Guided Bone Regeneration with Concentrated Growth Factor Enriched Bone Graft Matrix (Sticky Bone) vs. Bone-Shell Technique in Horizontal Ridge Augmentation: A Retrospective Study
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Study Population
- Age > 18 years without other age or gender restrictions;
- Horizontal ridge augmentation performed with SB or BS;
- Presence of CBCTs taken before surgery and six months after augmentation;
- Native bone crest width <5 mm (measured at 1 mm below the most cranial point of the alveolar crest);
- Implant-supported fixed prosthetic rehabilitation.
- Untreated or residual periodontal disease;
- Uncontrolled diabetes (HbA1c > 7.5%);
- Antiresorptive therapy;
- Head and/or neck radiotherapy;
- Immunosuppressive therapy;
- Incomplete or unavailable medical and periodontal charts (including radiographs);
- Post-operative complications (e.g., graft infection, flap dehiscence);
- Absence of signed informed consent;
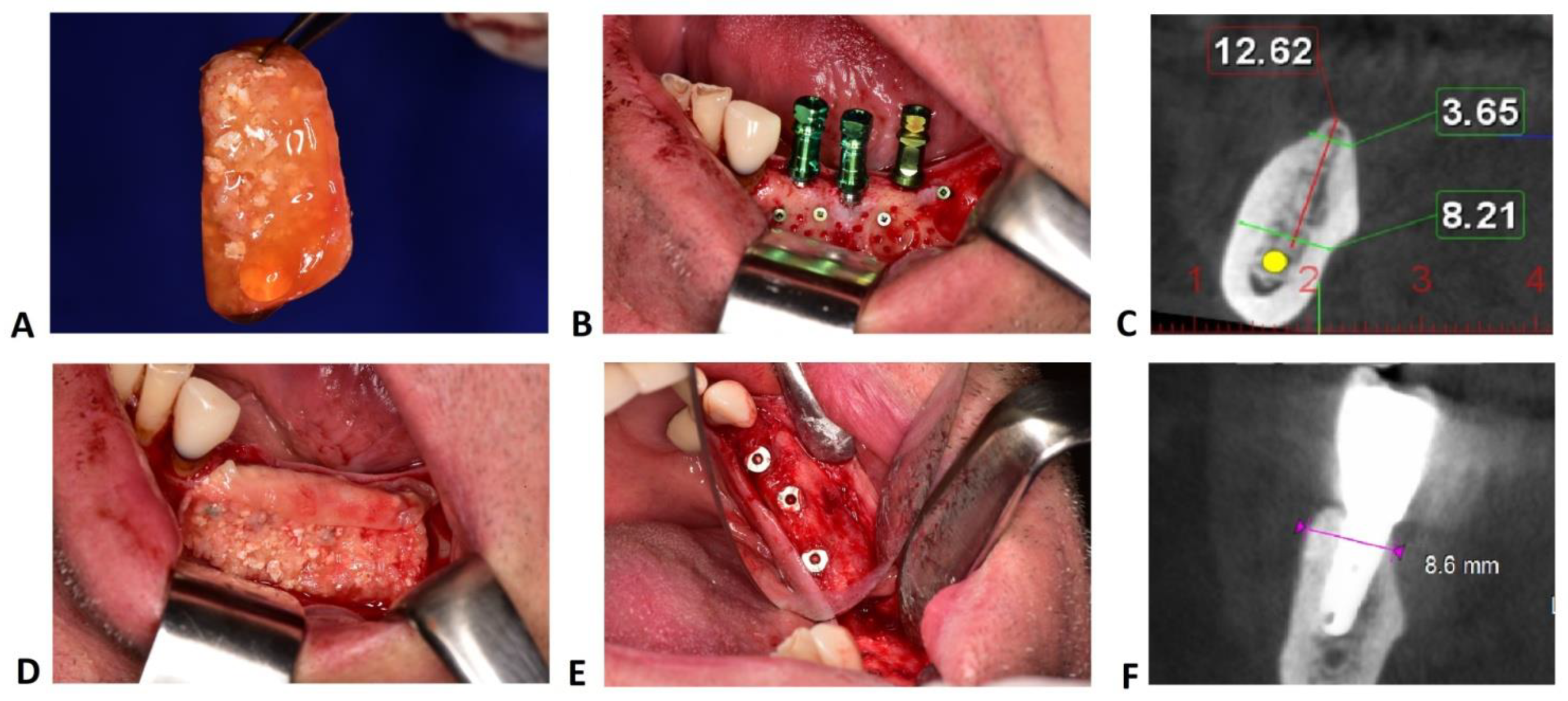
2.3. Surgical Protocol
2.4. Radiographic Measurements
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gowd, M.S.; Shankar, T.; Rajeev, R.; Singh, A. Prosthetic consideration in implant-supported prosthesis: A review of literature. J. Int. Soc. Prev. Community Dent. 2017, 7, S1–S7. [Google Scholar]
- Sharma, M.S.; Pandey, V.; Vartak, V.; Bondekar, V. Prosthetic Driven Implantology—A Review. Int. J. Res. Health Allied Sci. 2016, 2, 21–25. [Google Scholar]
- Gultekin, A.B.; Cansiz, E.; Yalcin, S. Ridge augmentation techniques in preprosthetic implant surgery. In A Textbook of Advanced Oral and Maxillofacial Surgery; Intech Open: London, UK, 2016; Volume 3. [Google Scholar]
- Rathee, M.; Bhoria, M.; Tamrakar, A.K. Restoration driven implant placement, key to predictable dental implant success: A Review. Res. Rev. J. Dent. Sci. 2013, 2, 2320–7949. [Google Scholar]
- Pagni, G.; Pellegrini, G.; Giannobile, W.V.; Rasperini, G. Postextraction alveolar ridge preservation: Biological basis and treatments. Int. J. Dent. 2012, 151030. [Google Scholar] [CrossRef] [Green Version]
- Schropp, L.; Wenzel, A.; Kostopoulos, L.; Karring, T. Bone healing and soft tissue contour changes following single-tooth extraction: A clinical and radiographic 12-month prospective study. Int. J. Periodontics Restor. Dent. 2003, 23, 313–323. [Google Scholar]
- Cha, H.S.; Kim, J.W.; Hwang, J.H.; Ahn, K.M. Frequency of bone graft in implant surgery. Maxillofac. Plast. Reconstr. Surg. 2016, 38, 19. [Google Scholar] [CrossRef] [Green Version]
- Farina, R.; Pramstraller, M.; Franceschetti, G.; Pramstraller, C.; Trombelli, L. Alveolar ridge dimensions in maxillary posterior sextants: A retrospective comparative study of dentate and edentulous sites using computerized tomography data. Clin. Oral Implant. Res. 2011, 22, 1138–1144. [Google Scholar] [CrossRef]
- Braut, V.; Bornstein, M.M.; Kuchler, U.; Buser, D. Bone dimensions in the posterior mandible: A retrospective radiographic study using cone beam computed tomography. Part 2-analysis of edentulous sites. Int. J. Periodontics Dent. 2014, 34, 639–647. [Google Scholar] [CrossRef] [Green Version]
