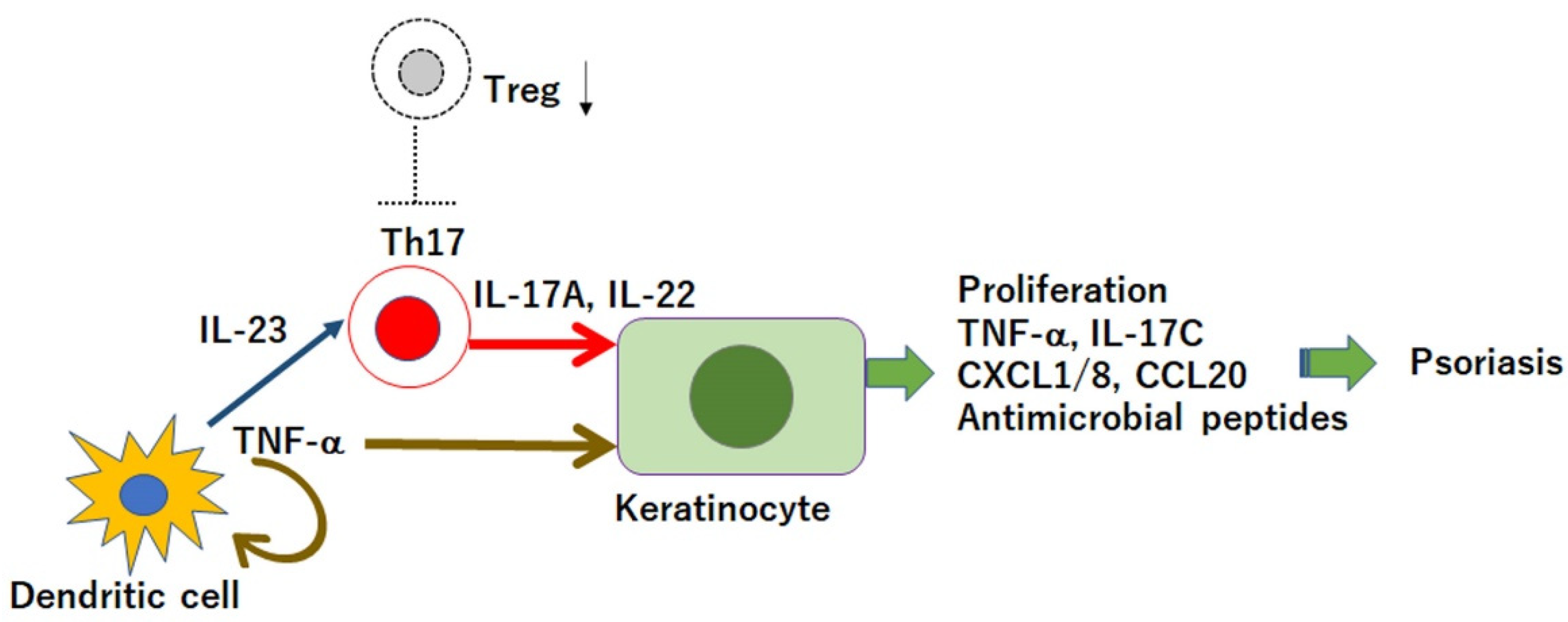
:1. Introduction
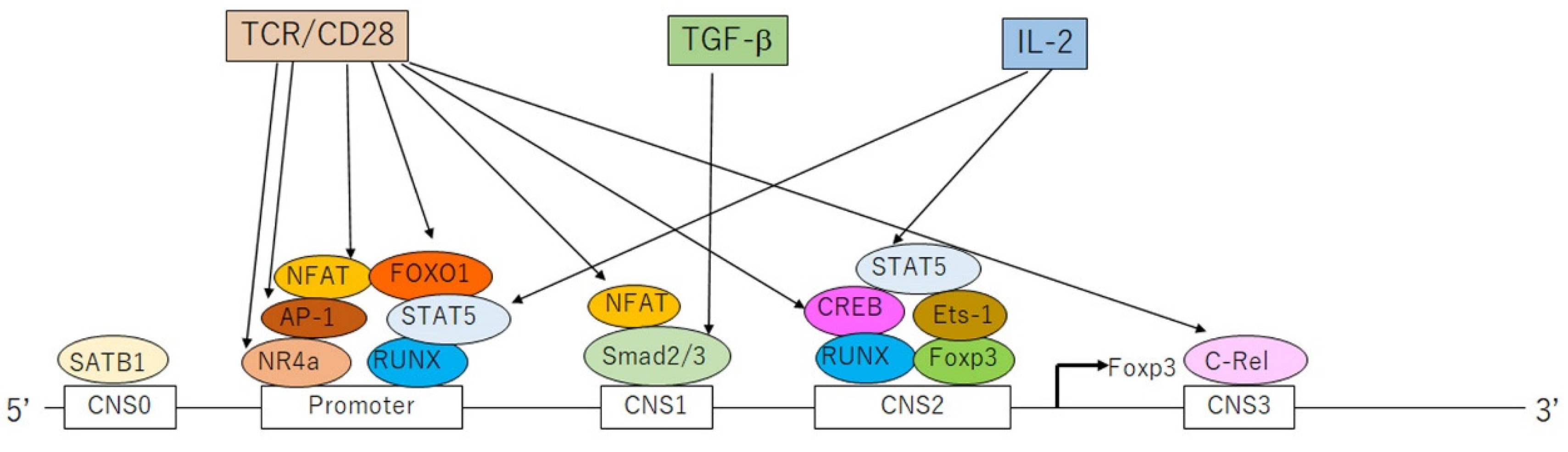
2. Genetic and Epigenetic Evidence for Defected Tregs in Psoriasis
3. Downregulation of Tregs by Cytokines or Mediators Which Are Upregulated in Psoriasis
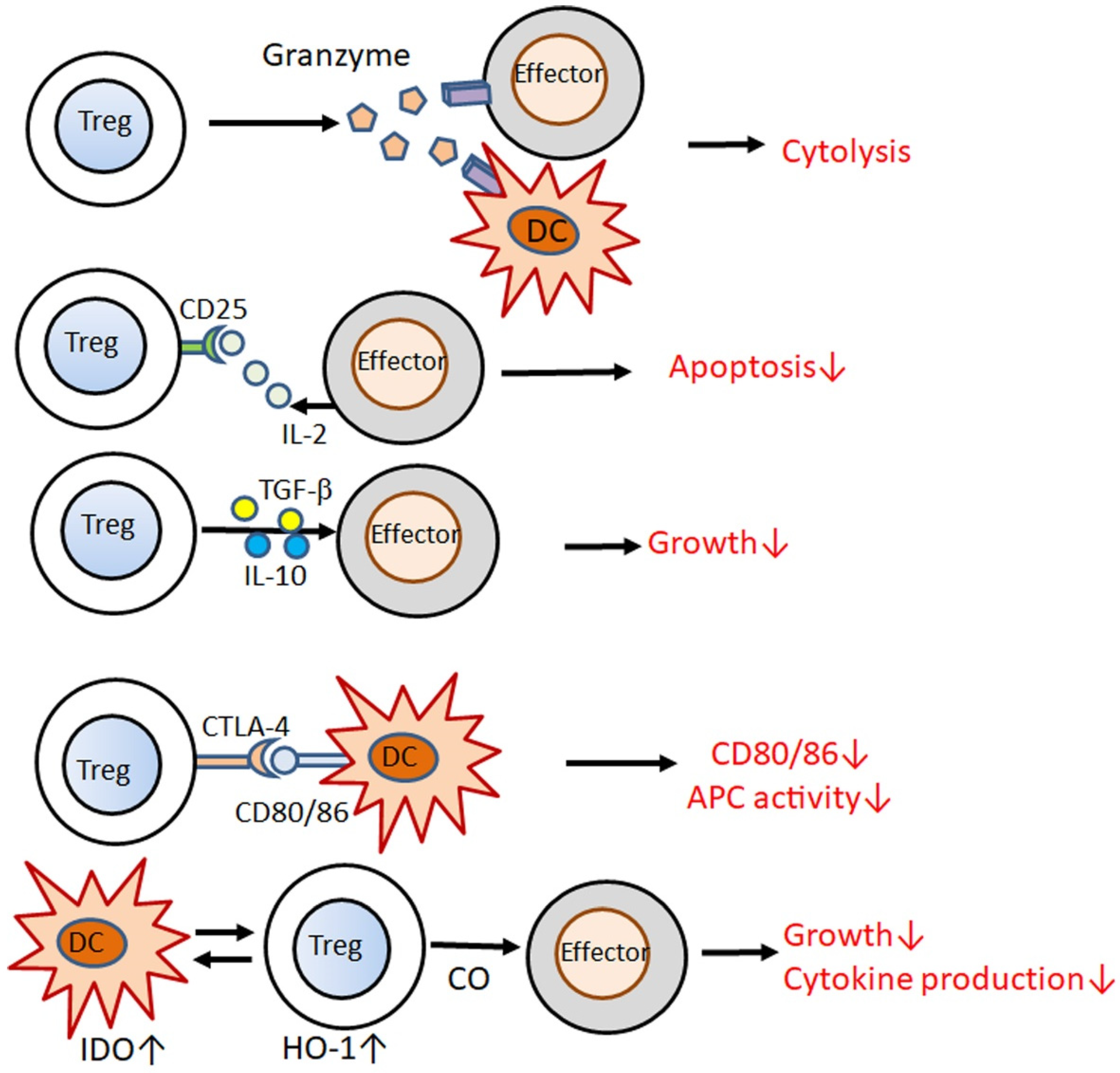
4. Stimulators for Tregs and Their Impairment in Psoriasis
5. Therapeutic Approach to Restore the Defect of Tregs in Psoriasis
5.1. Therapeutic Agents Currently Used for Psoriasis
5.1.1. Biologics
5.1.2. Vitamin D3
5.1.3. Retinoids
5.1.4. Narrow-Band Ultraviolet (UV) B Therapy
5.1.5. Dimethyl Fumarate (DMF)
5.1.6. Janus Kinase (JAK) Inhibitors
5.2. Therapeutic Agents under Development
5.2.1. SCFAs
5.2.2. STAT3 Inhibitors
5.2.3. Probiotics/Prebiotics
5.2.4. HDAC Inhibitors
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Takeshita, J.; Grewal, S.; Langan, S.; Mehta, N.N.; Ogdie, A.; Van Voorhees, A.S.; Gelfand, J. Psoriasis and comorbid diseases. J. Am. Acad. Dermatol. 2017, 76, 377–390. [Google Scholar] [CrossRef] [Green Version]
- Furue, K.; Ito, T.; Furue, M. Differential efficacy of biologic treatments targeting the TNF-α/IL-23/IL-17 axis in psoriasis and psoriatic arthritis. Cytokine 2018, 111, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, E.; Sato, Y.; Minagawa, A.; Okuyama, R. Pathogenesis of psoriasis and development of treatment. J. Dermatol. 2018, 45, 264–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Knethen, A.; Heinicke, U.; Weigert, A.; Zacharowski, K.; Brüne, B. Histone Deacetylation Inhibitors as Modulators of Regulatory T Cells. Int. J. Mol. Sci. 2020, 21, 2356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugiyama, H.; Gyulai, R.; Toichi, E.; Garaczi, E.; Shimada, S.; Stevens, S.R.; McCormick, T.S.; Cooper, K. Dysfunctional Blood and Target Tissue CD4+CD25high Regulatory T Cells in Psoriasis: Mechanism Underlying Unrestrained Pathogenic Effector T Cell Proliferation. J. Immunol. 2005, 174, 164–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komine, M. Recent Advances in Psoriasis Research; the Clue to Mysterious Relation to Gut Microbiome. Int. J. Mol. Sci. 2020, 21, 2582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalekar, L.A.; Rosenblum, M.D. Regulatory T cells in inflammatory skin disease: From mice to humans. Int. Immunol. 2019, 31, 457–463. [Google Scholar] [CrossRef] [Green Version]
- Nussbaum, L.; Chen, Y.; Ogg, G. Role of regulatory T cells in psoriasis pathogenesis and treatment. Br. J. Dermatol. 2021, 184, 14–24. [Google Scholar] [CrossRef]
- Shevach, E.M.; Thornton, A.M. tTregs, pTregs, and iTregs: Similarities and differences. Immunol. Rev. 2014, 259, 88–102. [Google Scholar] [CrossRef] [Green Version]
- Gagliani, N.; Magnani, C.F.; Huber, S.; Gianolini, M.E.; Pala, M.; Licona-Limon, P.; Guo, B.; Herbert, D.R.; Bulfone, A.; Trentini, F.; et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat. Med. 2013, 19, 739–746. [Google Scholar] [CrossRef]
