Association between Early Absolute Neutrophil Count and Level of D-Dimer among Patients with COVID-19 Infection in Central Taiwan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Subjects and Study Design
2.3. Managements
2.4. Sensitivity Analyses
2.5. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Management and Outcome in Patients with COVID-19 Infection
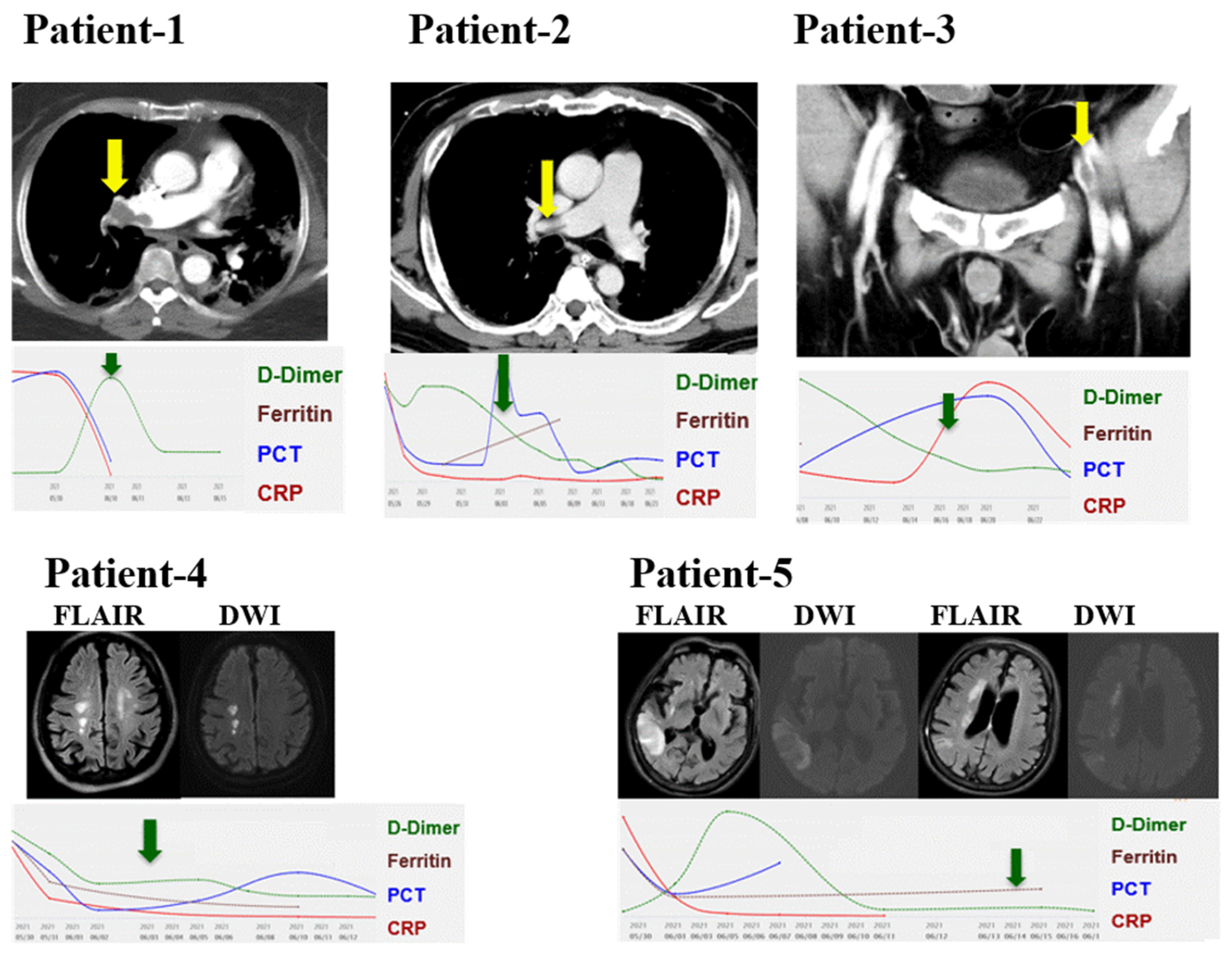
