Differential Impact of Cytochrome 2C19 Allelic Variants on Three Different Platelet Function Tests in Clopidogrel-Treated Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Laboratory Measurements
2.2.1. Multiple Electrode Impedance Aggregometry by Multiplate
2.2.2. VerifyNow
2.2.3. Light Transmission Aggregometry (LTA)
2.2.4. CYP2C19 Analysis
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Agreement between Platelet Function Tests
3.3. Residual Platelet Reactivity per Group of CYP2C19 Metabolism
3.4. Effect of Metabolizer Status on Platelet Reactivity
3.5. Relative Importance of Metabolizer Status on Platelet Reactivity
4. Discussion
5. Conclusions and Future Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Inclusion Criteria | Definition |
| PCI 30–90 days before study inclusion | Elective or emergency procedure |
| Dual/triple antithrombotic therapy | Including clopidogrel as P2Y12 inhibitor |
| Classified as ‘vulnerable’ by ≥ 3 predefined risk factors: | Age ≥ 75 years Female gender Renal dysfunction (MDRD-eGFR ≤ 60 mL/min) Body weight ≤ 60 kg Hypertension (previously diagnosed, or on medication) Diabetes mellitus Anemia (Hb < 8.2 mmol/L for men, <7.3 mmol/L for women) Previous stroke Previous major bleeding Liver dysfunction (known hepatitis or transplant) History of gastric/duodenal ulcers Daily use of NSAIDs or SSRIs Triple antithrombotic therapy (DAPT + oral anticoagulants) Previous in-stent thrombosis or high-risk coronary stent (e.g., last remaining vessel or left main coronary artery) |
| CYP2C19 polymorphism available | Analysis of CYP2C19 polymorphism performed by June 2018 |
| Exclusion criteria | Definition |
| Known platelet function disorders | Previously diagnosed platelet function disorders |
| Recent coronary intervention | PCI or CABG ≤ 7 days |
| Recent new ischemic event | ACS or stroke ≤ 7 days |
| Signs of active infection | Fever, antibiotic treatment or hospital admission during laboratory assessment of platelet function |
| Medication noncompliance | Confirmed noncompliance in antithrombotic medication by patient interview or pharmacy dispensing |
| Variables | LTA ADP20 (n = 300) | VerifyNow P2Y12 (n = 304) | Multiplate (n = 305) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate * | Univariate | Multivariate * | Univariate | Multivariate * | |||||||||||||
| B | 95% CI | p | B | 95% CI | p | B | p | B | 95% CI | p | B | 95% CI | p | B | 95% CI | p | ||
| Age ≥ 75 years | 3.456 | −0.072–6.983 | 0.055 | 3.746 | 0.27–7.23 | 0.035 | 28.013 | 11.050–44.976 | 0.001 | 11.381 | −3.88–26.64 | 0.143 | 3.121 | −2.373–8.615 | 0.264 | 3.493 | −1.99–8.97 | 0.211 |
| Gender, female | −1.292 | −4.793–2.209 | 0.468 | 3.973 | −12.950–20.986 | 0.644 | 2.306 | −3.097–7.709 | 0.402 | |||||||||
| Weight < 60 kg | −10.356 | −16.454–−4.259 | 0.001 | −5.386 | −11.64–0.87 | 0.091 | −51.291 | −79.540–−23.042 | 0.000 | −30.905 | −56.79–−5.02 | 0.019 | −3.585 | −13.115–5.944 | 0.460 | −6.025 | −15.64–3.59 | 0.218 |
| Diabetes Mellitus | 0.033 | −3.537–3.602 | 0.986 | −0.028 | −3.45–3.40 | 0.987 | 13.874 | −3.480–31.229 | 0.117 | 7.221 | −7.83–22.27 | 0.346 | 4.480 | −1.044–10.003 | 0.112 | 2.753 | −2.64–8.15 | 0.316 |
| Hypertension | −1.171 | −5.851–3.510 | 0.623 | 4.893 | −17.597–27.383 | 0.669 | −1.923 | −9.114–5.268 | 0.599 | |||||||||
| Renal dysfunction | −1.263 | −4.734–2.209 | 0.475 | −0.878 | −4.31–2.56 | 0.615 | 25.743 | 9.140–42.346 | 0.002 | 8.096 | −7.01–23.20 | 0.292 | 4.920 | −0.449–10.288 | 0.072 | 4.474 | −0.96–9.91 | 0.106 |
