Acute Coronary Syndrome in the Older Patient
Abstract
:1. Introduction
2. Diagnostic Approach in the Older Patient
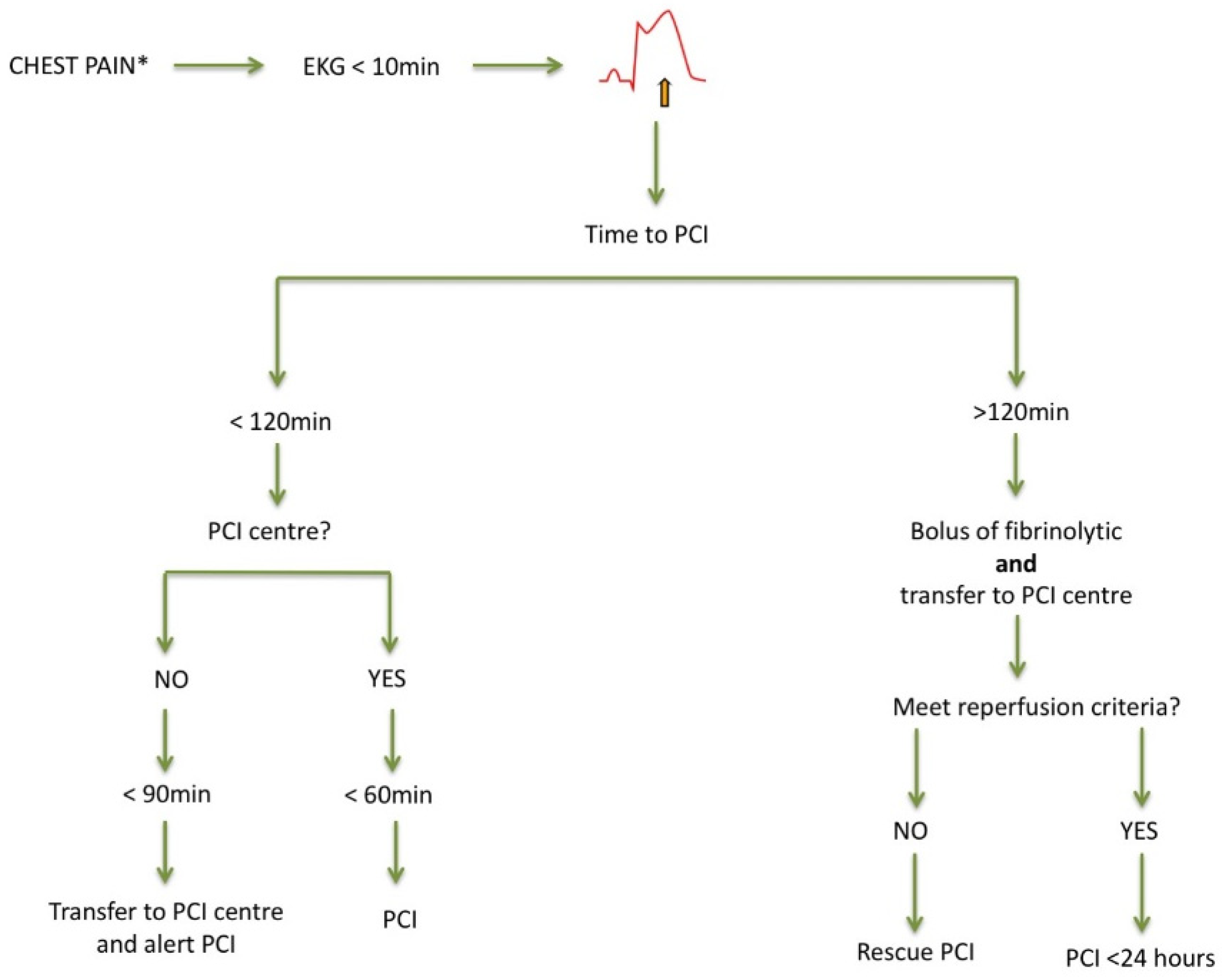
3. ST-Segment Elevation Myocardial Infarction (STEMI) Patients
4. Non-STEMI (NSTEMI) Patients: Invasive Versus Conservative Treatment?
5. Antithrombotic Treatment: Antiplatelets and Anticoagulants
5.1. Bleeding Risk in Elderly Patients with Acute Coronary Syndromes
5.2. Antiplatelet
5.3. Anticoagulants
6. Geriatric Conditions: Frailty and Comorbidity
7. Ethics Conditions
8. Secondary Prevention in the Elderly
9. Current and Future Research Directions
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics—2017 Update: A Report from the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef] [PubMed]
- Townsend, N.; Nichols, M.; Scarborough, P.; Rayner, M. Cardiovascular disease in Europe-epidemiological update 2015. Eur. Heart J. 2015, 36, 2696–2705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Topaz, G.; Finkelstein, A.; Flint, N.; Shacham, Y.; Banai, S.; Steinvil, A.; Arbel, Y.; Keren, G.; Yankelson, L. Comparison of 30-day and long-term outcomes and hospital complications among patients aged <75 versus ≥75 years with ST-elevation myocardial infarction undergoing percutaneous coronary intervention. Am. J. Cardiol. 2017, 119, 1897–1901. [Google Scholar] [CrossRef]
- Ariza-Solé, A.; Alegre, O.; Elola, F.J.; Fernández, C.; Formiga, F.; Martínez-Sellés, M.; Bernal, J.L.; Segura, J.V.; Iñíguez, A.; Bertomeu, V.; et al. Management of myocardial infarction in the elderly. Insights from Spanish Minimum Basic Data Set. Eur. Heart J. Acute Cardiovasc. Care 2019, 8, 242–251. [Google Scholar] [CrossRef]
- Alvarez Alvarez, B.; Cid Alvarez, A.B.; Redondo Dieguez, A.; Sanmartin Pena, X.; Lopez Otero, D.; Avila Carrillo, A.; Gomez Peña, F.; Trillo Nouche, R.; Martinez Selles, M.; Gonzalez-Juanatey, J. Short-term and long-term validation of the fastest score in patients with ST-elevation myocardial infarction after primary angioplasty. Int. J. Cardiol. 2018, 269, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Díez-Villanueva, P.; Ariza-Solé, A.; Bonanad, C.; Martínez-Sellés, M. Síndrome Coronario Agudo en el Paciente Anciano, 1st ed.; International Marketing & Communication, S.A.: Madrid, Spain, 2019. [Google Scholar]
- Madhavan, M.V.; Gersh, B.J.; Alexander, K.P.; Granger, C.B.; Stone, G.W. Coronary artery disease in patients ≥80 years of age. J. Am. Coll. Cardiol. 2018, 71, 2015–2040. [Google Scholar] [CrossRef] [PubMed]
- Welsh, P.; Preiss, D.; Shah, A.; McAllister, D.; Briggs, A.; Boachie, C.; McConnachie, A.; Hayward, C.; Padmanabhan, S.; Welsh, C.; et al. Comparison between high-sensitivity cardiac troponin T and cardiac troponin I in a large general population cohort. Clin. Chem. 2018, 64, 1607–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eggers, K.M.; Lind, L.; Venge, P.; Lindahl, B. Factors influencing the 99th percentile of cardiac troponin I evaluated in community-dwelling individuals at 70 and 75 years of age. Clin. Chem. 2013, 59, 1068–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gore, M.O.; Seliger, S.L.; Defilippi, C.R.; Nambi, V.; Christenson, R.H.; Hashim, I.A.; Hoogeveen, R.C.; Ayers, C.R.; Sun, W.; McGuire, D.K.; et al. Age- and sex-dependent upper reference limits for the high-sensitivity cardiac troponin T assay. J. Am. Coll. Cardiol. 2014, 63, 1441–1448. [Google Scholar] [CrossRef] [Green Version]