- Gregori, S.; Goudy, K.S.; Roncarolo, M.G. The Cellular and Molecular Mechanisms of Immuno-Suppression by Human Type 1 Regulatory T Cells. Front. Immunol. 2012, 3, 30. [Google Scholar] [CrossRef] [Green Version]
- Jacek, R.W. The characterization and role of regulatory T cells in immune reactions. Front. Biosci. 2008, 13, 2266–2274. [Google Scholar] [CrossRef]
- Gondek, D.C.; Lu, L.-F.; Quezada, S.; Sakaguchi, S.; Noelle, R.J. Cutting Edge: Contact-Mediated Suppression by CD4+CD25+ Regulatory Cells Involves a Granzyme B-Dependent, Perforin-Independent Mechanism. J. Immunol. 2005, 174, 1783–1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Khattar, M.; Guo, Z.; Miyahara, Y.; Linkes, S.P.; Sun, Z.; He, X.; Stepkowski, S.M.; Chen, W. IL-2-deprivation and TGF-β are two non-redundant suppressor mechanisms of CD4+CD25+ regulatory T cell which jointly restrain CD4+CD25− cell activation. Immunol. Lett. 2010, 132, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Pandiyan, P.; Zheng, L.; Ishihara, S.; Reed, J.; Lenardo, M.J. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation–mediated apoptosis of effector CD4+ T cells. Nat. Immunol. 2007, 8, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- Li, M.O.; Flavell, R.A. Contextual Regulation of Inflammation: A Duet by Transforming Growth Factor-β and Interleukin-10. Immunity 2008, 28, 468–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saraiva, M.; O’Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef] [Green Version]
- Wing, K.; Onishi, Y.; Prieto-Martin, P.; Yamaguchi, T.; Miyara, M.; Fehervari, Z.; Nomura, T.; Sakaguchi, S. CTLA-4 Control over Foxp3+ Regulatory T Cell Function. Science 2008, 322, 271–275. [Google Scholar] [CrossRef]
- Brusko, T.M.; Wasserfall, C.H.; Agarwal, A.; Kapturczak, M.H.; Atkinson, M.A. An Integral Role for Heme Oxygenase-1 and Carbon Monoxide in Maintaining Peripheral Tolerance by CD4+CD25+ Regulatory T Cells. J. Immunol. 2005, 174, 5181–5186. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Li, K.; Li, F.; Li, H.; Liu, L.; Wang, L.; Zhang, Z.; Gao, T.; Liu, Y. Polymorphisms in the FOXP3 gene in Han Chinese psoriasis patients. J. Dermatol. Sci. 2010, 57, 51–56. [Google Scholar] [CrossRef]
- Ngalamika, O.; Liang, G.; Zhao, M.; Yu, X.; Yang, Y.; Yin, H.; Liu, Y.; Yung, S.; Chan, T.M.; Lu, Q. Peripheral whole blood FOXP3 TSDR methylation: A potential marker in severity assessment of autoimmune diseases and chronic infections. Immunol. Investig. 2014, 44, 126–136. [Google Scholar] [CrossRef]