| History of bleeding | −0.288 | −5.095–4.520 | 0.906 | 8.777 | −14.269–31.824 | 0.454 | 4.724 | −2.634–12.082 | 0.207 | |||||||||
| Stroke in history # | 4.733 | 1.013–8.453 | 0.013 | 3.699 | 0.11– 7.29 | 0.043 | 17.203 | −0.919–35.325 | 0.063 | 17.793 | 2.02–33.57 | 0.027 | 8.439 | 2.641–14.236 | 0.004 | 8.390 | 2.70–14.08 | 0.004 |
| Current smoking # | −3.147 | −8.176–1.883 | 0.219 | −2.370 | −7.23–2.49 | 0.338 | −33.738 | −58.169–−9.308 | 0.007 | −15.310 | −36.84–6.22 | 0.163 | −0.760 | −8.635–7.116 | 0.850 | −0.019 | −7.65–7.62 | 0.996 |
| (Es-) omeprazole | 1.490 | −3.505–6.484 | 0.558 | 1.644 | −3.97 –7.26 | 0.565 | 24.248 | 0.236–48.259 | 0.048 | 39.726 | 14.96–64.49 | 0.002 | 8.702 | 1.036–16.369 | 0.026 | 13.40 | 4.49–22.32 | 0.003 |
| Aspirin | −6.837 | −10.478–−3.197 | 0.000 | −4.471 | −11.64–2.70 | 0.220 | 6.074 | −12.011–24.160 | 0.509 | −0.286 | −31.20–30.63 | 0.986 | −2.491 | −8.305–3.324 | 0.400 | −0.979 | −12.12–10.17 | 0.863 |
| Anticoagulant | 6.134 | 2.587–9.681 | 0.001 | 2.343 | −4.61–9.30 | 0.508 | −3.139 | −20.674–14.396 | 0.725 | −4.957 | −34.91–24.99 | 0.745 | 2.204 | −3.422–7.831 | 0.441 | 2.064 | −8.70–12.83 | 0.706 |
| Hemoglobin (mmol/L) | 2.347 | 0.774–3.920 | 0.004 | 2.145 | 0.50–3.79 | 0.011 | −20.674 | −28.101–−13.248 | 0.000 | −22.703 | −30.01–−15.40 | 0.000 | −3.396 | −5.864–−0.928 | 0.007 | −1.021 | −3.64–1.60 | 0.444 |
| Platelets (1×109/L) | −0.035 | −0.056–−0.014 | 0.001 | −0.013 | −0.04–0.01 | 0.246 | −0.223 | −0.324–−0.122 | 0.000 | −0.243 | −0.34–−0.15 | 0.000 | 0.070 | 0.038–0.103 | 0.000 | 0.076 | 0.04–0.11 | 0.000 |
| Metabolizer status PM IM EM RM | 6.895 | −3.222–17.012 | 0.181 | 8.644 | −1.16–18.44 | 0.084 | 79.859 | 28.945–130.773 | 0.002 | 47.029 | 1.49–92.56 | 0.043 | 3.859 | −12.154–19.872 | 0.636 | 6.243 | −9.30–21.78 | 0.430 |
| 3.173 | −1.147–7.492 | 0.149 | 2.958 | −1.23–7.14 | 0.165 | 30.234 | 9.804–50.664 | 0.004 | 23.981 | 5.64–42.32 | 0.011 | 8.264 | 1.488–15.040 | 0.017 | 8.792 | 2.21–15.37 | 0.009 | |
| ref | ref | - | ref | ref | - | ref | - | ref | ref | - | ref | - | ref | ref | - | |||
| −4.153 | −8.154–−0.151 | 0.042 | −4.648 | −8.49–−0.81 | 0.018 | −17.381 | −36.306–1.543 | 0.072 | −12.047 | −28.87–4.77 | 0.160 | 1.757 | −4.533–8.047 | 0.583 | 1.516 | −4.55–7.58 | 0.623 | |
References
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef] [PubMed]
- Amsterdam, E.A.; Wenger, N.K.; Brindis, R.G.; Casey, D.E., Jr.; Ganiats, T.G.; Holmes, D.R., Jr.; Jaffe, A.S.; Jneid, H.; Kelly, R.F.; Kontos, M.C.; et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 130, e344–e426. [Google Scholar] [CrossRef]
- Dayoub, E.J.; Seigerman, M.; Tuteja, S.; Kobayashi, T.; Kolansky, D.M.; Giri, J.; Groeneveld, P.W. Trends in Platelet Adenosine Diphosphate P2Y12 Receptor Inhibitor Use and Adherence Among Antiplatelet-Naive Patients After Percutaneous Coronary Intervention, 2008–2016. JAMA Intern. Med. 2018, 178, 943–950. [Google Scholar] [CrossRef] [Green Version]
