The Incidence of Inflammatory Bowel Disease in the Paediatric Population in the District of Lower Silesia, Poland
Abstract
:1. Introduction
2. Aim
3. Materials and Methods
4. Statistical Analysis
5. Results
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cosnes, J.; Gower, C.; Seksik, P.; Cortot, A. Epidemiology and Natural History of Inflammatory Bowel Diseases. Gastroenterology 2011, 140, 1785–1794.e4. [Google Scholar] [CrossRef] [PubMed]
- Day, A.S. Crohn’s and colitis in children and adolescents. World J. Gastroenterol. 2012, 18, 5862–5869. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, E.; Szymanska, S.; Szczepanski, M.; Landowski, P.; Czaja-Bulsa, G.; Jarocka-Cyrta, E.; Korczowski, B.; Krzesiek, E.; Kierkuś, J. Demographic characteristics of children with early clinical manifestation of inflammatory bowel disease. Gastroenterol. Rev. 2016, 11, 14–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- M’Koma, A.E. Inflammatory Bowel Disease: An expanding global health problem. Clin. Med. Insights Gastroenterol. 2013, 6, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Gasparetto, M.; Guariso, G. Highlights in IBD Epidemiology and Its Natural History in the Paediatric Age. Gastroenterol. Res. Pract. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Bernstein, C.N.; Vatn, M.H.; Lakatos, P.; Loftus, E.V., Jr.; Tysk, C.; O’Morain, C.; Moum, B.; Colombel, J.-F.; on behalf of the Epidemiology and Natural History Task Force of the International Organization of Inflammatory Bowel Disease (IOIBD). Geographical variability and environmental risk factors in inflammatory bowel disease. Gut 2013, 62, 630–649. [Google Scholar] [CrossRef] [PubMed]
- Karolewska-Bochenek, K.; Lazowska-Przeorek, I.; Albrecht, P.; Grzybowska, K.; Ryzko, J.; Szamotulska, K.; Radzikowski, A.; Landowski, P.; Krzesiek, E.; Ignys, I.; et al. Epidemiology of Inflammatory Bowel Disease among Children in Poland. A prospective, population-based, 2-year study, 2002–2004. Digestion 2009, 79, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Guariso, G.; Gasparetto, M.; Pozza, L.V.D.; D’Incà, R.; Zancan, L.; Sturniolo, G.; Brotto, F.; Facchin, P. Inflammatory Bowel Disease Developing in Paediatric and Adult Age. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, B.K.; Fell, J.M.; Beattie, R.M.; Mitton, S.G.; Wilson, D.C.; Jenkins, H. Guidelines for the Management of Inflammatory Bowel Disease in Children in the United Kingdom. J. Pediatr. Gastroenterol. Nutr. 2010, 50, S1–S13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, M.D. Epidemiology and Environmental Triggers of IBD. Inflamm. Bowel Dis. Monit. 2012, 13, 10–16. [Google Scholar]
- Ananthakrishnan, A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Basso, P.; Fonseca, M.T.; Bonfá, G.; Alves, V.; Sales-Campos, H.; Nardini, V.; Cardoso, C.R.D.B. Association among genetic predisposition, gut microbiota, and host immune response in the etiopathogenesis of inflammatory bowel disease. Braz. J. Med. Biol. Res. 2014, 47, 727–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruel, J.; Ruane, D.; Mehandru, S.; Gower, C.; Colombel, J.-F. IBD across the age spectrum—Is it the same disease? Nat. Rev. Gastroenterol. Hepatol. 2013, 11, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, H.H.; Schwerd, T.; Koletzko, S.; Shah, N.; Kammermeier, J.; Elkadri, A.; Ouahed, J.; Wilson, D.C.; Travis, S.P.; Turner, D.; et al. The Diagnostic Approach to Monogenic Very Early Onset Inflammatory Bowel Disease. Gastroenterology 2014, 147, 990–1007.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, A.; Koletzko, S.; Turner, D.; Escher, J.C.; Cucchiara, S.; de Ridder, L.; Kolho, K.-L.; Veres, G.; Russell, R.K.; Paerregaard, A.; et al. ESPGHAN Revised Porto Criteria for the Diagnosis of Inflammatory Bowel Disease in Children and Adolescents. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 795–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Główny Urząd Statystyczny. Available online: https://bdl.stat.gov.pl/BDL/dane/podgrup/wymiary (accessed on 21 October 2020).