- Bovenschen, H.J.; van de Kerkhof, P.C.; van Erp, P.E.; Woestenenk, R.; Joosten, I.; Koenen, H.J.P.M. Foxp3+ Regulatory T Cells of Psoriasis Patients Easily Differentiate into IL-17A-Producing Cells and Are Found in Lesional Skin. J. Investig. Dermatol. 2011, 131, 1853–1860. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Yao, Q.; Mariscal, A.G.; Wu, X.; Hülse, J.; Pedersen, E.; Helin, K.; Waisman, A.; Vinkel, C.; Thomsen, S.F.; et al. Epigenetic control of IL-23 expression in keratinocytes is important for chronic skin inflammation. Nat. Commun. 2018, 9, 1–18. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Li, B.; Yu, C.; Bai, X.; Xiao, C.; Wang, L.; Dang, E.; Yang, L.; Wang, G. A novel role of IL-17A in contributing to the impaired suppressive function of Tregs in psoriasis. J. Dermatol. Sci. 2021, 101, 84–92. [Google Scholar] [CrossRef]
- Chong, W.P.; Zhong, Y.; Mattapallil, M.; Chen, J.; Caspi, R.R. Essential role of IL-17A in Tregs induction in autoimmune uveitis. J. Immunol. 2019, 202, 116.6. [Google Scholar]
- Cunnusamy, K.; Chen, P.W.; Niederkorn, J.Y. IL-17A–Dependent CD4+CD25+ Regulatory T Cells Promote Immune Privilege of Corneal Allografts. J. Immunol. 2011, 186, 6737–6745. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Li, B.; Dang, E.; Jin, L.; Fan, X.; Wang, G. Impaired function of regulatory T cells in patients with psoriasis is mediated by phosphorylation of STAT3. J. Dermatol. Sci. 2016, 81, 85–92. [Google Scholar] [CrossRef]
- Goodman, W.A.; Levine, A.D.; Massari, J.V.; Sugiyama, H.; McCormick, T.S.; Cooper, K.D. IL-6 Signaling in Psoriasis Prevents Immune Suppression by Regulatory T Cells. J. Immunol. 2009, 183, 3170–3176. [Google Scholar] [CrossRef] [Green Version]
- Peluso, I.; Fantini, M.C.; Fina, D.; Caruso, R.; Boirivant, M.; Macdonald, T.T.; Pallone, F.; Monteleone, G. IL-21 counteracts the regulatory T cell-mediated suppression of human CD4+ T lymphocytes. J. Immunol. 2007, 178, 732–739. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Lei, J.; Yang, L.; Gao, C.; Dang, E.; Cao, T.; Xue, K.; Zhuang, Y.; Shao, S.; Zhi, D.; et al. Dysregulation of Akt-FOXO1 Pathway Leads to Dysfunction of Regulatory T Cells in Patients with Psoriasis. J. Investig. Dermatol. 2019, 139, 2098–2107. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Wang, L.-T.; Liang, G.-P.; Zhang, P.; Deng, X.-J.; Tang, Q.; Zhai, H.-Y.; Chang, C.C.; Su, Y.-W.; Lu, Q.-J. Up-regulation of microRNA-210 induces immune dysfunction via targeting FOXP3 in CD4+ T cells of psoriasis vulgaris. Clin. Immunol. 2014, 150, 22–30. [Google Scholar] [CrossRef]
- Kanda, N.; Hoashi, T.; Saeki, H. Nutrition and Psoriasis. Int. J. Mol. Sci. 2020, 21, 5405. [Google Scholar] [CrossRef]
- Keshari, S.; Wang, Y.; Herr, D.R.; Wang, S.-M.; Yang, W.-C.; Chuang, T.-H.; Chen, C.-L. Skin Cutibacterium acnes Mediates Fermentation to Suppress the Calcium Phosphate-Induced Itching: A Butyric Acid Derivative with Potential for Uremic Pruritus. J. Clin. Med. 2020, 9, 312. [Google Scholar] [CrossRef] [Green Version]
- Rozas, M.; de Ruijter, A.H.; Fabrega, M.; Zorgani, A.; Guell, M.; Paetzold, B.; Brillet, F. From Dysbiosis to Healthy Skin: Major Contributions of Cutibacterium acnes to Skin Homeostasis. Microorganisms 2021, 9, 628. [Google Scholar] [CrossRef]