- Boeddinghaus, J.; Nestelberger, T.; Twerenbold, R.; Neumann, J.T.; Lindahl, B.; Giannitsis, E.; Sörensen, N.A.; Badertscher, P.; Jann, J.E.; Wussler, D.; et al. Impact of age on the performance of the ESC 0/1h-algorithms for early diagnosis of myocardial infarction. Eur. Heart J. 2018, 39, 3780–3794. [Google Scholar] [CrossRef] [Green Version]
- Wu, A.; Christenson, R.H.; Greene, D.N.; Jaffe, A.S.; Kavsak, P.A.; Ordonez-Llanos, J.; Apple, F.S. Clinical laboratory practice recommendations for the use of cardiac troponin in acute coronary syndrome: Expert opinion from the academy of the american association for clinical chemistry and the task force on clinical applications of cardiac bio-markers of the international federation of clinical chemistry and laboratory medicine. Clin. Chem. 2018, 64, 645–655. [Google Scholar] [PubMed]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef] [PubMed]
- Puerto, E.; Viana-Tejedor, A.; Martínez-Sellés, M.; Domínguez-Pérez, L.; Moreno, G.; Martín-Asenjo, R.; Bueno, H. Temporal trends in mechanical complications of acute myocardial infarction in the elderly. J. Am. Coll. Cardiol. 2018, 72, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Bastante, T.; Rivero, F.; Cuesta, J.; García, M.; Antuña, P.; Díez-Villanueva, P. Síndrome Coronario Agudo con Elevación del Segmento ST. Díez-Villanueva, P. Manual de Cardiopatía en el Paciente Anciano, 1st ed.; International Marketing & Communication, S.A.: Madrid, Spain, 2018; pp. 89–95. [Google Scholar]
- Alexander, K.P.; Newby, L.K.; Armstrong, P.W.; Cannon, C.P.; Gibler, W.B.; Rich, M.W.; Van de Werf, F.; White, H.D.; Weaver, W.D.; Naylor, M.D.; et al. Acute coronary care in the elderly, Part II ST-segment–elevation myocardial infarction. A scientific statement for healthcare professionals from the american heart association council on clinical cardiology. Circulation 2007, 115, 2570–2589. [Google Scholar] [CrossRef] [Green Version]
- Rivero, F.; Bastante, T.; Cuesta, J.; Benedicto, A.; Salamanca, J.; Restrepo, J.A.; Aguilar, R.; Gordo, F.; Batlle, M.; Alfonso, F. Factors associated with delays in seeking medical attention in patients with ST-segment elevation acute coronary syndrome. Rev. Esp. Cardiol. 2016, 69, 279–285. [Google Scholar] [CrossRef] [PubMed]
- de la Torre Hernández, J.M.; Brugaletta, S.; Gómez Hospital, J.A.; Baz, J.A.; Pérez de Prado, A.; López Palop, R.; Cid, B.; García Camarero, T.; Diego, A.; Gimeno de Carlos, F.; et al. Primary angioplasty in patients older than 75 years. Profile of patients and procedures, outcomes, and predictors of prognosis in the ESTROFA IM + 75 Registry. Rev. Esp. Cardiol. 2017, 70, 81–87. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [Green Version]