- Iwańczak, F.; Krzesiek, E.; Iwańczak, B. Epidemiologia wrzodziejącego zapalenia jelita grubego i choroby Leśniowskiego-Crohna u dzieci z województwa dolnośląskiego i opolskiego. Pediatr. Pol. 2002, 77, 301–309. [Google Scholar]
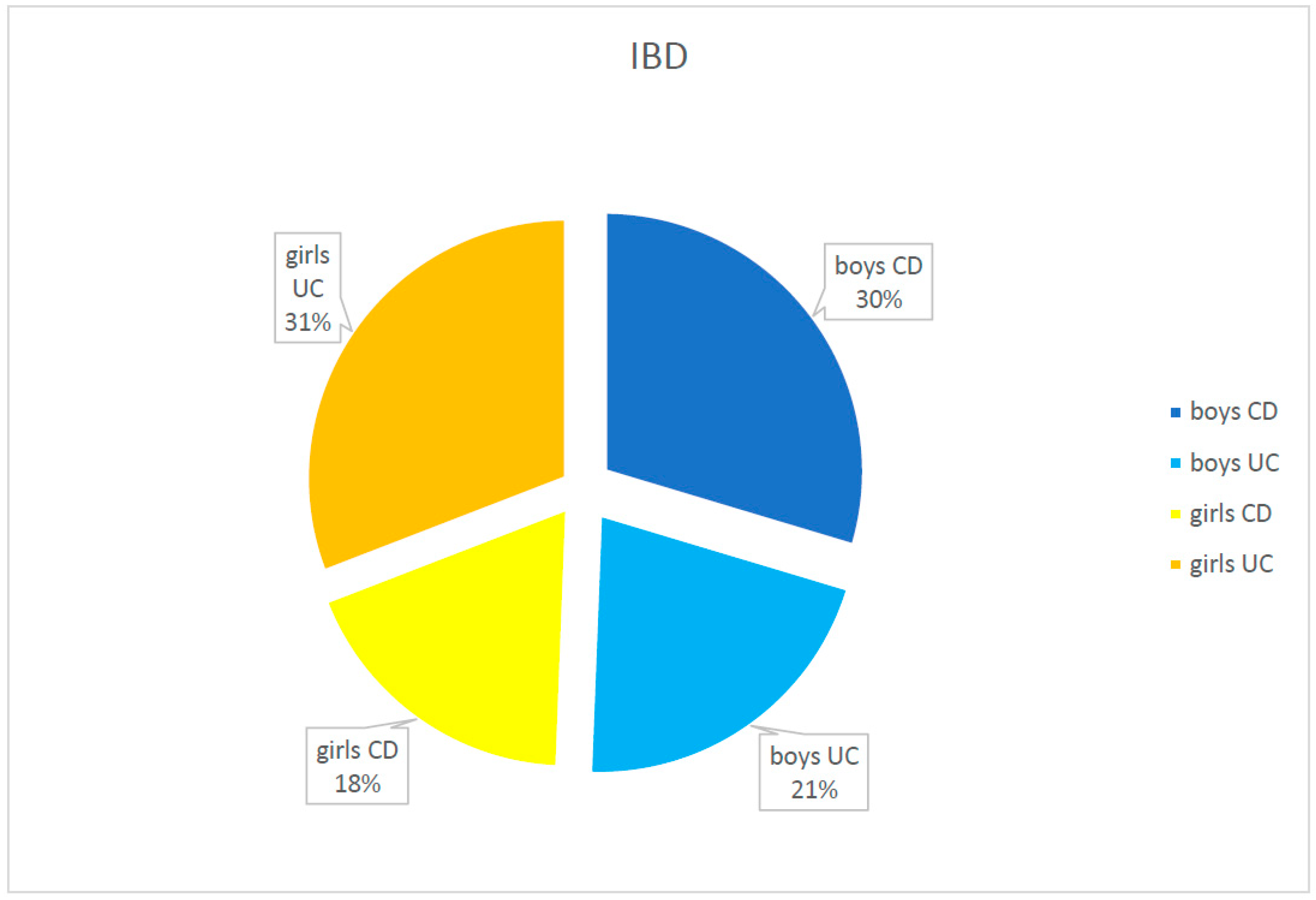
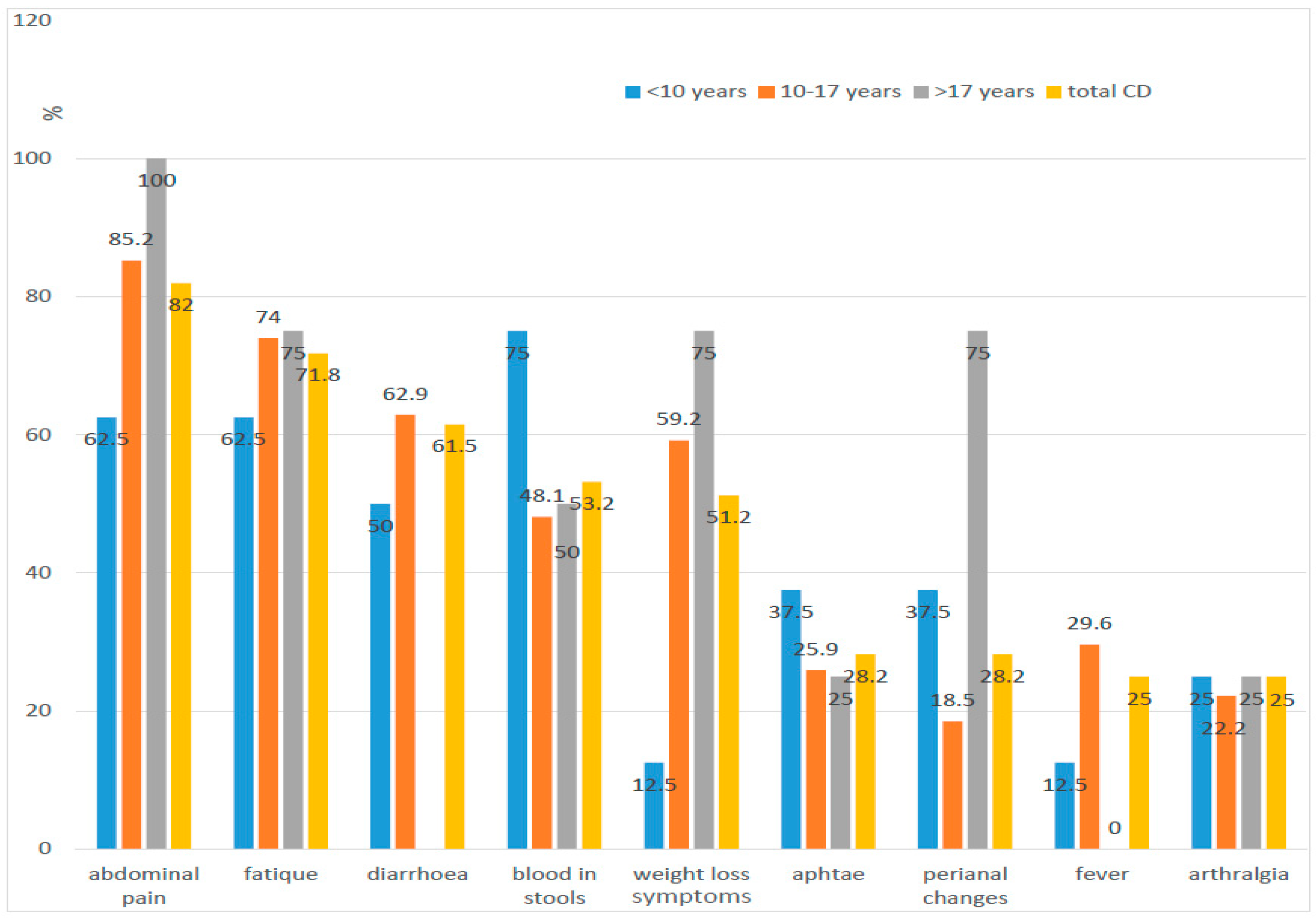
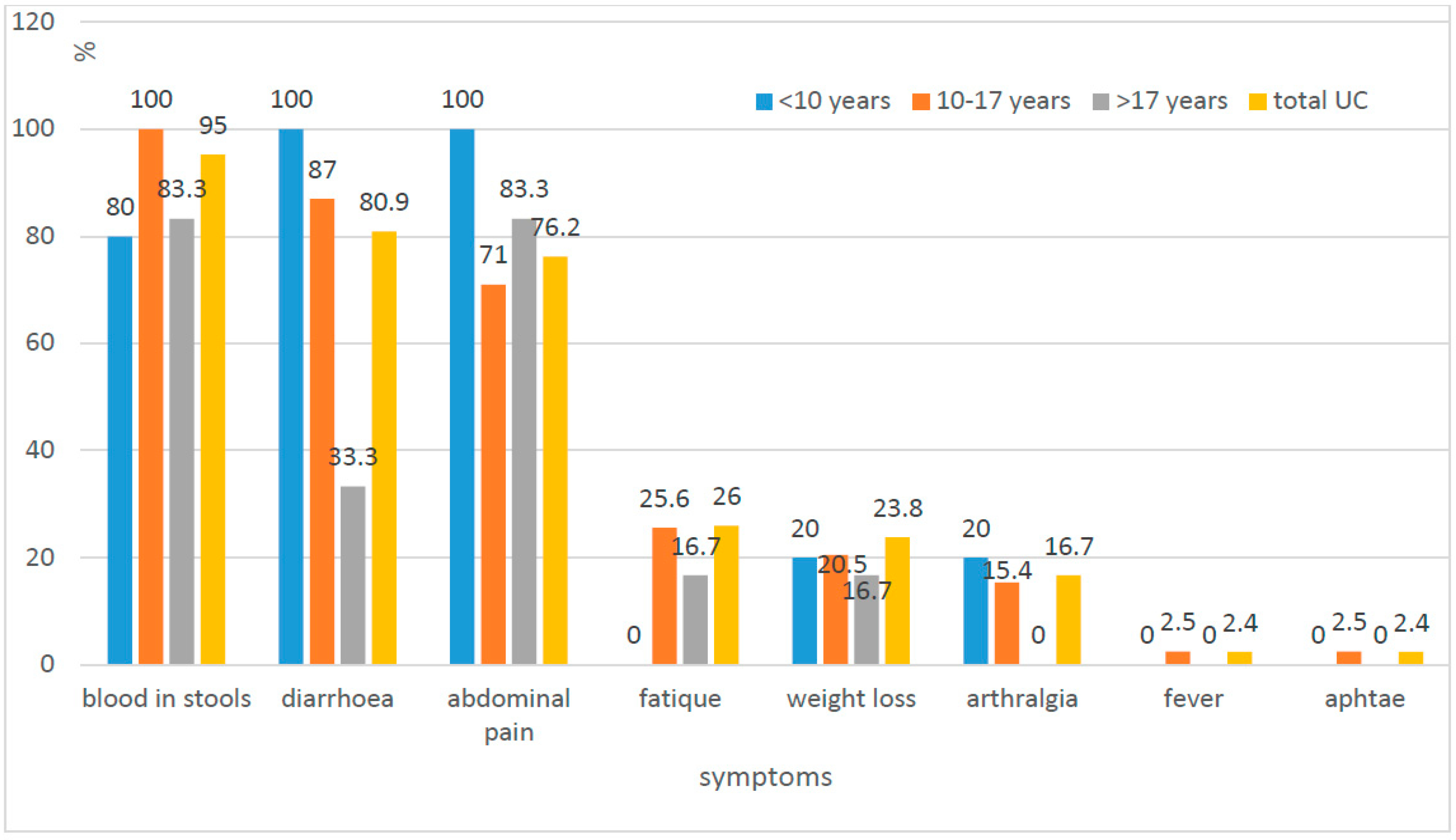
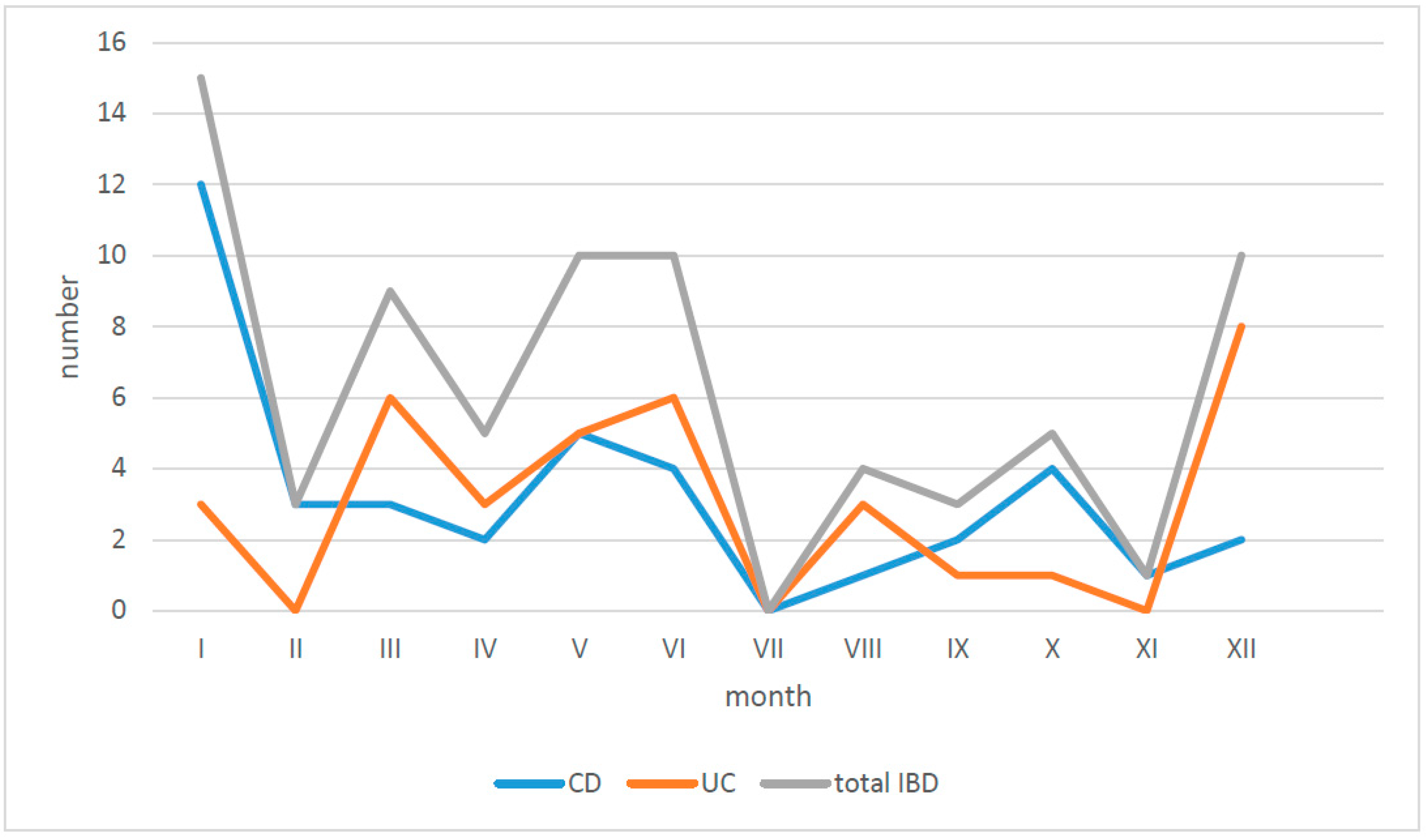
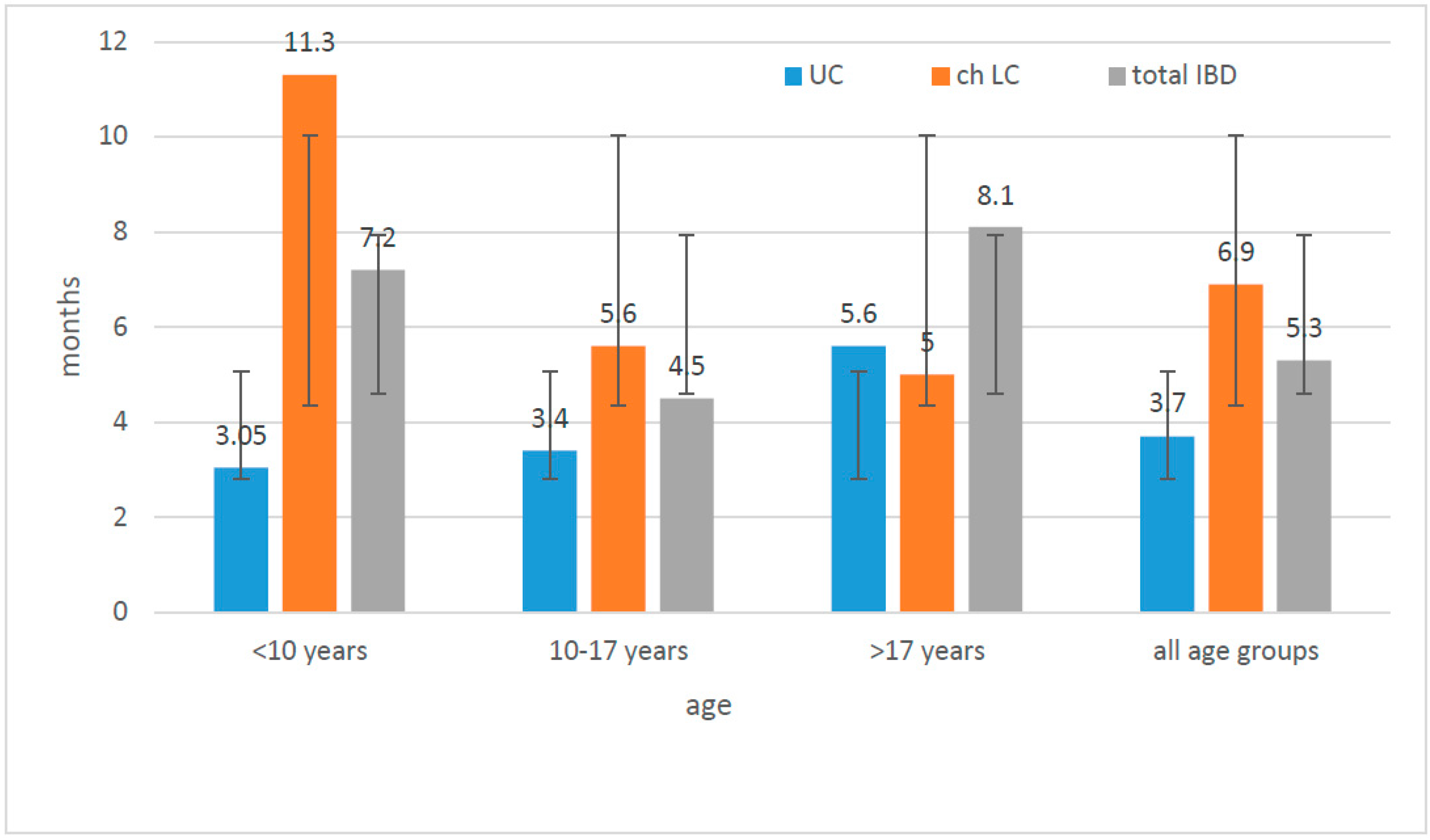
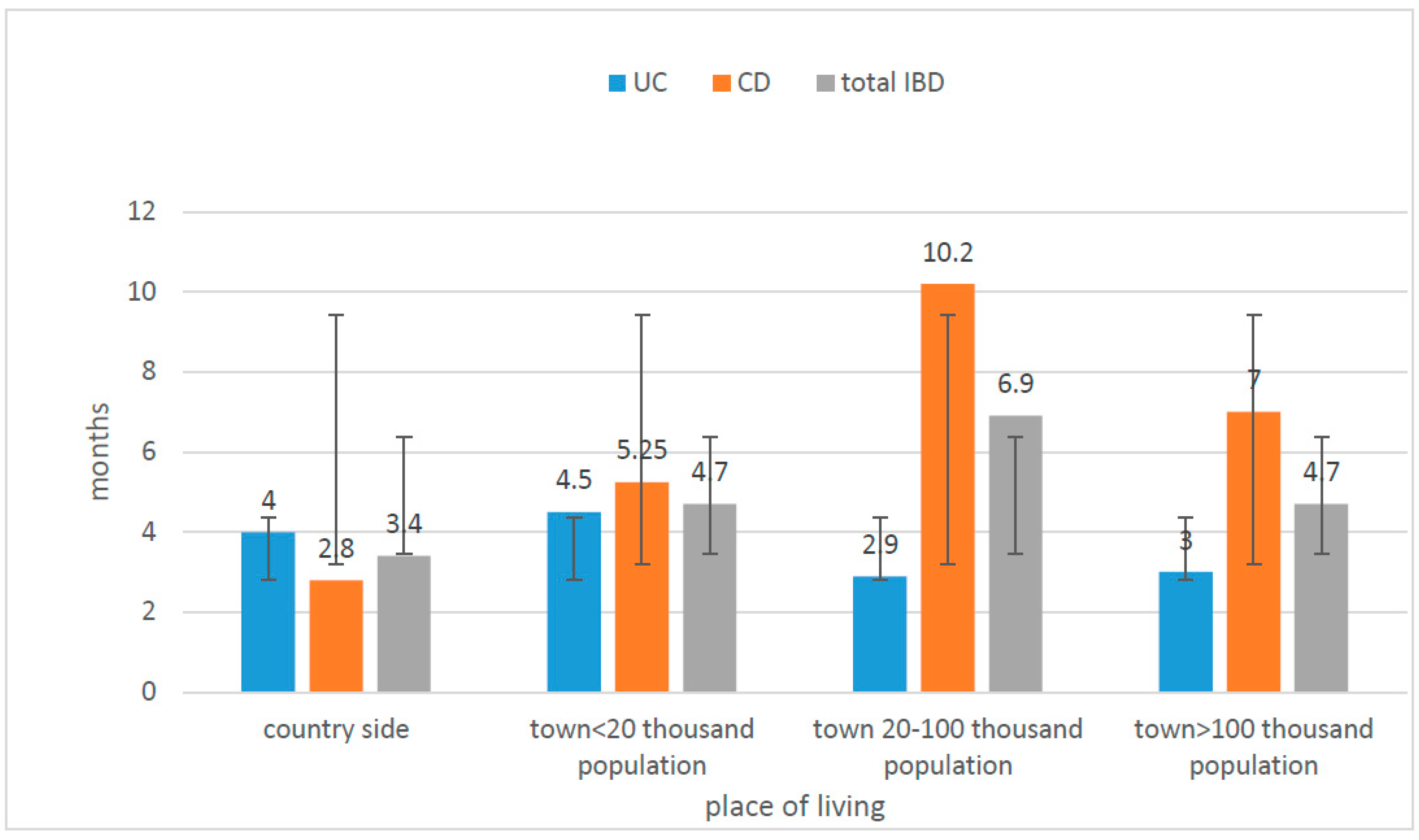
| Diagnosis | Number of Children N | Age at the Time of Diagnosis (In Years) | Time from First Symptoms to Diagnosis (Months) | IBD in Family Number of Children | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| From–To | Mean Age/SD | Girls | Boys | |||||||