3.3. Thromboembolic Events in 5 Patients with COVID-19 Infection
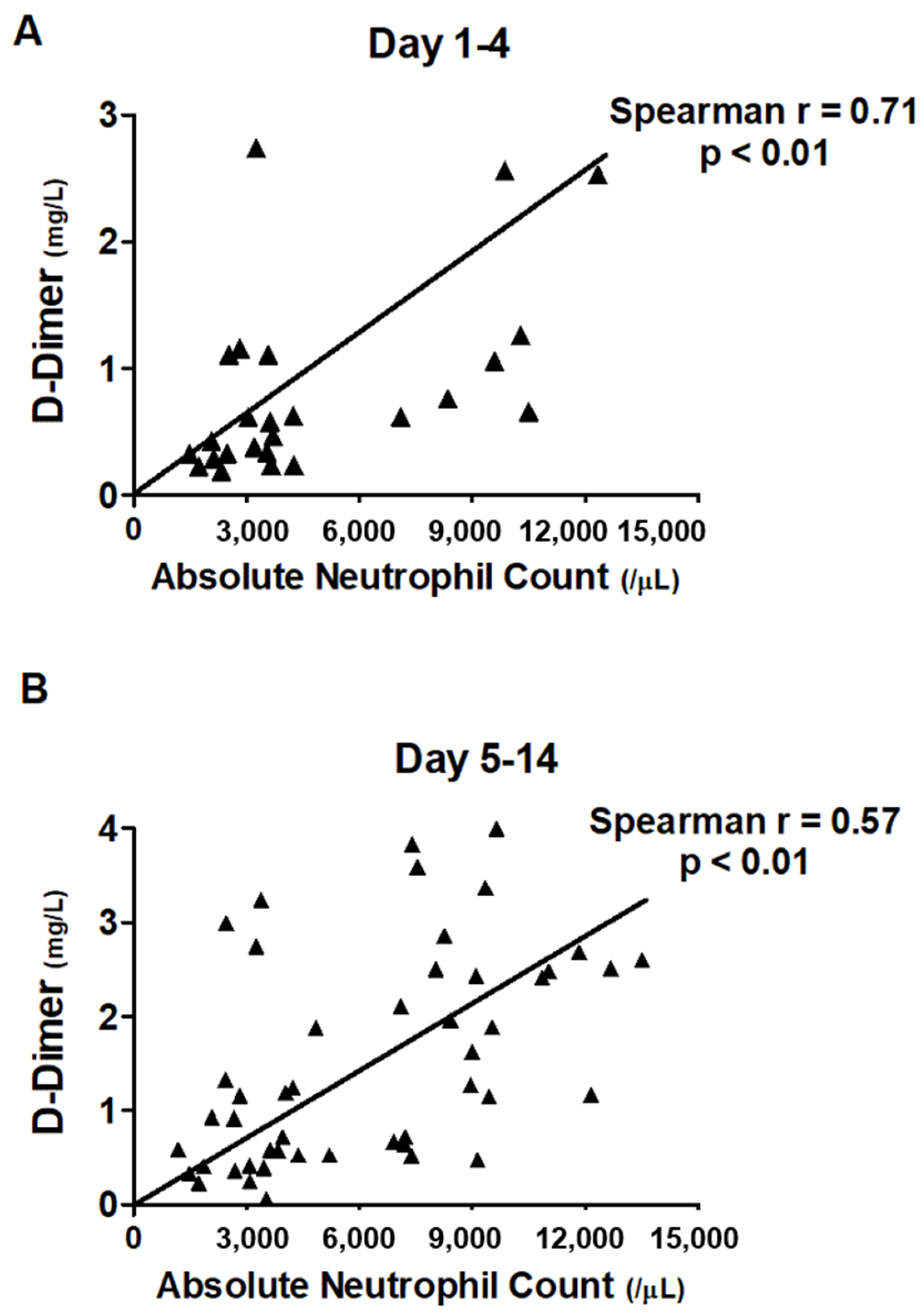
3.4. Correlation between ANC and Level of D-Dimer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- COVID-19 statistics in Taiwan. Taiwan Centers for Disease Control. Available online: https://data.cdc.gov.tw/en/dataset/covid19_tw__stats (accessed on 1 August 2021).