- Toleva, O.; Ibrahim, Q.; Brass, N.; Sookram, S.; Welsh, R. Treatment choices in elderly patients with ST: Elevation myocardial infarction-insights from the Vital Heart Response registry. Open Heart 2015, 2, e000235. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, P.W.; Gershlick, A.H.; Goldstein, P.; Wilcox, R.; Danays, T.; Lambert, Y.; Sulimov, V.; Rosell Ortiz, F.; Ostojic, M.; Welsh, R.C.; et al. Fibrinolysis or primary PCI in ST-segment elevation myocardial infarction. N. Engl. J. Med. 2013, 368, 1379–1387. [Google Scholar] [CrossRef] [Green Version]
- Puymirat, E.; Aissaoui, N.; Cayla, G.; Lafont, A.; Riant, E.; Mennuni, M.; Saint-Jean, O.; Blanchard, D.; Jourdain, P.; Elbaz, M.; et al. Changes in one-year mortality in elderly patients admitted with acute myocardial infarction in relation with early management. Am. J. Med. 2017, 130, 555–563. [Google Scholar] [CrossRef]
- Fernández-Bergés, D.; Degano, I.R.; Gonzalez Fernandez, R.; Subirana, I.; Vila, J.; Jiménez-Navarro, M.; Perez-Fernandez, S.; Roqué, M.; Bayes-Genis, A.; Fernandez-Aviles, F.; et al. Benefit of primary percutaneous coronary interventions in the elderly with ST segment elevation myocardial infarction. Open Heart 2020, 7, e001169. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, D.; Forman, D.E. Never too old for cardiac rehabilitation. Clin. Geriatr. Med. 2019, 35, 407. [Google Scholar] [CrossRef] [PubMed]
- Bueno, H.; Rossello, X.; Pocock, S.J.; Van de Werf, F.; Chin, C.T.; Danchin, N.; Lee, S.W.; Medina, J.; Huo, Y. In-hospital coronary revascularization rates and post-discharge mortality risk in non–ST-segment elevation acute coronary syndrome. J. Am. Coll. Cardiol. 2019, 74, 1454–1461. [Google Scholar] [CrossRef]
- Gabaldon-Perez, A.; Bonanad, C.; Garcia-Blas, S.; Gavara, J.; Rios-Navarro, C.; Perez-Sole, N.; de Dios, E.; Marcos-Garces, V.; Merenciano-Gonzalez, H.; Monmeneu, J.V.; et al. Stress cardiac magnetic resonance for mortality prediction and decision-making: Registry of 2496 elderly patients with chronic coronary syndrome. Rev. Esp. Cardiol. 2021, 66, 603–605. [Google Scholar] [CrossRef]
- Alvarez-Alvarez, B.; Abou Jokh Casas, C.; Garcia Acuña, J.M.; Cid Alvarez, B.; Agra Bermejo, R.M.; Cordero Fort, A.; Rodríguez Mañero, M.; Gude Sampedro, F.; González-Juanatey, J.R. Temporal trends between association of evidence-based treatment and outcomes in patients with non-ST-elevation myocardial infarction. Int. J. Cardiol. 2018, 260, 1–6. [Google Scholar] [CrossRef]
- Cordero, A.; Escribano, D.; García-Acuña, J.M.; Rodriguez-Mañero, M.; Agra-Bermejo, R.; Bertomeu-González, V.; Cid-Alvarez, B.; Alvarez-Alvarez, B.; Zuazola, P.; González-Juanatey, J.R. Long-term bleeding risk vs. mortality risk in acute coronary syndrome patients according to the 2019 ARC-HBR definition. Thromb. Res. 2020, 196, 516–518. [Google Scholar] [CrossRef]
- Simonsson, M.; Wallentin, L.; Alfredsson, J.; Erlinge, D.; Hellström Ängerud, K.; Hofmann, R.; Kellerth, T.; Lindhagen, L.; Ravn-Fischer, A.; Szummer, K.; et al. Temporal trends in bleeding events in acute myocardial infarction: Insights from the SWEDEHEART registry. Eur. Heart J. 2019, 41, 833–843. [Google Scholar] [CrossRef] [Green Version]
- Gargiulo, G.; Valgimigli, M.; Sunnåker, M.; Vranckx, P.; Frigoli, E.; Leonardi, S.; Spirito, A.; Gragnano, F.; Manavifar, N.; Galea, R.; et al. Choice of access site and type of anticoagulant in acute coronary syndromes with advanced Killip class or out-of-hospital cardiac arrest. Rev. Esp. Cardiol. 2020, 73, 893–901. [Google Scholar] [CrossRef]