- Mega, J.L.; Simon, T.; Collet, J.P.; Anderson, J.L.; Antman, E.M.; Bliden, K.; Cannon, C.P.; Danchin, N.; Giusti, B.; Gurbel, P.; et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: A meta-analysis. JAMA 2010, 304, 1821–1830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sibbing, D.; Stegherr, J.; Latz, W.; Koch, W.; Mehilli, J.; Dorrler, K.; Morath, T.; Schomig, A.; Kastrati, A.; von Beckerath, N. Cytochrome P450 2C19 loss-of-function polymorphism and stent thrombosis following percutaneous coronary intervention. Eur. Heart J. 2009, 30, 916–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shuldiner, A.R.; O’Connell, J.R.; Bliden, K.P.; Gandhi, A.; Ryan, K.; Horenstein, R.B.; Damcott, C.M.; Pakyz, R.; Tantry, U.S.; Gibson, Q.; et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA 2009, 302, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.A.; Sangkuhl, K.; Shuldiner, A.R.; Hulot, J.S.; Thorn, C.F.; Altman, R.B.; Klein, T.E. PharmGKB summary: Very important pharmacogene information for cytochrome P450, family 2, subfamily C, polypeptide 19. Pharm. Genom. 2012, 22, 159–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, S.A.; Sangkuhl, K.; Stein, C.M.; Hulot, J.S.; Mega, J.L.; Roden, D.M.; Klein, T.E.; Sabatine, M.S.; Johnson, J.A.; Shuldiner, A.R.; et al. Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin. Pharmacol. Ther. 2013, 94, 317–323. [Google Scholar] [CrossRef]
- Simon, T.; Verstuyft, C.; Mary-Krause, M.; Quteineh, L.; Drouet, E.; Meneveau, N.; Steg, P.G.; Ferrieres, J.; Danchin, N.; Becquemont, L.; et al. Genetic determinants of response to clopidogrel and cardiovascular events. N. Engl. J. Med. 2009, 360, 363–375. [Google Scholar] [CrossRef] [Green Version]
- Mega, J.L.; Close, S.L.; Wiviott, S.D.; Shen, L.; Hockett, R.D.; Brandt, J.T.; Walker, J.R.; Antman, E.M.; Macias, W.; Braunwald, E.; et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N. Engl. J. Med. 2009, 360, 354–362. [Google Scholar] [CrossRef] [Green Version]
- Collet, J.P.; Hulot, J.S.; Pena, A.; Villard, E.; Esteve, J.B.; Silvain, J.; Payot, L.; Brugier, D.; Cayla, G.; Beygui, F.; et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: A cohort study. Lancet 2009, 373, 309–317. [Google Scholar] [CrossRef]
- Sibbing, D.; Koch, W.; Gebhard, D.; Schuster, T.; Braun, S.; Stegherr, J.; Morath, T.; Schomig, A.; von Beckerath, N.; Kastrati, A. Cytochrome 2C19* 17 allelic variant, platelet aggregation, bleeding events, and stent thrombosis in clopidogrel-treated patients with coronary stent placement. Circulation 2010, 121, 512–518. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Tang, H.L.; Hu, Y.F.; Xie, H.G. The gain-of-function variant allele CYP2C19*17: A double-edged sword between thrombosis and bleeding in clopidogrel-treated patients. J. Thromb. Haemost. 2012, 10, 199–206. [Google Scholar] [CrossRef]
- Gross, L.; Trenk, D.; Jacobshagen, C.; Krieg, A.; Gawaz, M.; Massberg, S.; Baylacher, M.; Aradi, D.; Stimpfle, F.; Hromek, J.; et al. Genotype-Phenotype Association and Impact on Outcomes following Guided De-Escalation of Anti-Platelet Treatment in Acute Coronary Syndrome Patients: The TROPICAL-ACS Genotyping Substudy. Thromb. Haemost. 2018, 118, 1656–1667. [Google Scholar] [CrossRef] [Green Version]
- Tiroch, K.A.; Sibbing, D.; Koch, W.; Roosen-Runge, T.; Mehilli, J.; Schomig, A.; Kastrati, A. Protective effect of the CYP2C19*17 polymorphism with increased activation of clopidogrel on cardiovascular events. Am. Heart J. 2010, 160, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.R.; Thomas, C.D.; Beitelshees, A.L.; Tuteja, S.; Empey, P.E.; Lee, J.C.; Limdi, N.A.; Duarte, J.D.; Skaar, T.C.; Chen, Y.; et al. Impact of the CYP2C19*17 Allele on Outcomes in Patients Receiving Genotype-Guided Antiplatelet Therapy After Percutaneous Coronary Intervention. Clin. Pharmacol. Ther. 2021, 109, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.P.; Stephens, S.H.; Horenstein, R.B.; O’Connell, J.R.; Ryan, K.; Peer, C.J.; Figg, W.D.; Spencer, S.D.; Pacanowski, M.A.; Mitchell, B.D.; et al. The CYP2C19*17 variant is not independently associated with clopidogrel response. J. Thromb. Haemost. 