- Wadman, M.; Couzin-Frankel, J.; Kaiser, J.; Matacic, C. A rampage through the body. Science 2020, 368, 356–360. [Google Scholar] [CrossRef]
- COVID-ICU Group. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: A prospective cohort study. Intensive Care Med. 2021, 47, 60–73. [Google Scholar] [CrossRef]
- Ren, B.; Yan, F.; Deng, Z.; Zhang, S.; Xiao, L.; Wu, M.; Cai, L. Extremely High Incidence of Lower Extremity Deep Venous Thrombosis in 48 Patients with Severe COVID-19 in Wuhan. Circulation 2020, 142, 181–183. [Google Scholar] [CrossRef]
- Poissy, J.; Goutay, J.; Caplan, M.; Parmentier, E.; Duburcq, T.; Lassalle, F.; Jeanpierre, E.; Rauch, A.; Labreuche, J.; Susen, S. Pulmonary Embolism in Patients With COVID-19: Awareness of an Increased Prevalence. Circulation 2020, 142, 184–186. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Wang, M.; Zhou, Y.; Chang, J.; Xian, Y.; Wang, D.; Mao, L.; Jin, H.; Hu, B. Acute cerebrovascular disease following COVID-19: A single center, retrospective, observational study. Stroke Vasc. Neurol. 2020, 5, 279–284. [Google Scholar] [CrossRef]
- Miró, Ò.; Jiménez, S.; Mebazaa, A.; Freund, Y.; Burillo-Putze, G.; Martín, A.; Martín-Sánchez, F.J.; García-Lamberechts, E.J.; Alquézar-Arbé, A.; Jacob, J.; et al. Pulmonary embolism in patients with COVID-19: Incidence, risk factors, clinical characteristics, and outcome. Eur. Heart J. 2021, 42, ehab314. [Google Scholar] [CrossRef] [PubMed]
- Bilaloglu, S.; Aphinyanaphongs, Y.; Jones, S.; Iturrate, E.; Hochman, J.; Berger, J.S. Thrombosis in Hospitalized Patients With COVID-19 in a New York City Health System. JAMA 2020, 324, 799. [Google Scholar] [CrossRef] [PubMed]
- Iba, T.; Connors, J.M.; Spyropoulos, A.C.; Wada, H.; Levy, J.H. Ethnic differences in thromboprophylaxis for COVID-19 patients: Should they be considered? Int. J. Hematol. 2021, 113, 330–336. [Google Scholar] [CrossRef]
- Yamashita, Y.; Yamada, N.; Mo, M. The Primary Prevention of Venous Thromboembolism in Patients with COVID-19 in Japan: Current Status and Future Perspective. Ann. Vasc. Dis. 2021, 14, 1–4. [Google Scholar] [CrossRef]
- Barnes, B.J.; Adrover, J.M.; Baxter-Stoltzfus, A.; Borczuk, A.; Cools-Lartigue, J.; Crawford, J.M.; Daßler-Plenker, J.; Guerci, P.; Huynh, C.; Knight, J.S.; et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J. Exp. Med. 2020, 6, 217. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, A.; Vecchié, A.; Dagna, L.; Martinod, K.; Dixon, D.L.; Van Tassell, B.W.; Dentali, F.; Montecucco, F.; Massberg, S.; Levi, M.; et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 2021, 21, 319–329. [Google Scholar] [CrossRef]