- Mittal, Y.; Jindal, G.; Garg, S. Bone manipulation procedures in dental implants. Indian J. Dent. 2016, 7, 86–94. [Google Scholar] [CrossRef] [Green Version]
- Pommer, B.; Busenlechner, D.; Fürhauser, R.; Watzek, G.; Mailath-Pokorny, G.; Haas, R. Trends in techniques to avoid bone augmentation surgery: Application of short implants, narrow-diameter implants and guided surgery. J. Craniomaxillofac. Surg. 2016, 44, 1630–1634. [Google Scholar] [CrossRef]
- Vercellotti, T.; Troiano, G.; Oreglia, F.; Lombardi, T.; Gregorig, G.; Morella, E.; Rapani, A.; Stacchi, C. Wedge-shaped implants for minimally invasive treatment of narrow ridges: A multicenter prospective cohort study. J. Clin. Med. 2020, 9, 3301. [Google Scholar] [CrossRef]
- Cortés-Bretón Brinkmann, J.; García-Gil, I.; Pedregal, P.; Peláez, J.; Prados-Frutos, J.C.; Suárez, M.J. Long-term clinical behavior and complications of intentionally tilted dental implants compared with straight implants supporting fixed restorations: A systematic review and meta-analysis. Biology 2021, 10, 509. [Google Scholar] [CrossRef]
- Misch, C. Dental Implant Prosthetics, 2nd ed.; Mosby: St. Louis, MO, USA, 2014; p. 45. [Google Scholar]
- Al-Jasser, R.; Andreana, S. An overview of bone augmentation techniques. Clin. Case Rep. Rev. 2016, 2, 393–398. [Google Scholar] [CrossRef] [Green Version]
- Palacio García-Ochoa, A.; Pérez-González, F.; Negrillo Moreno, A.; Sánchez-Labrador, L.; Cortés-Bretón Brinkmann, J.; Martínez-González, J.M.; López-Quiles Martínez, J. Complications associated with inferior alveolar nerve reposition technique for simultaneous implant-based rehabilitation of atrophic mandibles. A systematic literature review. J. Stomatol. Oral Maxillofac. Surg. 2020, 121, 390–396. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, N.; Barona-Dorado, C.; Cortés-Breton Brinkmann, J.; Martín Ares, M.; Calvo-Guirado, J.L.; Martínez-González, J.M. Clinical and radiographic evaluation of implants placed by means of inferior alveolar nerve lateralization: A 5-year follow-up study. Clin. Oral Implant. Res. 2018, 29, 779–784. [Google Scholar] [CrossRef]
- Motamedian, S.R.; Khojaste, M.; Khojasteh, A. Success rate of implants placed in autogenous bone blocks versus allogenic bone blocks: A systematic literature review. Ann. Maxillofac. Surg. 2016, 6, 78–90. [Google Scholar]
- Orsini, G.; Stacchi, C.; Visintini, E.; Di Iorio, D.; Putignano, A.; Breschi, L.; Di Lenarda, R. Clinical and histologic evaluation of fresh frozen human bone grafts for horizontal reconstruction of maxillary alveolar ridges. Int. J. Periodontics Restor. Dent. 2011, 31, 535–544. [Google Scholar]
- Khoury, F.; Hanser, T. Mandibular bone block harvesting from the retromolar region: A 10-year prospective clinical study. Int. J. Oral Maxillofac. Implant. 2015, 30, 688–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tunkel, J.; de Stavola, L.; Kloss-Brandstätter, A. Alveolar ridge augmentation using the shell technique with allogeneic and autogenous bone plates in a split-mouth design—A retrospective case report from five patients. Clin. Case Rep. 2020, 9, 947–959. [Google Scholar] [CrossRef]