2013, 11, 1640–1646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Braunwald, E.; McCabe, C.H.; Montalescot, G.; Ruzyllo, W.; Gottlieb, S.; Neumann, F.J.; Ardissino, D.; De Servi, S.; Murphy, S.A.; et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2007, 357, 2001–2015. [Google Scholar] [CrossRef] [Green Version]
- Sibbing, D.; Aradi, D.; Alexopoulos, D.; Ten Berg, J.; Bhatt, D.L.; Bonello, L.; Collet, J.P.; Cuisset, T.; Franchi, F.; Gross, L.; et al. Updated Expert Consensus Statement on Platelet Function and Genetic Testing for Guiding P2Y12 Receptor Inhibitor Treatment in Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2019, 12, 1521–1537. [Google Scholar] [CrossRef]
- Price, M.J.; Berger, P.B.; Teirstein, P.S.; Tanguay, J.F.; Angiolillo, D.J.; Spriggs, D.; Puri, S.; Robbins, M.; Garratt, K.N.; Bertrand, O.F.; et al. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: The GRAVITAS randomized trial. JAMA 2011, 305, 1097–1105. [Google Scholar] [CrossRef]
- Collet, J.P.; Cuisset, T.; Range, G.; Cayla, G.; Elhadad, S.; Pouillot, C.; Henry, P.; Motreff, P.; Carrie, D.; Boueri, Z.; et al. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N. Engl. J. Med. 2012, 367, 2100–2109. [Google Scholar] [CrossRef] [Green Version]
- Cayla, G.; Cuisset, T.; Silvain, J.; Leclercq, F.; Manzo-Silberman, S.; Saint-Etienne, C.; Delarche, N.; Bellemain-Appaix, A.; Range, G.; El Mahmoud, R.; et al. Platelet function monitoring to adjust antiplatelet therapy in elderly patients stented for an acute coronary syndrome (ANTARCTIC): An open-label, blinded-endpoint, randomised controlled superiority trial. Lancet 2016, 388, 2015–2022. [Google Scholar] [CrossRef]
- Vries, M.J.; Bouman, H.J.; Olie, R.H.; Veenstra, L.F.; Zwaveling, S.; Verhezen, P.W.; Ten Cate-Hoek, A.J.; Ten Cate, H.; Henskens, Y.M.; van der Meijden, P.E. Determinants of agreement between proposed therapeutic windows of platelet function tests in vulnerable patients. Eur. Heart J. Cardiovasc. Pharmacother. 2016, 3, 11–17. [Google Scholar] [CrossRef]
- Lordkipanidze, M.; Pharand, C.; Nguyen, T.A.; Schampaert, E.; Palisaitis, D.A.; Diodati, J.G. Comparison of four tests to assess inhibition of platelet function by clopidogrel in stable coronary artery disease patients. Eur. Heart J. 2008, 29, 2877–2885. [Google Scholar] [CrossRef] [Green Version]
- Gremmel, T.; Steiner, S.; Seidinger, D.; Koppensteiner, R.; Panzer, S.; Kopp, C.W. Comparison of methods to evaluate clopidogrel-mediated platelet inhibition after percutaneous intervention with stent implantation. Thromb. Haemost. 2009, 101, 333–339. [Google Scholar]
- Paniccia, R.; Antonucci, E.; Gori, A.M.; Marcucci, R.; Giglioli, C.; Antoniucci, D.; Gensini, G.F.; Abbate, R.; Prisco, D. Different methodologies for evaluating the effect of clopidogrel on platelet function in high-risk coronary artery disease patients. J. Thromb. Haemost. 2007, 5, 1839–1847. [Google Scholar] [CrossRef]