- Cesaro, A.; Moscarella, E.; Gragnano, F.; Perrotta, R.; Diana, V.; Pariggiano, I.; Concilio, C.; Alfieri, A.; Cesaro, F.; Mercone, G.; et al. Transradial access versus transfemoral access: A comparison of outcomes and efficacy in reducing hemorrhagic events. Expert Rev. Cardiovasc. Ther. 2019, 17, 435–447. [Google Scholar] [CrossRef]
- Tegn, N.; Abdelnoor, M.; Aaberge, L.; Endresen, K.; Smith, P.; Aakhus, S.; Gjertsen, E.; Dahl-Hofseth, O.; Ranhoff, A.H.; Gullestad, L.; et al. Invasive versus conservative strategy in patients aged 80 years or older with non-ST-elevation myocardial infarction or unstable angina pectoris (After Eighty study): An open-label randomised controlled trial. Lancet 2016, 387, 1057–1065. [Google Scholar] [CrossRef]
- Kaura, A.; Sterne, J.; Trickey, A.; Abbott, S.; Mulla, A.; Glampson, B.; Panoulas, V.; Davies, J.; Woods, K.; Omigie, J.; et al. Invasive versus non-invasive management of older patients with non-ST elevation myocardial infarction (SENIOR-NSTEMI): A cohort study based on routine clinical data. Lancet 2020, 396, 623–634. [Google Scholar] [CrossRef]
- Cordero, A.; Rodríguez-Mañero, M.; Bertomeu-González, V.; Gonzalez-Juanatey, J.R. Managing NSTEMI in older patients. Lancet 2021, 397, 370–371. [Google Scholar] [CrossRef]
- Sanchis, J.; García Acuña, J.M.; Raposeiras, S.; Barrabés, J.A.; Cordero, A.; Martínez-Sellés, M.; Bardají, A.; Díez-Villanueva, P.; Marín, F.; Ruiz-Nodar, J.M.; et al. Comorbidity burden and revascularization benefit in elderly patients with acute coronary syndrome. Rev. Esp. Cardiol. 2020, 74, 765–772. [Google Scholar] [CrossRef] [PubMed]
- de Belder, A.; de la Torre Hernandez, J.M.; Lopez-Palop, R.; O’Kane, P.; Hernandez Hernandez, F.; Strange, J.; Gimeno, F.; Cotton, J.; Diaz Fernandez, J.F.; Carrillo Saez, P.; et al. A prospective randomized trial of everolimus-eluting stents versus bare-metal stents in octogenarians: The XIMA Trial (Xience or Vision Stents for the Management of Angina in the Elderly). J. Am. Coll. Cardiol. 2014, 63, 1371–1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urban, P.; Meredith, I.T.; Abizaid, A.; Pocock, S.J.; Carrié, D.; Naber, C.; Lipiecki, J.; Richardt, G.; Iñiguez, A.; Brunel, P.; et al. Polymer-free drug-coated coronary stents in patients at high bleeding risk. N. Engl. J. Med. 2015, 373, 2038–2047. [Google Scholar] [CrossRef] [Green Version]
- Windecker, S.; Latib, A.; Kedhi, E.; Kirtane, A.J.; Kandzari, D.E.; Mehran, R.; Price, M.J.; Abizaid, A.; Simon, D.I.; Worthley, S.G.; et al. Polymer-based or polymer-free stents in patients at high bleeding risk. N. Engl. J. Med. 2020, 382, 1208–1218. [Google Scholar] [CrossRef]
- Agra-Bermejo, R.; Cordero, A.; Veloso, P.R.; Álvarez, D.I.; Álvarez, B.Á.; Díaz, B.; Rodríguez, L.A.; Abou-Jokh, C.; Álvarez, B.C.; González-Juanatey, J.R.; et al. Long term prognostic benefit of complete revascularization in elderly presenting with NSTEMI: Real world evidence. Rev. Cardiovasc. Med. 2021, 22, 475–482. [Google Scholar] [CrossRef]