| Number of Children | Mean Age/SD | Number of Children | Mean Age/SD | From–To | Mean Lenght/SD | |||||
| UC | 42 (51.9%) | 3.6–17.7 | 13.2/3.6 | 25 (59.5%) | 13.6/3.2 | 17 (38.4%) | 12.2/4.2 | 0.25–17 | 3.7/3.2 | 3 (7.1%) |
| CD | 39 (48.1%) | 2.6–17.0 | 13.1/3.9 | 15 (40.5%) | 13.9/2.7 | 24 (61.6%) | 12.5/4.6 | 1–60 | 6.9/9.5 | 2 (5.1%) |
| IBD | 81 (100.0%) | 2.6–17.7 | 13.1/3.8 | 40 (49.4%) | 13.8/2.8 | 41 (50.6%) | 12.4/4.4 | 0.25–60 | 5.8/8.0 | 5 (6.2%) |
| Extent of Changes | Number of Patients (Total 42) | |
|---|---|---|
| Paris classification (UC) | E1: ulcerative proctitis | 11 |
| E2: Left-sided UC (distal to splenic flexure) | 18 | |
| E3: Extensive (hepatic flexure distally) | 4 | |
| E4: Pancolitis (proximal to hepatic flexure) | 9 | |
| Mayo score (UC) | Endoscopic severity of changes | |
| Mayo 1: erythema, decreased vascular pattern, mild friability | 7 | |
| Mayo 2: marked erythema, absent vascular pattern, friability, erosions | 28 | |
| Mayo 3: spontaneous bleeding, ulceration | 7 | |
| Paediatric ulcerative colitis activity index (PUCAI) | Clinic activity (points) | |
| <10 | 5 | |
| 10–34 | 24 | |
| 35–64 | 8 | |
| 65–85 | 5 | |
| Nutritional statement | Body mass index BMI (kg/m2) | |
| <15 | 2 | |
| 15–17.9 | 16 | |
| 18–20.9 | 14 | |
| 21–25 | 7 | |
| >25 | 3 |
| Paris Classification (CD) | Location of Changes | Number of Patients (Total 39) |
|---|---|---|
| L1: terminal ileal/limited caecal diseases | 9 | |
| L2: colonic | 15 | |