- Mumoli, N.; Conte, G.; Cei, M.; Vitale, J.; Capra, R.; Rotiroti, G.; Porta, C.; Monolo, D.; Colombo, A.; Mazzone, A.; et al. In-hospital fatality and venous thromboembolism during the first and second COVID-19 waves at a center opting for standard-dose thromboprophylaxis. Thromb. Res. 2021, 203, 82–84. [Google Scholar] [CrossRef]
- Liao, S.; Woulfe, T.; Hyder, S.; Merriman, E.; Simpson, D.; Chunilal, S. Incidence of venous thromboembolism in different ethnic groups: A regional direct comparison study. J. Thromb. Haemost. 2014, 12, 214–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicole Tran, H.; Klatsky, A.L. Lower risk of venous thromboembolism in multiple Asian ethnic groups. Prev. Med. Rep. 2019, 13, 268–269. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-L.; Lip, G.Y.H.; Chiang, C.-E. Stroke prevention in atrial fibrillation: An Asian perspective. Thromb. Haemost. 2014, 111, 789–797. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.-L.; Yap, E.S.; Goto, S.; Zhang, S.; Siu, C.-W.; Chiang, C.-E. The diagnosis and treatment of venous thromboembolism in Asian patients. Thromb. J. 2018, 16, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Bang, S.-M.; Jang, M.J.; Kim, K.H.; Yhim, H.-Y.; Kim, Y.-K.; Nam, S.-H.; Hwang, H.G.; Bae, S.H.; Kim, S.-H.; Mun, Y.-C.; et al. Prevention of Venous Thromboembolism, 2nd Edition: Korean Society of Thrombosis and Hemostasis Evidence-Based Clinical Practice Guidelines. J. Korean Med. Sci. 2014, 29, 164–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chew, T.-W.; Gau, C.-S.; Wen, Y.-W.; Shen, L.-J.; Mullins, C.D.; Hsiao, F.-Y. Epidemiology, clinical profile and treatment patterns of venous thromboembolism in cancer patients in Taiwan: A population-based study. BMC Cancer 2015, 15, 298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.-C.; Chen, S.-C.; Huang, C.-M.; Hu, Y.-F.; Chen, Y.-Y.; Chang, S.-L.; Lo, L.-W.; Lin, Y.-J.; Chen, S.-A. Clinical features and diagnosis of new malignancy in patients with acute pulmonary embolism and without a history of cancer. J. Chin. Med. Assoc. 2020, 83, 245–250. [Google Scholar] [CrossRef]
- Moll, M.; Zon, R.L.; Sylvester, K.W.; Chen, E.C.; Cheng, V.; Connell, N.; Fredenburgh, L.E.; Baron, R.M.; Cho, M.H.; Woolley, A.E.; et al. VTE in ICU Patients With COVID-19. Chest 2020, 158, 2130–2135. [Google Scholar] [CrossRef]
- Yamashita, Y.; Maruyama, Y.; Satokawa, H.; Nishimoto, Y.; Tsujino, I.; Sakashita, H.; Nakata, H.; Okuno, Y.; Ogihara, Y.; Yachi, S.; et al. Incidence and Clinical Features of Venous Thromboembolism in Hospitalized Patients With Coronavirus Disease 2019 (COVID-19) in Japan. Circ. J. 2021, CJ-21, 85. [Google Scholar] [CrossRef]