- Rumiz, E.; Berenguer, A.; Vilar, J.V.; Valero, E.; Facila, L.; Cubillos, A.; Sanmiguel, D.; Almela, P.; Morell, S. Long-term outcomes and predictors of morbi-mortality according to age in stemi patients with multivessel disease: Impact of an incomplete revascularization. Catheter. Cardiovasc. Interv. 2018, 92, E512–E517. [Google Scholar] [CrossRef]
- Harada, M.; Miura, T.; Kobayashi, T.; Kobayashi, H.; Kobayashi, M.; Nakajima, H.; Kimura, H.; Akanuma, H.; Mawatari, E.; Sato, T.; et al. Clinical impact of complete revascularization in elderly patients with multi-vessel coronary artery disease undergoing percutaneous coronary intervention: A sub-analysis of the SHINANO registry. Int. J. Cardiol. 2017, 230, 413–419. [Google Scholar] [CrossRef]
- Ariza-Solé, A.; Guerrero, C.; Formiga, F.; Aboal, J.; Abu-Assi, E.; Marín, F.; Bueno, H.; Alegre, O.; López-Palop, R.; Vidán, M.T.; et al. Global geriatric assessment and in-hospital bleeding risk in elderly patients with acute coronary syndromes: Insights from the LONGEVO-SCA registry. Thromb. Haemost. 2018, 118, 581–590. [Google Scholar] [CrossRef]
- Ndrepepa, G.; Berger, P.B.; Mehilli, J.; Seyfarth, M.; Neumann, F.J.; Schomig, A.; Kastrati, A. Periprocedural bleeding and 1-year outcome after percutaneous coronary interventions: Appropriateness of including bleeding as a component of a quadruple end point. J. Am. Coll. Cardiol. 2008, 51, 690697. [Google Scholar] [CrossRef] [Green Version]
- Eikelboom, J.W.; Mehta, S.R.; Anand, S.S.; Xie, C.; Fox, K.A.; Yusuf, S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation 2006, 114, 774–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manoukian, S.V.; Feit, F.; Mehran, R.; Voeltz, M.D.; Ebrahimi, R.; Hamon, M.; Dangas, G.D.; Lincoff, A.M.; White, H.D.; Moses, J.W.; et al. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: An analysis from the ACUITY Trial. J. Am. Coll. Cardiol. 2007, 49, 1362–1368. [Google Scholar] [CrossRef] [Green Version]
- Ariza-Solé, A.; Formiga, F.; Lorente, V.; Sánchez-Salado, J.C.; Sánchez-Elvira, G.; Roura, G.; Sánchez-Prieto, R.; Vila, M.; Moliner, P.; Cequier, A. Efficacy of bleeding risk scores in elderly patients with acute coronary syndromes. Rev. Esp. Cardiol. 2014, 67, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; van Klaveren, D.; James, S.; Heg, D.; Räber, L.; Feres, F.; Pilgrim, T.; Hong, M.K.; Kim, H.S.; Colombo, A.; et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: A pooled analysis of individual-patient datasets from clinical trials. Lancet 2017, 389, 1025–1034. [Google Scholar] [CrossRef]
- Guerrero, C.; Ariza-Solé, A.; Formiga, F.; Martínez-Sellés, M.; Vidán, M.; Aboal, J. Applicability of the PRECISE-DAPT score in elderly patients with myocardial infarction. J. Geriatr. Cardiol. 2018, 15, 713. [Google Scholar] [PubMed]
- Aradi, D.; Kirtane, A.; Bonello, L.; Gurbel, P.A.; Tantry, U.S.; Huber, K.; Freynhofer, M.K.; ten Berg, J.; Janssen, P.; Angiolillo, D.J.; et al. Bleeding and stent thrombosis on P2Y12-inhibitors: Collaborative analysis on the role of platelet reactivity for risk stratification after percutaneous coronary intervention. Eur. Heart J. 2015, 36, 17621771. [Google Scholar] [CrossRef] [Green Version]