- Isobe, J.; Maeda, S.; Obata, Y.; Iizuka, K.; Nakamura, Y.; Fujimura, Y.; Kimizuka, T.; Hattori, K.; Kim, Y.-G.; Morita, T.; et al. Commensal-bacteria-derived butyrate promotes the T-cell-independent IgA response in the colon. Int. Immunol. 2019, 32, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Kaisar, M.M.M.; Pelgrom, L.; van der Ham, A.; Yazdanbakhsh, M.; Everts, B. Butyrate Conditions Human Dendritic Cells to Prime Type 1 Regulatory T Cells via both Histone Deacetylase Inhibition and G Protein-Coupled Receptor 109A Signaling. Front. Immunol. 2017, 8, 1429. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Béguet-Crespel, F.; Marinelli, L.; Jamet, A.; LeDue, F.; Blottière, H.M.; Lapaque, N. Butyrate produced by gut commensal bacteria activates TGF-beta1 expression through the transcription factor SP1 in human intestinal epithelial cells. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic Treg Cell Homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, A.; Kaga, N.; Nakanishi, Y.; Ohno, H.; Miyamoto, J.; Kimura, I.; Hori, S.; Sasaki, T.; Hiramatsu, K.; Okumura, K.; et al. Maternal High Fiber Diet during Pregnancy and Lactation Influences Regulatory T Cell Differentiation in Offspring in Mice. J. Immunol. 2017, 199, 3516–3524. [Google Scholar] [CrossRef] [Green Version]
- Hu, M.; Eviston, D.; Hsu, P.; Mariño, E.; Chidgey, A.; Santner-Nanan, B.; Wong, K.; Richards, J.L.; Yap, Y.-A.; The BIS Investigator Group; et al. Decreased maternal serum acetate and impaired fetal thymic and regulatory T cell development in preeclampsia. Nat. Commun. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Olejniczak-Staruch, I.; Ciążyńska, M.; Sobolewska-Sztychny, D.; Narbutt, J.; Skibińska, M.; Lesiak, A. Alterations of the Skin and Gut Microbiome in Psoriasis and Psoriatic Arthritis. Int. J. Mol. Sci. 2021, 22, 3998. [Google Scholar] [CrossRef]
- Scher, J.U.; Ubeda, C.; Artacho, A.; Attur, M.; Isaac, S.; Reddy, S.; Marmon, S.; Neimann, A.; Brusca, S.; Patel, T.; et al. Decreased Bacterial Diversity Characterizes the Altered Gut Microbiota in Patients with Psoriatic Arthritis, Resembling Dysbiosis in Inflammatory Bowel Disease. Arthritis Rheumatol. 2015, 67, 128–139. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.; Zhao, S.; Zhu, W.; Wu, L.; Li, J.; Sheng, M.; Lei, L.; Chen, X.; Peng, C. The Akkermansia muciniphila is a gut microbiota signature in psoriasis. Exp. Dermatol. 2018, 27, 144–149. [Google Scholar] [CrossRef]
- Eppinga, H.; Weiland, C.J.S.; Thio, H.B.; van der Woude, C.J.; Nijsten, T.E.C.; Peppelenbosch, M.P.; Konstantinov, S.R. Similar Depletion of Protective Faecalibacterium prausnitziiin Psoriasis and Inflammatory Bowel Disease, but not in Hidradenitis Suppurativa. J. Crohns Coliti 2016, 10, 1067–1075. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Ho, H.J.; Tseng, C.; Lai, Z.; Shieh, J.-J.; Wu, C.-Y. Intestinal microbiota profiling and predicted metabolic dysregulation in psoriasis patients. Exp. Dermatol. 2018, 27, 1336–1343. [Google Scholar] [CrossRef]