| L3: ileocolonic | 13 | |
| L4: isolated upper disease | 2 | |
| SES-CD | Endoscopic severity of changes (points) | |
| <5 | 11 | |
| 5–10 | 8 | |
| >10 | 16 | |
| Evaluated outside the clinic—lack of date | 4 | |
| PCDAI | Clinic activity (points) | |
| <10 | 8 | |
| 10–25 | 18 | |
| 26–50 | 10 | |
| >50 | 3 | |
| Nutritional statement | Body mass index BMI (kg/m2) | |
| <15 | 4 | |
| 15–17.9 | 20 | |
| 18–20.9 | 12 | |
| 21–25 | 2 | |
| >25 | 1 |
| Diagnosis | UC | CD | IBD | IBD | ||
|---|---|---|---|---|---|---|
| Boys | ||||||
| Girls | ||||||
| Number of new cases in 2016–2018 | 42 | 39 | 81 | 41 | ||
| 40 | ||||||
| Number of children aged 0–19 (years) in the region of Lower Silesia | 544,201 | |||||
| Boys (M) | 277,941 | |||||
| Girls (F) | 266,260 | |||||
| Incidence rate—the number of IBD children aged 0–19 years, per 100 000, per year | 2.57 | 2.38 | 4.96 | 4.9 | 5.0 | |
| Symptom | UC n (%) | CD n (%) | p-Value |
|---|---|---|---|
| Diarrhoea | 34 (89.95) | 24 (61.54) | 0.0528 |
| Abdominal pain | 32 (76.15) | 32 (82.05) | 0.5175 |
| Blood in stool | 40 (95.24) | 2 (4.76) | 0.00002 |
| Weakness | 11 (26.19) | 28 (71.79) | 0.00004 |
| Weight loos | 10 (23.81) | 20 (51.28) | 0.0105 |
| Joints involvement | 7 (16.67) | 9 (23.08) | 0.469 |
| Skin changes | 0 (0) | 5 (12.82) | 0.0532 * |
| Mucosal changes in oral cavity | 1 (2.38) | 11 (28.21) | 0.0011 |
| Perianal changes | 0 (0) | 11 (28.21) | 0.00021 |
| Place of Residence | Number of Patients | Statistical Analysis | ||||