- Oldgren, J.; Healey, J.S.; Ezekowitz, M.; Commerford, P.; Avezum, A.; Pais, P.; Zhu, J.; Jansky, P.; Sigamani, A.; Morillo, C.A.; et al. Variations in Cause and Management of Atrial Fibrillation in a Prospective Registry of 15 400 Emergency Department Patients in 46 Countries: The RE-LY Atrial Fibrillation Registry. Circulation 2014, 129, 1568–1576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, W.-H.; Chan, Y.-H.; Liao, J.-N.; Kuo, L.; Chen, S.-A.; Chao, T.-F. Optimal Management of Anticoagulation Therapy in Asian Patients with Atrial Fibrillation. Circ. J. 2021, CJ-21, 85. [Google Scholar] [CrossRef]
- Kremer, S.; Lersy, F.; De Sèze, J.; Ferré, J.-C.; Maamar, A.; Carsin-Nicol, B.; Collange, O.; Bonneville, F.; Adam, G.; Martin-Blondel, G.; et al. Brain MRI Findings in Severe COVID-19: A Retrospective Observational Study. Radiology 2020, 297, E242–E251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Qin, L.; Li, K.; Wang, Q.; Zhao, Y.; Xu, B.; Liang, L.; Dai, Y.; Feng, Y.; Sun, J.; et al. A Novel Scoring System for Prediction of Disease Severity in COVID-19. Front. Cell. Infect. Microbiol. 2020, 10, 318. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, X.; Zhu, C.; Song, Y.; Feng, F.; Qiu, Y.; Feng, J.; Jia, Q.; Song, Q.; Zhu, B.; et al. Immune Phenotyping Based on the Neutrophil-to-Lymphocyte Ratio and IgG Level Predicts Disease Severity and Outcome for Patients With COVID-19. Front. Mol. Biosci. 2020, 7, 157. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Xiang, P.; Pu, L.; Xiong, H.; Li, C.; Zhang, M.; Tan, J.; Xu, Y.; Song, R.; et al. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J. Transl. Med. 2020, 18, 206. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, C.; Mao, Z.; Xiao, M.; Wang, L.; Qi, S.; Zhou, F. Predictive values of neutrophil-to-lymphocyte ratio on disease severity and mortality in COVID-19 patients: A systematic review and meta-analysis. Crit. Care 2020, 24, 1–10. [Google Scholar] [CrossRef]
- Middleton, E.A.; He, X.-Y.; Denorme, F.; Campbell, R.A.; Ng, D.; Salvatore, S.P.; Mostyka, M.; Baxter-Stoltzfus, A.; Borczuk, A.C.; Loda, M.; et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 2020, 136, 1169–1179. [Google Scholar] [CrossRef]
- Blanch-Ruiz, M.A.; Ortega-Luna, R.; Martinez-Cuesta, M.A.; Alvarez, A. The Neutrophil Secretome as a Crucial Link between Inflammation and Thrombosis. Int. J. Mol. Sci. 2021, 22, 4170. [Google Scholar] [CrossRef]