- Paniccia, R.; Antonucci, E.; Maggini, N.; Miranda, M.; Gori, A.M.; Marcucci, R.; Giusti, B.; Balzi, D.; Prisco, D.; Abbate, R. Comparison of methods for monitoring residual platelet reactivity after clopidogrel by point-of-care tests on whole blood in high-risk patients. Thromb. Haemost. 2010, 104, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Claassens, D.M.F.; Vos, G.J.A.; Bergmeijer, T.O.; Hermanides, R.S.; van’t Hof, A.W.J.; van der Harst, P.; Barbato, E.; Morisco, C.; Tjon Joe Gin, R.M.; Asselbergs, F.W.; et al. A Genotype-Guided Strategy for Oral P2Y12 Inhibitors in Primary PCI. N. Engl. J. Med. 2019, 381, 1621–1631. [Google Scholar] [CrossRef]
- Pereira, N.L.; Farkouh, M.E.; So, D.; Lennon, R.; Geller, N.; Mathew, V.; Bell, M.; Bae, J.H.; Jeong, M.H.; Chavez, I.; et al. Effect of Genotype-Guided Oral P2Y12 Inhibitor Selection vs Conventional Clopidogrel Therapy on Ischemic Outcomes After Percutaneous Coronary Intervention: The TAILOR-PCI Randomized Clinical Trial. JAMA 2020, 324, 761–771. [Google Scholar] [CrossRef]
- Hulot, J.S.; Chevalier, B.; Belle, L.; Cayla, G.; Khalife, K.; Funck, F.; Berthier, R.; Piot, C.; Tafflet, M.; Montalescot, G.; et al. Routine CYP2C19 Genotyping to Adjust Thienopyridine Treatment After Primary PCI for STEMI: Results of the GIANT Study. JACC Cardiovasc. Interv. 2020, 13, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Pereira, N.L.; Rihal, C.; Lennon, R.; Marcus, G.; Shrivastava, S.; Bell, M.R.; So, D.; Geller, N.; Goodman, S.G.; Hasan, A.; et al. Effect of CYP2C19 Genotype on Ischemic Outcomes During Oral P2Y12 Inhibitor Therapy: A Meta-Analysis. JACC Cardiovasc. Interv. 2021, 14, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Olie, R.H.; Van der Meijden, P.E.J.; Vries, M.J.A.; Veenstra, L.; van’t Hof, A.W.J.; Ten Berg, J.M.; Henskens, Y.M.C.; Ten Cate, H. Antithrombotic therapy in high-risk patients after percutaneous coronary intervention; study design, cohort profile and incidence of adverse events. Neth. Heart J. 2021. [Google Scholar] [CrossRef]
- Bank, P.C.D.; Caudle, K.E.; Swen, J.J.; Gammal, R.S.; Whirl-Carrillo, M.; Klein, T.E.; Relling, M.V.; Guchelaar, H.J. Comparison of the Guidelines of the Clinical Pharmacogenetics Implementation Consortium and the Dutch Pharmacogenetics Working Group. Clin. Pharmacol. Ther. 2018, 103, 599–618. [Google Scholar] [CrossRef]
- Hochholzer, W.; Trenk, D.; Fromm, M.F.; Valina, C.M.; Stratz, C.; Bestehorn, H.P.; Buttner, H.J.; Neumann, F.J. Impact of cytochrome P450 2C19 loss-of-function polymorphism and of major demographic characteristics on residual platelet function after loading and maintenance treatment with clopidogrel in patients undergoing elective coronary stent placement. J. Am. Coll. Cardiol. 2010, 55, 2427–2434. [Google Scholar] [CrossRef] [Green Version]
- Gremmel, T.; Steiner, S.; Seidinger, D.; Koppensteiner, R.; Panzer, S.; Kopp, C.W. The influencing factors for clopidogrel-mediated platelet inhibition are assay-dependent. Thromb. Res. 2011, 128, 352–357. [Google Scholar] [CrossRef]
- Wallen, N.H.; Ladjevardi, M.; Albert, J.; Broijersen, A. Influence of different anticoagulants on platelet aggregation in whole blood; a comparison between citrate, low molecular mass heparin and hirudin. Thromb. Res. 1997, 87, 151–157. [Google Scholar] [CrossRef]
- van Werkum, J.W.; Harmsze, A.M.; Elsenberg, E.H.; Bouman, H.J.; Ten Berg, J.M.; Hackeng, C.M. The use of the VerifyNow system to monitor antiplatelet therapy: A review of the current evidence. Platelets 2008, 19, 479–488. [Google Scholar] [CrossRef]