- Shapiro, J.; Cohen, N.A.; Shalev, V.; Uzan, A.; Koren, O.; Maharshak, N. Psoriatic patients have a distinct structural and functional fecal microbiota compared with controls. J. Dermatol. 2019, 46, 595–603. [Google Scholar] [CrossRef]
- Umar, M.; Sastry, K.S.; Al Ali, F.; Al-Khulaifi, M.; Wang, E.; Chouchane, A.I. Vitamin D and the Pathophysiology of Inflammatory Skin Diseases. Ski. Pharmacol. Physiol. 2018, 31, 74–86. [Google Scholar] [CrossRef]
- Filoni, A.; Vestita, M.; Congedo, M.; Giudice, G.; Tafuri, S.; Bonamonte, D. Association between psoriasis and vitamin D. Medicine 2018, 97, e11185. [Google Scholar] [CrossRef]
- Shimizu, T.; Kamata, M.; Fukaya, S.; Hayashi, K.; Fukuyasu, A.; Tanaka, T.; Ishikawa, T.; Ohnishi, T.; Tada, Y. Anti-IL-17A and IL-23p19 antibodies but not anti-TNFα antibody induce expansion of regulatory T cells and restoration of their suppressive function in imiquimod-induced psoriasiform dermatitis. J. Dermatol. Sci. 2019, 95, 90–98. [Google Scholar] [CrossRef]
- Hau, C.S.; Shimizu, T.; Tada, Y.; Kamata, M.; Takeoka, S.; Shibata, S.; Mitsui, A.; Asano, Y.; Sugaya, M.; Kadono, T.; et al. The vitamin D3 analog, maxacalcitol, reduces psoriasiform skin inflammation by inducing regulatory T cells and downregulating IL-23 and IL-17 production. J. Dermatol. Sci. 2018, 92, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Perez, A.; Raab, R.; Chen, T.C.; Turner, A.; Holick, M.F. Safety and efficacy of oral calcitriol (1,25-dihydroxyvitamin D3) for the treatment of psoriasis. Br. J. Dermatol. 1996, 134, 1070–1078. [Google Scholar] [CrossRef]
- Theodoridis, X.; Grammatikopoulou, M.G.; Stamouli, E.-M.; Talimtzi, P.; Pagkalidou, E.; Zafiriou, E.; Haidich, A.-B.; Bogdanos, D.P. Effectiveness of oral vitamin D supplementation in lessening disease severity among patients with psoriasis: A systematic review and meta-analysis of randomized controlled trials. Nutrition 2021, 82, 111024. [Google Scholar] [CrossRef]
- Khalil, S.; Bardawil, T.; Stephan, C.; Darwiche, N.; Abbas, O.; Kibbi, A.G.; Nemer, G.; Kurban, M. Retinoids: A journey from the molecular structures and mechanisms of action to clinical uses in dermatology and adverse effects. J. Dermatol. Treat. 2017, 28, 684–696. [Google Scholar] [CrossRef]
- Wang, X.; Wang, G.; Gong, Y.; Liu, Y.; Gu, J.; Chen, W.; Shi, Y. Disruption of Circulating CD4+ T-Lymphocyte Subpopulations in Psoriasis Patients is Ameliorated by Narrow-Band UVB Therapy. Cell Biophys. 2014, 71, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Loser, K.; Mehling, A.; Loeser, S.; Apelt, J.; Kuhn, A.; Grabbe, S.; Schwarz, T.; Penninger, J.; Beissert, S. Epidermal RANKL controls regulatory T-cell numbers via activation of dendritic cells. Nat. Med. 2006, 12, 1372–1379. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Shinzawa, M.; Akiyama, N. RANKL-RANK interaction in immune regulatory systems. World J. Orthop. 2012, 3, 142–150. [Google Scholar] [CrossRef]