- Sohn, D.S.; Huang, B.; Kim, J.; Park, W.E. Utilization of autologous Concentrated Growth Factors (CGF) enriched bone graft matrix (sticky bone) and CGF-enriched fibrin membrane in implant dentistry. J. Implant. Adv. Clin. Dent. 2015, 7, 11–29. [Google Scholar]
- Sbricoli, L.; Guazzo, R.; Annunziata, M.; Gobbato, L.; Bressan, E.; Nastri, L. Selection of collagen membranes for bone regeneration: A literature review. Materials 2020, 13, 786. [Google Scholar] [CrossRef] [Green Version]
- Ronda, M.; Rebaudi, A.; Torelli, L.; Stacchi, C. Expanded vs. dense polytetrafluoroethylene membranes in vertical ridge augmentation around dental implants: A prospective randomized controlled clinical trial. Clin. Oral Implant. Res. 2014, 25, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.A. Evolution, current status and advances in application of platelet concentrate in periodontics and implantology. World J. Clin. Cases 2017, 5, 159–171. [Google Scholar] [CrossRef]
- Upadhayaya, V.; Arora, A.; Goyal, A. Bioactive platelet aggregates: Prp, Prgf, Prf, Cgf and sticky bone. IOSR J. Dent. Med. Sci. 2017, 16, 5–11. [Google Scholar] [CrossRef]
- Lokwani, B.V.; Gupta, D.; Agrawal, R.S.; Mehta, S.; Nirmal, N.J. The use of concentrated growth factor in dental implantology: A systematic review. J. Indian Prosthodont. Soc. 2020, 20, 3–10. [Google Scholar]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. STROBE Initiative. The Strengthening the Reporting of Observational studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. PLoS Med. 2007, 4, e296. [Google Scholar] [CrossRef] [Green Version]
- Romanos, G.E. Periosteal releasing incision for successful coverage of augmented sites. A technical note. J. Oral Implantol. 2020, 36, 25–30. [Google Scholar] [CrossRef]
- Ronda, M.; Stacchi, C. Management of a coronally advanced lingual flap in regenerative osseous surgery: A case series introducing a novel technique. Int. J. Periodontics Restor. Dent. 2011, 31, 505–513. [Google Scholar]
- Ronda, M.; Stacchi, C. A novel approach for the coronal advancement of the buccal flap. Int. J. Periodontics Restor. Dent. 2015, 35, 795–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sentineri, R.; Lombardi, T.; Berton, F.; Stacchi, C. Laurell-Gottlow suture modified by Sentineri for tight closure of a wound with a single line of sutures. Br. J. Oral Maxillofac. Surg. 2016, 54, e18–e19. [Google Scholar] [CrossRef]
- Gomez-Roman, G.; Launer, S. Peri-implant bone changes in immediate and non-immediate root-analog stepped implants-a matched comparative prospective study up to 10 years. Int. J. Implant. Dent. 2016, 2, 15. [Google Scholar] [CrossRef] [Green Version]
- Shrout, P.E.; Fleiss, J.L. Intraclass correlations: Uses in assessing rater reliability. Psychol. Bull. 1979, 86, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Heinze, G.; Dunkler, D. Five myths about variable selection. Transpl. Int. Off. J. Eur. Soc. Organ Transplant. 2017, 30, 6–10. [Google Scholar] [CrossRef]
- Spray, J.R.; Black, C.G.; Morris, H.F.; Ochi, S. The influence of bone thickness on facial marginal bone response: Stage 1 placement through stage 2 uncovering. Ann. Periodontol. 2000, 5, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.T.; Darby, I.B.; Reynolds, E.C. A prospective clinical study of non-submerged immediate implants: Clinical outcomes and esthetic results. Clin. Oral Implant. Res. 2007, 18, 552–562. [Google Scholar] [CrossRef]