- Hulshof, A.M.; Vries, M.; Verhezen, P.; Wetzels, R.; Haartmans, M.; Olie, R.; Ten Cate, H.; Henskens, Y. The Influence of Prostaglandin E1 and Use of Inhibitor Percentage on the Correlation between the Multiplate and VerifyNow in Patients on Dual Antiplatelet Therapy. Platelets 2020, 32, 463–468. [Google Scholar] [CrossRef] [Green Version]
- Sibbing, D.; Braun, S.; Jawansky, S.; Vogt, W.; Mehilli, J.; Schomig, A.; Kastrati, A.; von Beckerath, N. Assessment of ADP-induced platelet aggregation with light transmission aggregometry and multiple electrode platelet aggregometry before and after clopidogrel treatment. Thromb. Haemost. 2008, 99, 121–126. [Google Scholar] [CrossRef]
- Harmsze, A.M.; van Werkum, J.W.; Hackeng, C.M.; Ruven, H.J.; Kelder, J.C.; Bouman, H.J.; Breet, N.J.; Ten Berg, J.M.; Klungel, O.H.; de Boer, A.; et al. The influence of CYP2C19*2 and *17 on on-treatment platelet reactivity and bleeding events in patients undergoing elective coronary stenting. Pharm. Genom. 2012, 22, 169–175. [Google Scholar] [CrossRef]
- Frere, C.; Cuisset, T.; Gaborit, B.; Alessi, M.C.; Hulot, J.S. The CYP2C19*17 allele is associated with better platelet response to clopidogrel in patients admitted for non-ST acute coronary syndrome. J. Thromb. Haemost. 2009, 7, 1409–1411. [Google Scholar] [CrossRef] [PubMed]
- Gremmel, T.; Kopp, C.W.; Seidinger, D.; Koppensteiner, R.; Panzer, S.; Sunder-Plassmann, R.; Mannhalter, C.; Steiner, S. Differential impact of cytochrome 2C9 allelic variants on clopidogrel-mediated platelet inhibition determined by five different platelet function tests. Int. J. Cardiol. 2013, 166, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Notarangelo, F.M.; Maglietta, G.; Bevilacqua, P.; Cereda, M.; Merlini, P.A.; Villani, G.Q.; Moruzzi, P.; Patrizi, G.; Malagoli Tagliazucchi, G.; Crocamo, A.; et al. Pharmacogenomic Approach to Selecting Antiplatelet Therapy in Patients With Acute Coronary Syndromes: The PHARMCLO Trial. J. Am. Coll. Cardiol. 2018, 71, 1869–1877. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Ma, Y.T.; Yang, Y.N.; Li, X.M.; Zheng, Y.Y.; Ma, X.; Fu, Z.Y.; Ba, B.; Li, Y.; Yu, Z.X.; et al. Personalized antiplatelet therapy according to CYP2C19 genotype after percutaneous coronary intervention: A randomized control trial. Int. J. Cardiol. 2013, 168, 3736–3740. [Google Scholar] [CrossRef]
- Levine, G.N.; Bates, E.R.; Bittl, J.A.; Brindis, R.G.; Fihn, S.D.; Fleisher, L.A.; Granger, C.B.; Lange, R.A.; Mack, M.J.; Mauri, L.; et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients with Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation 2016, 134, e123–e155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthelemy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2020. [Google Scholar] [CrossRef] [PubMed]
- Cavallari, L.H.; Lee, C.R.; Beitelshees, A.L.; Cooper-DeHoff, R.M.; Duarte, J.D.; Voora, D.; Kimmel, S.E.; McDonough, C.W.; Gong, Y.; Dave, C.V.; et al. Multisite Investigation of Outcomes with Implementation of CYP2C19 Genotype-Guided Antiplatelet Therapy After Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2018, 11, 181–191. [Google Scholar] [CrossRef]
- Price, M.J.; Angiolillo, D.J. Pharmacogenomic Testing to Select Antiplatelet Therapy. J. Am. Coll. Cardiol. 2018, 71, 1878–1881. [Google Scholar] [CrossRef]

| All Patients (n = 308) |
RM (n = 107) |
EM (n = 112) |
IM (n = 80) |
PM (n = 9) | p ^ | |
|---|---|---|---|---|---|---|