- Mrowietz, U.; Van De Kerkhof, P.; Schoenenberger, A.; Ryzhkova, A.; Pau-Charles, I.; Llamas-Velasco, M.; Daudén, E.; Carrascosa, J.M.; De La Cueva, P.; Salgado-Boquete, L.; et al. Efficacy of dimethyl fumarate treatment for moderate-to-severe plaque psoriasis: Presentation extracts from the 29th EADV virtual congress, 29–31 October 2020. Expert Rev. Clin. Immunol. 2021, 17, 1–11. [Google Scholar] [CrossRef]
- Brück, J.; Dringen, R.; Amasuno, A.; Pau-Charles, I.; Ghoreschi, K. A review of the mechanisms of action of dimethylfumarate in the treatment of psoriasis. Exp. Dermatol. 2018, 27, 611–624. [Google Scholar] [CrossRef] [Green Version]
- Pitarokoili, K.; Bachir, H.; Sgodzai, M.; Grüter, T.; Haupeltshofer, S.; Duscha, A.; Pedreiturria, X.; Motte, J.; Gold, R. Induction of Regulatory Properties in the Intestinal Immune System by Dimethyl Fumarate in Lewis Rat Experimental Autoimmune Neuritis. Front. Immunol. 2019, 10, 2132. [Google Scholar] [CrossRef]
- Ma, N.; Wu, Y.; Xie, F.; Du, K.; Wang, Y.; Shi, L.; Ji, L.; Liu, T.; Ma, X. Dimethyl fumarate reduces the risk of mycotoxins via improving intestinal barrier and microbiota. Oncotarget 2017, 8, 44625–44638. [Google Scholar] [CrossRef]
- Sulaimani, J.; Cluxton, D.; Clowry, J.; Petrasca, A.; Molloy, O.; Moran, B.; Sweeney, C.; Malara, A.; McNicholas, N.; McGuigan, C.; et al. Dimethyl fumarate modulates the Treg–Th17 cell axis in patients with psoriasis. Br. J. Dermatol. 2021, 184, 495–503. [Google Scholar] [CrossRef]
- Mougiakakos, D.; Johansson, C.C.; Kiessling, R. Naturally occurring regulatory T cells show reduced sensitivity toward oxidative stress–induced cell death. Blood 2009, 113, 3542–3545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mougiakakos, D.; Johansson, C.C.; Jitschin, R.; Böttcher, M.; Kiessling, R. Increased thioredoxin-1 production in human naturally occurring regulatory T cells confers enhanced tolerance to oxidative stress. Blood 2011, 117, 857–861. [Google Scholar] [CrossRef] [Green Version]
- Virtanen, A.T.; Haikarainen, T.; Raivola, J.; Silvennoinen, O. Selective JAKinibs: Prospects in Inflammatory and Autoimmune Diseases. BioDrugs 2019, 33, 15–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Feng, X.; Han, P.; Lei, Y.; Xia, Y.; Tian, D.; Yan, W. The JAK inhibitor tofacitinib ameliorates immune-mediated liver injury in mice. Mol. Med. Rep. 2019, 20, 4883–4892. [Google Scholar] [CrossRef]