- Aradi, D.; Storey, R.F.; Komocsi, A.; Trenk, D.; Gulba, D.; Kiss, R.G.; Husted, S.; Bonello, L.; Sibbing, D.; Collet, J.P.; et al. Expert position paper on the role of platelet function testing in patients undergoing percutaneous coronary intervention. Eur. Heart J. 2014, 35, 209215. [Google Scholar] [CrossRef] [Green Version]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; et al. Ticagrelor versus Clopidogrel in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Braunwald, E.; McCabe, C.H.; Montalescot, G.; Ruzyllo, W.; Gottlieb, S.; Neumann, F.J.; Ardissino, D.; De Servi, S.; Murphy, S.A.; et al. Prasugrel versus Clopidogrel in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 2007, 357, 2001–2015. [Google Scholar] [CrossRef] [Green Version]
- Danchin, N.; Lettino, M.; Zeymer, U.; Widimsky, P.; Bardaji, A.; Barrabes, J.A.; Cequier, A.; Claeys, M.J.; De Luca, L.; Dörler, J.; et al. Use, patient selection and outcomes of P2Y12 receptor inhibitor treatment in patients with STEMI based on contemporary European registries. Eur. Heart J. Cardiovasc. Pharmacother. 2016, 2, 152–167. [Google Scholar] [CrossRef] [PubMed]
- Husted, S.; James, S.; Becker, R.C.; Horrow, J.; Katus, H.; Storey, R.F.; Cannon, C.P.; Heras, M.; Lopes, R.D.; Morais, J.; et al. Ticagrelor versus clopidogrel in elderly patients with acute coronary syndromes: A substudy from the prospective randomized PLATelet inhibition and patient Outcomes (PLATO) trial. Circ. Cardiovasc. Qual. Outcomes 2012, 5, 680–688. [Google Scholar] [CrossRef] [Green Version]
- Garay, A.; Ariza-Solé, A.; Formiga, F.; Raposeiras-Roubín, S.; Abu-Assi, E.; Sánchez-Salado, J.C.; Lorente, V.; Alegre, O.; Henriques, J.; D’Ascenzo, F.; et al. Prediction of post-discharge bleeding in elderly patients with acute coronary syndromes: Insights from the BleeMACS registry. Thromb. Haemost. 2018, 118, 929–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szummer, K.; Montez-Rath, M.E.; Alfredsson, J.; Erlinge, D.; Lindahl, B.; Hofmann, R.; Ravn-Fischer, A.; Svensson, P.; Jernberg, T. Comparison between ticagrelor and clopidogrel in elderly patients with an acute coronary syndrome: Insights from the SWEDEHEART registry. Circulation 2020, 142, 1700–1708. [Google Scholar] [CrossRef]
- Cayla, G.; Cuisset, T.; Silvain, J.; Leclercq, F.; Manzo-Silberman, S.; Saint-Etienne, C.; Delarche, N.; Bellemain-Appaix, A.; Range, G.; El Mahmoud, R.; et al. Platelet function monitoring to adjust antiplatelet therapy in elderly patients stented for an acute coronary syndrome (ANTARCTIC): An open-label, blinded-endpoint, randomised controlled superiority trial. Lancet 2016, 388, 2015–2022. [Google Scholar] [CrossRef]
- Gimbel, M.; Qaderdan, K.; Willemsen, L.; Hermanides, R.; Bergmeijer, T.; de Very, E.; Heestermans, T.; Tjon Joe Gin, M.; Waalewijn, R.; Hofma, S.; et al. Clopidogrel versus ticagrelor or prasugrel in patients aged 70 years or older with non-ST-elevation acute coronary syndrome (POPular AGE): The randomised, open-label, non-inferiority trial. Lancet 2020, 395, 1374–1381. [Google Scholar] [CrossRef]