- Bautista-Becerril, B.; Campi-Caballero, R.; Sevilla-Fuentes, S.; Hernández-Regino, L.; Hanono, A.; Flores-Bustamante, A.; González-Flores, J.; García-Ávila, C.; Aquino-Gálvez, A.; Castillejos-López, M.; et al. Immunothrombosis in COVID-19: Implications of Neutrophil Extracellular Traps. Biomolecules 2021, 11, 694. [Google Scholar] [CrossRef]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef] [Green Version]
- Rosales, C. Neutrophils at the crossroads of innate and adaptive immunity. J. Leukoc. Biol. 2020, 108, 377–396. [Google Scholar] [CrossRef] [PubMed]
- Döring, Y.; Soehnlein, O.; Weber, C. Neutrophil Extracellular Traps in Atherosclerosis and Atherothrombosis. Circ. Res. 2017, 120, 736–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Feng, X.; Zhang, D.; Jiang, C.; Mei, H.; Wang, J.; Zhang, C.; Li, H.; Xia, X.; Kong, S.; et al. Deep Vein Thrombosis in Hospitalized Patients With COVID-19 in Wuhan, China: Prevalence, Risk Factors, and Outcome. Circulation 2020, 142, 114–128. [Google Scholar] [CrossRef]
- Bekelis, K.; Missios, S.; Ahmad, J.; Labropoulos, N.; Schirmer, C.M.; Calnan, D.R.; Skinner, J.; MacKenzie, T.A. Ischemic Stroke Occurs Less Frequently in Patients With COVID-19: A Multicenter Cross-Sectional Study. Stroke 2020, 51, 3570–3576. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.I.; Baskett, W.I.; Huang, W.; Shyu, D.; Myers, D.; Raju, M.; Lobanova, I.; Suri, M.F.; Naqvi, S.H.; French, B.R.; et al. Acute Ischemic Stroke and COVID-19: An Analysis of 27,676 Patients. Stroke 2021, 52, 905–912. [Google Scholar] [CrossRef]
| All | Mechanical Ventilation (+) | Mechanical Ventilation (−) | p-Value | |
|---|---|---|---|---|
| n = 28 | n = 9 | n = 19 | ||
| COVID severity | ||||
| Mechanical ventilation | 9 | 9 | ||
| High flow nasal cannula | 4 | 4 | ||
| Venturi mask | 1 | 1 | ||
| Nasal cannula | 7 | 7 | ||
| Room air, lung infiltration | 2 | 2 | ||
| Room air, no pulmonary infiltration | 5 | 5 | ||
| Prone ventilation | ||||
| Awake prone | 6 | 6 | ||
| Intubated prone ventilation | 2 | 2 | ||
| ARDS grade | ||||
| Mild | 3 | 3 (33.3%) | NA | |
| Mod | 3 | 3 (33.3%) | NA | |
| Severe | 3 | 3 (33.3%) | NA | |
| Basic data | ||||
| Age, years | 60.0 (50.3–69.5) | 62.0 (59.5–73.5) | 58.0 (40.0–67.0) | 0.05 |
| Sex, male | 11 (39.3%) | 5 (55.6%) | 6 (31.6%) | 0.41 |
| Body weight, kg | 62.5 (59.0–71.8) | 70.0 (66.0–77.5) | 60.0 (57.0–71.0) | 0.04 |
| Symptom-admit, day | 5.0 (2.0–8.0) | 7.0 (6.0–8.5) | 4.5 ± 1.0 | 0.03 |
| Symptom-intubation, day | 7 (3.0–7.0) | 7.0 (3.0–7.0) | NA | |
| Comorbidities | ||||
| Diabetes mellitus | 8 (28.6%) | 4 (44.4%) | 4 (21.1%) | 0.20 |
| Hypertension | 8 (28.6%) | 3 (33.3%) | 5 (26.3%) | 0.52 |
| Coronary artery disease | 2 (7.1%) | 1 (11.1%) | 1 (5.3%) | 0.55 |
| Old cerebrovascular diseases | 2 (7.1%) | 1 (11.1%) | 1 (5.3%) | 0.55 |