- Sewgobind, V.D.K.D.; Quaedackers, M.E.; van der Laan, L.; Kraaijeveld, R.; Korevaar, S.S.; Chan, G.; Weimar, W.; Baan, C.C. The Jak Inhibitor CP-690,550 Preserves the Function of CD4+CD25brightFoxP3+ Regulatory T Cells and Inhibits Effector T Cells. Arab. Archaeol. Epigr. 2010, 10, 1785–1795. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, A.; Philippsen, R.; Schwarz, T. Induction of regulatory T cells and correction of cytokine dysbalance by short chain fatty acids—Implications for the therapy of psoriasis. J. Investig. Dermatol. 2020, 141, 95.e2–104.e2. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, K.; Takaishi, M.; Nakajima, K.; Ikeda, M.; Kanda, T.; Tarutani, M.; Iiyama, T.; Asao, N.; DiGiovanni, J.; Sano, S. Stat3 as a Therapeutic Target for the Treatment of Psoriasis: A Clinical Feasibility Study with STA-21, a Stat3 Inhibitor. J. Investig. Dermatol. 2011, 131, 108–117. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-S.; Kwok, S.-K.; Lim, M.-A.; Kim, E.-K.; Ryu, J.-G.; Kim, S.-M.; Oh, H.-J.; Ju, J.H.; Park, S.-H.; Kim, H.-Y.; et al. STA-21, a Promising STAT-3 Inhibitor That Reciprocally Regulates Th17 and Treg Cells, Inhibits Osteoclastogenesis in Mice and Humans and Alleviates Autoimmune Inflammation in an Experimental Model of Rheumatoid Arthritis. Arthritis Rheumatol. 2014, 66, 918–929. [Google Scholar] [CrossRef]
- Park, J.-S.; Kim, S.-M.; Hwang, S.-H.; Choi, S.-Y.; Kwon, J.Y.; Kwok, S.-K.; Cho, M.-L.; Park, S.-H. Combinatory treatment using tacrolimus and a STAT3 inhibitor regulate Treg cells and plasma cells. Int. J. Immunopathol. Pharmacol. 2018, 32, 2058738418778724. [Google Scholar] [CrossRef] [Green Version]
- Alzahrani, Y.A.; Alesa, D.I.; Alshamrani, H.M.; Alamssi, D.N.; Alzahrani, N.S.; Almohammadi, M.E. The role of gut microbiome in the pathogenesis of psoriasis and the therapeutic effects of probiotics. J. Fam. Med. Prim. Care 2019, 8, 3496–3503. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Van Bergenhenegouwen, J.; Overbeek, S.; Van De Kant, H.J.G.; Garssen, J.; Folkerts, G.; Vos, P.; Morgan, M.E.; Kraneveld, A.D. Bifidobacterium breve Attenuates Murine Dextran Sodium Sulfate-Induced Colitis and Increases Regulatory T Cell Responses. PLoS ONE 2014, 9, e95441. [Google Scholar] [CrossRef] [Green Version]
- Salehipour, Z.; Haghmorad, D.; Sankian, M.; Rastin, M.; Nosratabadi, R.; Dallal, M.M.S.; Tabasi, N.; Khazaee, M.; Nasiraii, L.R.; Mahmoudi, M. Bifidobacterium animalis in combination with human origin of Faecalibacterium prausnitziiin ameliorate neuroinflammation in experimental model of multiple sclerosis by altering CD4+ T cell subset balance. Biomed. Pharmacother. 2017, 95, 1535–1548. [Google Scholar] [CrossRef] [PubMed]
- Kanda, N.; Hoashi, T.; Saeki, H. Nutrition and atopic dermatitis. J. Nippon. Med Sch. 2021, 88, 171–177. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, H.; Xiao, X.; Hu, L.; Xin, F.; Yu, X. Inulin-type fructan improves diabetic phenotype and gut microbiota profiles in rats. PeerJ 2018, 6, e4446. [Google Scholar] [CrossRef] [PubMed]
- Groeger, D.; O’Mahony, L.; Murphy, E.F.; Bourke, J.F.; Dinan, T.; Kiely, B.; Shanahan, F.; Quigley, E.M. Bifidobacterium infantis35624 modulates host inflammatory processes beyond the gut. Gut Microbes 2013, 4, 325–339. [Google Scholar] [CrossRef] [Green Version]