- Gragnano, F.; Moscarella, E.; Calabrò, P.; Cesaro, A.; Pafundi, P.C.; Ielasi, A.; Patti, G.; Cavallari, I.; Antonucci, E.; Cirillo, P.; et al. Clopidogrel versus ticagrelor in high-bleeding risk patients presenting with acute coronary syndromes: Insights from the multicenter START-ANTIPLATELET registry. Intern. Emerg. Med. 2021, 16, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.D.; Hong, H.; Harskamp, R.E.; Bhatt, D.L.; Mehran, R.; Cannon, C.P.; Granger, C.B.; Verheugt, F.; Li, J.; Ten Berg, J.M.; et al. Safety and efficacy of antithrombotic strategies in patients with atrial fibrillation undergoing percutaneous coronary intervention. JAMA Cardiol. 2019, 4, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, G.; Goette, A.; Tijssen, J.; Eckardt, L.; Lewalter, T.; Vranckx, P.; Valgimigli, M. Safety and efficacy outcomes of double vs. triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: A systematic review and meta-analysis of non-vitamin K antagonist oral anticoagulant-based randomized clinical trials. Eur. Heart J. 2019, 40, 3757–3767. [Google Scholar]
- Khandelwal, D.; Goel, A.; Kumar, U.; Gulati, V.; Narang, R.; Dey, A.B. Frailty is associated with longer hospital stay and increased mortality in hospitalized older patients. J. Nutr. Health Aging 2012, 16, 732–735. [Google Scholar] [CrossRef]
- Afilalo, J.; Alexander, K.P.; Mack, M.J.; Maurer, M.S.; Green, P.; Allen, L.A.; Popma, J.J.; Ferrucci, L.; Forman, D.E. Frailty assessment in the cardiovascular care of older adults. J. Am. Coll. Cardiol. 2014, 63, 747–762. [Google Scholar] [CrossRef] [Green Version]
- Abellan van Kan, G.; Rolland, Y.M.; Morley, J.E.; Vellas, B. Frailty: Toward a clinical definition. J. Am. Med. Dir. Assoc. 2008, 9, 71–72. [Google Scholar] [CrossRef]
- Díez-Villanueva, P.; Arizá-Solé, A.; Vidán, M.T.; Bonanad, C.; Formiga, F.; Sanchis, J.; Martín-Sánchez, F.J.; Ruiz-Ros, V.; Sanmartín-Fernández, M.; Bueno, H.; et al. Recommendations of the Geriatric Cardiology Section of the Spanish Society of Cardiology for the assessment of frailty in elderly patients with heart disease. Rev. Esp. Cardiol. 2019, 72, 63–71. [Google Scholar] [CrossRef]
- Morley, J.E.; Vellas, B.; van Kan, G.A.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.C.; Doehner, W.; Evans, J.; et al. Frailty consensus: A call to action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [Green Version]
- Cesari, M.; Calvani, R.; Marzetti, E. Frailty in older persons. Clin. Geriatr. Med. 2017, 33, 293–303. [Google Scholar] [CrossRef]
- Bebb, O.; Smith, F.G.; Clegg, A.; Hall, M.; Gale, C.P. Frailty and acute coronary syndrome: A structured literature review. Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Ekerstad, N.; Swahn, E.; Janzon, M.; Alfredsson, J.; Löfmark, R.; Lindenberger, M.; Carlsson, P. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation 2011, 124, 2397–2404. [Google Scholar] [CrossRef]
- Graham, M.M.; Galbraith, P.D.; O’Neill, D.; Rolfson, D.B.; Dando, C.; Norris, C.M. Frailty and outcome in elderly patients with acute coronary syndrome. Can. J. Cardiol. 2013, 29, 1610–1615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchis, J.; Bonanad, C.; Ruiz, V.; Fernández, J.; García-Blas, S.; Mainar, L.; Ventura, S.; Rodríguez-Borja, E.; Chorro, F.J.; Hermenegildo, C.; et al. Frailty and other geriatric conditions for risk stratification of older patients with acute coronary syndrome. Am. Heart J. 2014, 168, 784–791. [Google Scholar] [CrossRef]