| Age, years | 75.2 (8.5) | 74.9 (9.6) | 74.7 (7.9) | 76.5 (7.4) | 74.3 (9.4) | 0.478 |
| Female | 127 (41.2) | 47 (43.9) | 43 (38.4) | 34 (42.5) | 3 (33.3) | 0.807 |
| BMI, kg/m2 | 27.4 (4.5) | 27.8 (4.5) | 27.2 (4.6) | 27.3 (4.6) | 25.6 (3.9) | 0.477 |
| Current smoking | 41 (13.3) | 12 (11.2) | 18 (16.5) | 11 (14.5) | 0 (0.0) | 0.429 |
| Index PCI–ACS | 172 (55.8) | 59 55.1 | 60 (53.6) | 47 (58.8) | 6 (66.7) | 0.809 |
| Index PCI–Elective | 136 (44.2) | 48 (44.9) | 52 (46.4) | 33 (41.2) | 3 (33.3) | |
| Medication | ||||||
| Clopidogrel | 308 (100.0) | 107 (100.0) | 112(100.0) | 80(100.0) | 9 (100.0) | 1.000 |
| Aspirin | 214 (69.5) | 71 (66.4) | 77 (68.8) | 57 (71.3) | 9 (100.0) | 0.204 |
| VKA | 72 (23.4) | 28 (26.2) | 27 (24.1) | 17 (21.3) | 0 (0.0) | 0.328 |
| DOAC | 32 (10.4) | 10 (9.3) | 12 (10.7) | 10 (12.5) | 0 (0.0) | 0.668 |
| (es-)omeprazole use | 28 (9.1) | 9 (8.4) | 13 (11.6) | 5 (6.3%) | 1 (11.1) | 0.646 |
| Risk factors | ||||||
| Age ≥ 75 years | 193 (62.7) | 65 (60.7) | 67 (59.8) | 56 (70.0) | 5 (55.6) | 0.459 |
| Women | 127 (41.2) | 47 (43.9) | 43 (38.3) | 34 (43.) | 3 (33.3) | 0.807 |
| Weight < 60 kg | 28 (9.1) | 11 (10.3) | 10 (8.9) | 7 (8.8) | 0 (0.0) | 0.778 |
| Diabetes mellitus | 110 (35.7) | 35 (32.7) | 39 (34.8) | 29 (36.3) | 7 (77.8) | 0.060 |
| Hypertension | 258 (83.8) | 94 (87.9) | 92 (82.1) | 66 (82.5) | 6 (66.7) | 0.313 |
| Anemia | 107 (34.7) | 37 (34.6) | 39 (34.8) | 27 (33.8) | 4 (44.4) | 0.938 |
| Renal dysfunction | 178 (57.8) | 52 (48.6) | 67 (59.8) | 53 (66.3) | 6 (66.7) | 0.088 |
| Liver failure | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Peptic ulcer disease | 42 (13.6) | 12 (11.2) | 15 (13.4) | 13 (16.3) | 2 (22.2) | 0.667 |
| Prior major bleeding | 47 (15.3) | 21 (19.6) | 13 (11.6) | 11 (13.8) | 2 (22.2) | 0.360 |
| Previous stroke | 89 (28.9) | 31 (29.0) | 32 (28.8) | 24 (30.0) | 2 (22.2) | 0.971 |
| Use of NSAIDs | 14 (4.5) | 4 (3.7) | 6 (5.4) | 4 (5.0) | 0 (0.0) | 0.850 |
| Use of SSRIs | 15 (4.9) | 4 (3.7) | 7 (6.3) | 4 (5.0) | 0 (0.0) | 0.748 |
| Triple therapy | 34 (11.0) | 11 (10.3) | 15 (13.4) | 7 (8.8) | 1 (11.1) | 0.772 |
| High-risk PCI | 17 (5.5) | 9 (8.4) | 5 (4.5) | 3 (3.8) | 0 (0.0) | 0.398 |
| Previous history | ||||||
| Prior PCI | 113 (36.7) | 31 (29.0) | 42 (37.5) | 35 (43.8) | 5 (55.6) | 0.118 |
| Prior CABG | 69 (22.4) | 22 (20.6) | 30 (26.8) | 16 (20.0) | 1 (11.1) | 0.520 |
| Atrial fibrillation | 90 (29.2) | 33 (30.8) | 30 (26.8) | 27 (33.8) | 0 (0.0) | 0.174 |
| Active malignancy | 16 (5.2) | 4 (3.7) | 6 f(5.4) | 5 (6.3) | 1 (11.1) | 0.732 |
| Laboratory test | ||||||
| Hemoglobin, mmol/L | 8.2 (1.1) | 8.3 (1.1) | 8.2 (1.0) | 8.1 (1.1) | 7.9 (1.0) | 0.582 |
| Platelet count, 1×109/L | 257 (76) | 269 (85) | 256 (73) | 246 (66) | 218 (45) | 0.080 |
| Creatinine, µmol/L | 117 (67) | 104 (48) | 126 (85) | 120 (55) | 131 (85) | 0.102 |
| MDRD-eGFR, mL/min/1.73 m2 | 56.1 (20.1) | 59.9 (18.6) | 54.9 (21.6) | 52 (19.1) | 55.4 (23.1) | 0.082 |
| Metabolism | CYP2C19 Alleles | Frequency n (%), n = 308 | Group Total n (%) |
|---|---|---|---|
| Rapid metabolizer (RM) | *1/*17 | 91 (29.5) | 107 (34.7) |
| *17/*17 | 16 (5.2) | ||
| Extensive metabolizer (EM) | *1/*1 | 112 (36.4) | 112 (36.4) |
| Intermediate metabolizer (IM) | *1/*2 | 67 (21.8) | 80 (26.0) |
| *2/*17 | 12 (3.9) | ||
| *1/*3 | 1 (0.3) | ||
| Poor metabolizer (PM) | *2/*2 | 9 (2.9) | 9 (2.9) |
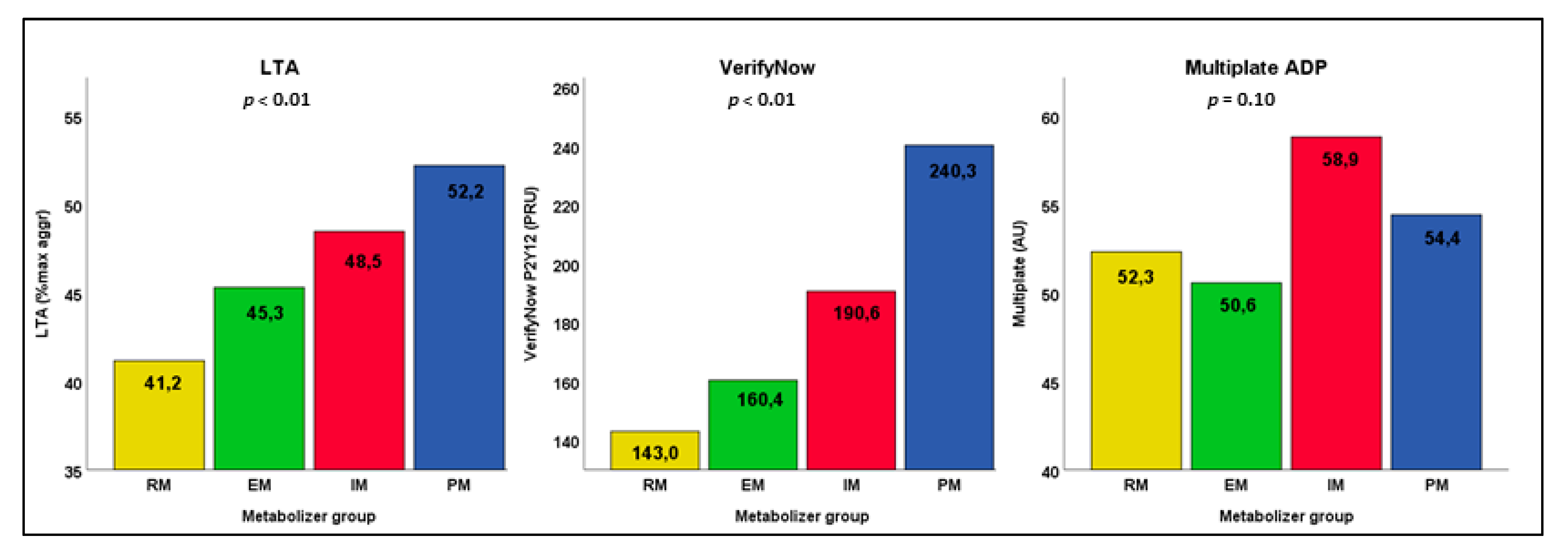