- Navarro-López, V.; Martínez-Andrés, A.; Ramírez-Boscá, A.; Ruzafa-Costas, B.; Núñez-Delegido, E.; Carrión-Gutiérrez, M.; Prieto-Merino, D.; Codoñer-Cortés, F.; Ramón-Vidal, D.; Genovés-Martínez, S.; et al. Efficacy and Safety of Oral Administration of a Mixture of Probiotic Strains in Patients with Psoriasis: A Randomized Clinical Trial. Acta Derm. Venereol. 2019, 99, 1078–1084. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, M.; Takahashi, K.; Abe, S.; Yamada, K.; Suzuki, M.; Masahisa, M.; Endo, M.; Abe, K.; Inoue, R.; Hoshi, H. Improvement of Psoriasis by Alteration of the Gut Environment by Oral Administration of Fucoidan from Cladosiphon Okamuranus. Mar. Drugs 2020, 18, 154. [Google Scholar] [CrossRef] [Green Version]
- Koenen, H.J.P.M.; Smeets, R.L.; Vink, P.M.; Van Rijssen, E.; Boots, A.M.H.; Joosten, I. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17–producing cells. Blood 2008, 112, 2340–2352. [Google Scholar] [CrossRef] [Green Version]
| Therapeutic Agents | Mechanisms for Restoring Tregs |
|---|---|
| Currently Used | |
| Anti-IL-23p19 or anti-IL-12/23p40 antibodies | Reversing conversion from Tregs into Th17 cells |
| Anti-IL-17A or anti-IL-17RA antibodies | Increasing TGF-β secretion, Foxp3 expression, and suppressive function of Tregs |
| Topical vitamin D3 | Increasing Foxp3 expression through VDR |
| Retinoids | Increasing Foxp3 expression through RAR |
| Narrow-band UVB | Increasing RANKL expression in keratinocytes and inducing DCs to expand Tregs |
| Dimethyl fumarate | Increasing the frequency of Tregs resistant to dimethyl fumarate-induced oxidative stress or increasing SCFA-producing bacteria in the gut |
| Under development | |
| SCFAs | Increasing Foxp3 expression via HDAC inhibition, TGF-β production in IEC, RA synthesis in DCs, and proliferation of tTregs |
| STAT3 inhibitors | Increasing Foxp3 expression via induction of STAT5 |
| Probiotics | Increasing SCFA production in the gut |
| Prebiotics | Increasing SCFA-producing bacteria in the gut |
| HDAC inhibitors | Increasing Foxp3 expression and stabilization |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanda, N.; Hoashi, T.; Saeki, H. The Defect in Regulatory T Cells in Psoriasis and Therapeutic Approaches. J. Clin. Med. 2021, 10, 3880. https://doi.org/10.3390/jcm10173880
Kanda N, Hoashi T, Saeki H. The Defect in Regulatory T Cells in Psoriasis and Therapeutic Approaches. Journal of Clinical Medicine. 2021; 10(17):3880. https://doi.org/10.3390/jcm10173880
Chicago/Turabian StyleKanda, Naoko, Toshihiko Hoashi, and Hidehisa Saeki. 2021. "The Defect in Regulatory T Cells in Psoriasis and Therapeutic Approaches" Journal of Clinical Medicine 10, no. 17: 3880. https://doi.org/10.3390/jcm10173880
APA StyleKanda, N., Hoashi, T., & Saeki, H. (2021). The Defect in Regulatory T Cells in Psoriasis and Therapeutic Approaches. Journal of Clinical Medicine, 10(17), 3880. https://doi.org/10.3390/jcm10173880