|---|---|---|---|---|---|---|
| CD (n = 39) | % | UC (n = 42) | % | Test χ2 | ||
| village | 9 | 23.08 | 7 | 16.67 | χ2 = 3.139 | |
| town < 20,000 residence | 8 | 20.51 | 15 | 35.71 | Df = 3 | |
| city 20—100,000 residence | 15 | 38.46 | 11 | 26.19 | P = 0.3707 | |
| city >100,000 residence | 7 | 17.95 | 9 | 21.43 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzesiek, E.; Kofla-Dlubacz, A.; Akutko, K.; Stawarski, A. The Incidence of Inflammatory Bowel Disease in the Paediatric Population in the District of Lower Silesia, Poland. J. Clin. Med. 2021, 10, 3994. https://doi.org/10.3390/jcm10173994
Krzesiek E, Kofla-Dlubacz A, Akutko K, Stawarski A. The Incidence of Inflammatory Bowel Disease in the Paediatric Population in the District of Lower Silesia, Poland. Journal of Clinical Medicine. 2021; 10(17):3994. https://doi.org/10.3390/jcm10173994
Chicago/Turabian StyleKrzesiek, Elzbieta, Anna Kofla-Dlubacz, Katarzyna Akutko, and Andrzej Stawarski. 2021. "The Incidence of Inflammatory Bowel Disease in the Paediatric Population in the District of Lower Silesia, Poland" Journal of Clinical Medicine 10, no. 17: 3994. https://doi.org/10.3390/jcm10173994
APA StyleKrzesiek, E., Kofla-Dlubacz, A., Akutko, K., & Stawarski, A. (2021). The Incidence of Inflammatory Bowel Disease in the Paediatric Population in the District of Lower Silesia, Poland. Journal of Clinical Medicine, 10(17), 3994. https://doi.org/10.3390/jcm10173994