| Platelet Function Test | CYP2C19 Metabolism * | Patients, n(%) Total n = 308 | Median [IQR] | Jonckheere -Terpstra Test ^ |
|---|---|---|---|---|
| LTA (n = 300), % max aggr | Metabolizer status | <0.0001 | ||
| Rapid metabolizer | 103 (34.3%) | 41.0 (30.0–51.0) | 0.02 | |
| Extensive metabolizer | 110 (36.7%) | 46.0 (36.0–54.0) | Ref. | |
| Intermediate metabolizer | 78 (26.0%) | 49.0 (39.8–57.0) | 0.03 | |
| Poor metabolizer | 9 (3.0%) | 48.0 (43.0–66.5) | 0.08 | |
| VerifyNow (n = 304), PRU | Metabolizer status | <0.0001 | ||
| Rapid metabolizer | 106 (34.9%) | 150.0 (89.3–195.0] | 0.06 | |
| Extensive metabolizer | 110 (36.2%) | 152.0 (112.0–217.5] | Ref. | |
| Intermediate metabolizer | 80 (26.3%) | 193.5 (144.0–236.5] | <0.01 | |
| Poor metabolizer | 8 (2.6%) | 247.0 (144.0–236.5] | <0.01 | |
| Multiplate (n = 305), AU | Metabolizer status | 0.10 | ||
| Rapid metabolizer | 105 (34.4%) | 49.0 (37.5–64.0) | n/a | |
| Extensive metabolizer | 111 (36.3%) | 47.0 (33.0–64.0) | ||
| Intermediate metabolizer | 80 (26.3%) | 55.5 (41.0–72.8) | ||
| Poor metabolizer | 9 (3.0%) | 54.0 (38.5–68.5) |
| Variables | LTA | VerifyNow | Multiplate |
|---|---|---|---|
| Beta Weight | Beta Weight | Beta Weight | |
| Age > 75 years | 0.119 | 0.077 | 0.079 |
| Body weight < 60 kg | −0.099 | −0.119 | −0.068 |
| Diabetes | 0.003 | 0.049 | 0.054 |
| Renal dysfunction | −0.029 | 0.053 | 0.090 |
| Previous stroke | 0.112 | 0.111 | 0.163 * |
| Current smoking | −0.054 | −0.073 | −0.002 |
| (es-) omeprazole use | 0.035 | 0.156 | 0.156 * |
| Aspirin use | −0.139 * | 0.005 | −0.013 |
| Anticoagulant use | 0.073 | −0.033 | 0.049 |
| Hemoglobin | 0.155 * | −0.337 * | −0.046 |
| Platelet count | −0.072 | −0.268 * | 0.265 * |
| Metabolizer group | 0.227 * | 0.212 * | 0.110 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olie, R.H.; Hensgens, R.R.K.; Wijnen, P.A.H.M.; Veenstra, L.F.; de Greef, B.T.A.; Vries, M.J.A.; van der Meijden, P.E.J.; ten Berg, J.M.; ten Cate, H.; Bekers, O.; et al. Differential Impact of Cytochrome 2C19 Allelic Variants on Three Different Platelet Function Tests in Clopidogrel-Treated Patients. J. Clin. Med. 2021, 10, 3992. https://doi.org/10.3390/jcm10173992
Olie RH, Hensgens RRK, Wijnen PAHM, Veenstra LF, de Greef BTA, Vries MJA, van der Meijden PEJ, ten Berg JM, ten Cate H, Bekers O, et al. Differential Impact of Cytochrome 2C19 Allelic Variants on Three Different Platelet Function Tests in Clopidogrel-Treated Patients. Journal of Clinical Medicine. 2021; 10(17):3992. https://doi.org/10.3390/jcm10173992
Chicago/Turabian StyleOlie, Renske H., Rachelle R. K. Hensgens, Petal A. H. M. Wijnen, Leo F. Veenstra, Bianca T. A. de Greef, Minka J. A. Vries, Paola E. J. van der Meijden, Jurriën M. ten Berg, Hugo ten Cate, Otto Bekers, and et al. 2021. "Differential Impact of Cytochrome 2C19 Allelic Variants on Three Different Platelet Function Tests in Clopidogrel-Treated Patients" Journal of Clinical Medicine 10, no. 17: 3992. https://doi.org/10.3390/jcm10173992
APA StyleOlie, R. H., Hensgens, R. R. K., Wijnen, P. A. H. M., Veenstra, L. F., de Greef, B. T. A., Vries, M. J. A., van der Meijden, P. E. J., ten Berg, J. M., ten Cate, H., Bekers, O., & Henskens, Y. M. C. (2021). Differential Impact of Cytochrome 2C19 Allelic Variants on Three Different Platelet Function Tests in Clopidogrel-Treated Patients. Journal of Clinical Medicine, 10(17), 3992. https://doi.org/10.3390/jcm10173992