| History of cancer | 1 (3.6%) | 0 (0%) | 1 (5.3%) | 0.68 |
| Hepatitis | 2 (7.1%) | 1 (11.1%) | 1 (5.3%) | 0.55 |
| COPD | 2 (7.1%) | 2 (22.2%) | 0 (0%) | 0.10 |
| Autoimmune disease | 1 (3.6%) | 0 (0%) | 1 (5.3%) | 0.70 |
| Laboratory data | ||||
| White blood cell counts (/μL) | 5430 (4040–8902) | 9480 (5300–12,070) | 4540 (3950–7200) | 0.02 |
| Absolute neutrophil counts (/μL) | 3678 (2652–7210) | 8225 (4146–12,491) | 3427 (2329–4255) | <0.01 |
| Absolute lymphocyte counts (/μL) | 913 (604–1260) | 843 (312–1232) | 958 (618–1341) | 0.47 |
| Hemoglobin (g/dL) | 13.1 (12.2–14.3) | 12.3 (10.6–13.2) | 13.3 (12.6–14.4) | 0.04 |
| Platelet (103/μL) | 201 (156–251) | 230 (159–300) | 200 (155–234) | 0.16 |
| C-reactive protein (mg/dl) | 4.4 (12.5–9.39) | 9.6 (1.3–15.8) | 3.0 (1.1–6.7) | 0.12 |
| Ferritin (ng/mL) | 875 (162–1328) | 1023 (437–1341) | 504 (111–504) | 0.24 |
| D-Dimer (mg/L) | 1.0 (0.4–5.8) | 6.0 (4.4–24.6) | 0.6 (0.3–1.2) | <0.01 |
| LDH (U/L) | 426 (281–570) | 533 (496–613) | 370 (245–522) | 0.02 |
| Procalcitonin (ng/mL) | 0.07 (0.05–0.20) | 0.12(0.06–0.35) | 0.06 (0.05–0.14) | 0.19 |
| Creatinine (mg/dL) | 0.7 (0.7–0.9) | 0.8 (0.7–1.5) | 0.7 (0.7–0.8) | 0.6 |
| All | Mechanical Ventilation (+) | Mechanical Ventilation (−) | p-Value | |
|---|---|---|---|---|
| n = 28 | n = 9 | n = 19 | ||
| Management | ||||
| Remdesivir, 5-day course | 21 (75%) | 9 (100%) | 12 (63.2%) | 0.06 |
| Dexamethasone, 6 mg/day 10 days | 21 (75%) | 9 (100%) | 12 (63.2%) | 0.06 |
| Tocilizumab, 8 mg per kg | 12 (42.9%) | 9 (100%) | 3 (15.8%) | <0.01 |
| Vasopressor | 6 (21.4%) | 6 (66.7%) | 0 (0%) | <0.01 |
| Vasopressor-day | 3.0 (2.5–4.3) | 3.0 (2.5–4.3) | ||
| Sedation | 6 (21.4%) | 6 (66.7%) | 0 (0%) | <0.01 |
| Sedation-day | 8.0 (5.8–12.0) | 8.0 (5.8–12.0) | ||
| NMBA | 6 (21.4%) | 6 (66.7%) | 0 (0%) | <0.01 |
| NMBA-day | 1.0 (0.0–3.5) | 1.0 (0.0–3.5) | ||
| Coagulation-associated variables | ||||
| Thromembolic events | 5 (17.9%) | 4 (44.4%) | 1 (5.3%) | 0.04 |
| Pulmonary embolism | 2 (7.1%) | 2 (22.2%) | 0 (0%) | |
| Ischemic stroke | 2 (7.1%) | 1 (11.1% | 1 (5.3%) | |
| Venous thrombosis | 1 (3.6%) | 1 (11.1%) | 0 (0%) | |
| Anticoagulant | 12 (42.9%) | 9 (100%) | 3 (15.8%) | <0.01 |
| Bleeding events | 0.03 | |||
| Major bleeding | 1 (3.6%) | 1 (11.1%) | 0 (0%) | |
| Minor bleeding | 2 (7.1%) | 2 (22.2%) | 0 (0%) | |
| Infection-associated events | ||||
| Co-infection | 0 (0%) | 0 (0%) | 0 (0%) | >0.99 |
| Secondary infection | 3 (10.7%) | 3 (33.3%) | 0 (0%) | 0.03 |
| Blood stream infection | 1 (3.6%) | 1 (11.1%) | 0 (0%) | |
| Urinary tract infection | 1 (3.6%) | 1 (11.1%) | 0 (0%) | |
| Ventilator-associated pneumonia | 1 (3.6%) | 1 (11.1%) | 0 (0%) | |