- Nohra, J.; Dagher, M.; Matni, G.; Mokbel, N.; Jobaili, E.; Naaman, N. Effect of primary stability and soft-and hard-tissue thickness on marginal bone loss: A prospective pilot study. Implant. Dent. 2018, 27, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Katsoulis, J.; Enkling, N.; Takeichi, T.; Urban, I.A.; Mericske-Stern, R.; Avrampou, M. Relative bone width of the edentulous maxillary ridge. Clinical implications of digital assessment in presurgical implant planning. Clin. Implant. Dent. Relat. Res. 2012, 14, e213–e223. [Google Scholar] [CrossRef]
- Sánchez-Labrador, L.; Molinero-Mourelle, P.; Pérez-González, F.; Saez-Alcaide, L.M.; Brinkmann, J.C.; Martínez, J.L.; Martínez-González, J.M. Clinical performance of alveolar ridge augmentation with xenogeneic bone block grafts versus autogenous bone block grafts. A systematic review. J. Stomatol. Oral Maxillofac. Surg. 2021, 122, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, A.; Wilde, F.; Heufelder, M.; Winter, K.; Schramm, A. Autogenous bone grafts in oral implantology-is it still a "gold standard"? A consecutive review of 279 patients with 456 clinical procedures. Int. J. Implant. Dent. 2017, 3, 23. [Google Scholar] [CrossRef]
- Korsch, M.; Kasprzyk, S.; Walther, W.; Bartols, A. Lateral alveolar ridge augmentation with autogenous block grafts fixed at a distance vs resorbable poly-D-L-lactide foil fixed at a distance: 5-year results of a single-blind, randomised controlled trial. Int. J. Oral Implantol. 2019, 12, 299–312. [Google Scholar]
- Al-Azem, R.; Ali, N.; Mostafa, D. The effectiveness of platelet concentrates in periodontal surgeries. Int. J. Dent. Res. 2018, 6, 61–65. [Google Scholar] [CrossRef]
- Amorfini, L.; Migliorati, M.; Signori, A.; Silvestrini-Biavati, A.; Benedicenti, S. Block allograft technique versus standard guided bone regeneration: A randomized clinical trial. Clin. Implant. Dent. Relat. Res. 2014, 16, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Elnayef, B.; Porta, C.; Suárez-López Del Amo, F.; Mordini, L.; Gargallo-Albiol, J.; Hernández-Alfaro, F. The fate of lateral ridge augmentation: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Implant. 2018, 33, 622–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbu, H.M.; Andreescu, C.F.; Lorean, A.; Kolerman, R. Comparison of two techniques for lateral ridge augmentation in mandible with ramus block graft. J. Craniofacial Surg. 2016, 27, 662–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendoza-Azpur, G.; de la Fuente, A.; Chavez, E.; Valdivia, E.; Khouly, I. Horizontal ridge augmentation with guided bone regeneration using particulate xenogenic bone substitutes with or without autogenous block grafts: A randomized controlled trial. Clin. Implant. Dent. Relat. Res. 2019, 21, 521–530. [Google Scholar] [CrossRef]