| Outcomes | ||||
| Ventilator-day | 10.0 (7.5–19.5) | 10.0 (7.5–19.5) | NA | NA |
| Hospital-day | 15.5 (13.0–22.8) | 27.0 (17.5–34.0) | 15.0 (11.0–19.0) | <0.01 |
| Hospital-mortality | 0 (0%) | 0 (0%) | 0 (0%) | >0.99 |
| ANC | ALC | CRP | Ferritin | LDH | D-Dimer | ||
|---|---|---|---|---|---|---|---|
| ANC | Spearman r | 1.00 | 0.01 | 0.10 | 0.22 | 0.47 | 0.58 |
| Sig. (2-tailed) | 0.91 | 0.40 | 0.20 | <0.01 | <0.01 | ||
| N | 94 | 94 | 74 | 35 | 78 | 94 | |
| ALC | Spearman r | 0.01 | 1.00 | −0.42 | −0.08 | −0.20 | −0.07 |
| Sig. (2-tailed) | 0.91 | <0.01 | 0.64 | 0.08 | 0.52 | ||
| N | 94 | 95 | 74 | 35 | 79 | 95 | |
| CRP | Spearman r | 0.10 | −0.42 | 1.00 | 0.57 | 0.33 | 0.06 |
| Sig. (2-tailed) | 0.40 | <0.01 | <0.01 | 0.01 | 0.57 | ||
| N | 74 | 74 | 82 | 39 | 70 | 82 | |
| Ferritin | Spearman r | 0.22 | −0.08 | 0.57 | 1.00 | 0.49 | 0.38 |
| Sig. (2-tailed) | 0.20 | 0.64 | <0.01 | <0.01 | 0.01 | ||
| N | 35 | 35 | 39 | 42 | 39 | 42 | |
| LDH | Spearman r | 0.47 | −0.20 | 0.35 | 0.49 | 1.00 | 0.47 |
| Sig. (2-tailed) | <0.01 | 0.08 | 0.01 | 0.00 | <0.01 | ||
| N | 78 | 79 | 70 | 39 | 85 | 85 | |
| D Dimer | Spearman r | 0.58 | −0.07 | 0.06 | 0.38 | 0.47 | 1.00 |
| Sig. (2-tailed) | <0.01 | 0.52 | 0.57 | 0.01 | <0.01 | ||
| N | 94 | 95 | 82 | 42 | 85 | 104 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chao, W.-C.; Wu, C.-L.; Huang, J.-A.; Chai, J.-W.; Teng, C.-L.; Lee, W.-L.; Fu, Y.-C.; Chen, S.-A. Association between Early Absolute Neutrophil Count and Level of D-Dimer among Patients with COVID-19 Infection in Central Taiwan. J. Clin. Med. 2021, 10, 3891. https://doi.org/10.3390/jcm10173891
Chao W-C, Wu C-L, Huang J-A, Chai J-W, Teng C-L, Lee W-L, Fu Y-C, Chen S-A. Association between Early Absolute Neutrophil Count and Level of D-Dimer among Patients with COVID-19 Infection in Central Taiwan. Journal of Clinical Medicine. 2021; 10(17):3891. https://doi.org/10.3390/jcm10173891
Chicago/Turabian StyleChao, Wen-Cheng, Chieh-Liang Wu, Jin-An Huang, Jyh-Wen Chai, Chieh-Lin Teng, Wen-Lieng Lee, Yun-Ching Fu, and Shih-Ann Chen. 2021. "Association between Early Absolute Neutrophil Count and Level of D-Dimer among Patients with COVID-19 Infection in Central Taiwan" Journal of Clinical Medicine 10, no. 17: 3891. https://doi.org/10.3390/jcm10173891
APA StyleChao, W.-C., Wu, C.-L., Huang, J.-A., Chai, J.-W., Teng, C.-L., Lee, W.-L., Fu, Y.-C., & Chen, S.-A. (2021). Association between Early Absolute Neutrophil Count and Level of D-Dimer among Patients with COVID-19 Infection in Central Taiwan. Journal of Clinical Medicine, 10(17), 3891. https://doi.org/10.3390/jcm10173891