- Korsch, M.; Peichl, M. Retrospective Study: Lateral ridge augmentation using autogenous dentin: Tooth-shell technique vs. bone-shell technique. Int. J. Environ. Res. Public Health 2021, 18, 3174. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.; Lin, G.H.; Monje, A.; Chan, H.L.; Wang, H.L. Wound healing complications following guided bone regeneration for ridge augmentation: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Implant. 2018, 33, 41–50. [Google Scholar] [CrossRef]
- Tay, J.; Lu, X.J.; Lai, W.; Fu, J.H. Clinical and histological sequelae of surgical complications in horizontal guided bone regeneration: A systematic review and proposal for management. Int. J. Implant. Dent. 2020, 6, 76. [Google Scholar] [CrossRef]

| Test | Control | Significance | |
|---|---|---|---|
| Age | |||
| Years | 51.0 ± 11.9 | 47.4 ± 9.7 | NS |
| Gender | |||
| Male | 12 | 11 | NS |
| Female | 28 | 29 | |
| Smoking Status | |||
| Smoker | 17 | 15 | NS |
| Non Smoker | 23 | 25 | |
| History of Periodontitis | |||
| Yes | 12 | 13 | NS |
| No | 28 | 27 |
| Number of Cases = 80 | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Post-op Complications | OR | [95% CI] | p-value | OR | [95% CI] | p-value |
| Age | 0.980 | [0.921–1.043] | 0.530 | |||
| History of Periodontitis | 0.935 | [0.221–3.961] | 0.927 | |||
| Gender | 0.214 | [0.054–0.848] | 0.028 * | 0.504 | [0.047–0.887] | 0.074 |
| Smoking | 1.593 | [0.421–6.020] | 0.493 | |||
| Jaw Area (maxilla/mandible) | 1.296 | [0.308–5.461] | 0.724 | |||
| Ridge Width | 0.264 | [0.087–0.802] | 0.019 * | 0.249 | [0.074–0.837] | 0.025 * |
| Surgical Technique (SB/BS) | 1.000 | [0.266–3.763] | 1.000 |
| Sticky Bone | Bone-Shell Technique | Significance | |
|---|---|---|---|
| Preoperative Ridge Width (mm) | 3.7 ± 0.8 | 3.0 ± 0.6 | NS |
| Post-operative Ridge Width (mm) | 7.4 ± 1.1 | 6.7 ± 1.2 | NS |
| Ridge Width Gain (mm) | 3.7 ± 1.2 | 3.7 ± 1.1 | NS |
| Maximum Width Gain (mm) | 6.2 | 6.3 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbu, H.M.; Iancu, S.A.; Rapani, A.; Stacchi, C. Guided Bone Regeneration with Concentrated Growth Factor Enriched Bone Graft Matrix (Sticky Bone) vs. Bone-Shell Technique in Horizontal Ridge Augmentation: A Retrospective Study. J. Clin. Med. 2021, 10, 3953. https://doi.org/10.3390/jcm10173953
Barbu HM, Iancu SA, Rapani A, Stacchi C. Guided Bone Regeneration with Concentrated Growth Factor Enriched Bone Graft Matrix (Sticky Bone) vs. Bone-Shell Technique in Horizontal Ridge Augmentation: A Retrospective Study. Journal of Clinical Medicine. 2021; 10(17):3953. https://doi.org/10.3390/jcm10173953
Chicago/Turabian StyleBarbu, Horia Mihail, Stefania Andrada Iancu, Antonio Rapani, and Claudio Stacchi. 2021. "Guided Bone Regeneration with Concentrated Growth Factor Enriched Bone Graft Matrix (Sticky Bone) vs. Bone-Shell Technique in Horizontal Ridge Augmentation: A Retrospective Study" Journal of Clinical Medicine 10, no. 17: 3953. https://doi.org/10.3390/jcm10173953
APA StyleBarbu, H. M., Iancu, S. A., Rapani, A., & Stacchi, C. (2021). Guided Bone Regeneration with Concentrated Growth Factor Enriched Bone Graft Matrix (Sticky Bone) vs. Bone-Shell Technique in Horizontal Ridge Augmentation: A Retrospective Study. Journal of Clinical Medicine, 10(17), 3953. https://doi.org/10.3390/jcm10173953