- Alonso Salinas, G.L.; Sanmartín Fernández, M.; Pascual Izco, M.; Martín Asenjo, R.; Recio-Mayoral, A.; Salvador Ramos, L.; Marzal Martín, D.; Camino López, A.; Jiménez Mena, M.; Zamorano Gómez, J.L. Frailty is a short- term prognostic marker in acute coronary syndrome of elderly patients. Eur. Heart J. Acute Cardiovasc. Care 2016, 5, 434–440. [Google Scholar] [CrossRef]
- Dodson, J.A.; Arnold, S.V.; Gosch, K.L.; Gill, T.M.; Spertus, J.A.; Krumholz, H.M.; Rich, M.W.; Chaudhry, S.I.; Forman, D.E.; Masoudi, F.A.; et al. Slow gait speed and risk of mortality or hospital readmission after myocardial infarction in the translational research investigating underlying disparities in recovery from acute myocardial infarction: Patients’ health status registry. J. Am. Geriatr. Soc. 2016, 64, 596–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Núñez, J.; Ruiz, V.; Bonanad, C.; Miñana, G.; García-Blas, S.; Valero, E.; Núñez, E.; Sanchis, J. Percutaneous coronary intervention and recurrent hospitalizations in elderly patients with non ST-segment acute coronary syndrome: The role of frailty. Int. J. Cardiol. 2017, 228, 456–458. [Google Scholar] [CrossRef]
- Blanco, S.; Ferrières, J.; Bongard, V.; Toulza, O.; Sebai, F.; Billet, S.; Biendel, C.; Lairez, O.; Lhermusier, T.; Boudou, N.; et al. Prognosis impact of frailty assessed by the Edmonton Frail Scale in the setting of acute coronary syndrome in the elderly. Can. J. Cardiol. 2017, 33, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, J.; Ruiz, V.; Bonanad, C.; Valero, E.; Ruescas-Nicolau, M.A.; Ezzatvar, Y.; Sastre, C.; García-Blas, S.; Mollar, A.; Bertomeu-González, V.; et al. Prognostic value of geriatric conditions beyond age after acute coronary syndrome. Mayo Clin. Proc. 2017, 92, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Ekerstad, N.; Swahn, E.; Janzon, M.; Alfredsson, J.; Löfmark, R.; Lindenberger, M.; Andersson, D.; Carlsson, P. Frailty is independently associated with 1-year mortality for elderly patients with non-ST-segment elevation myocardial infarction. Eur. J. Prev. Cardiol. 2014, 21, 1216–1224. [Google Scholar] [CrossRef]
- Alegre, O.; Formiga, F.; López-Palop, R.; Marín, F.; Vidán, M.T.; Martínez-Sellés, M.; Carol, A.; Sionis, A.; Díez-Villanueva, P.; Aboal, J.; et al. An easy assessment of frailty at baseline independently predicts prognosis in very elderly patients with acute coronary syndromes. J. Am. Med. Dir. Assoc. 2018, 19, 296–303. [Google Scholar] [CrossRef] [Green Version]
- Sanchis, J.; Ruiz, V.; Sastre, C.; Bonanad, C.; Ruescas, A.; Fernández-Cisnal, A.; Mollar, A.; Valero, E.; Blas, S.G.; González, J.; et al. Frailty tools for assessment of long-term prognosis after acute coronary syndrome. Mayo Clin. Proc. Innov. Qual. Outcomes 2020, 4, 642–648. [Google Scholar] [CrossRef]
- Llaó, I.; Ariza-Solé, A.; Sanchis, J.; Alegre, O.; López-Palop, R.; Formiga, F.; Marín, F.; Vidán, M.T.; Martínez-Sellés, M.; Sionis, A.; et al. Invasive strategy and frailty in very elderly patients with acute coronary syndromes. EuroIntervention 2018, 14, e336–e342. [Google Scholar] [CrossRef]
- Sanchis, J.; Ariza-Solé, A.; Abu-Assi, E.; Alegre, O.; Alfonso, F.; Barrabés, J.A.; Baz, J.A.; Carol, A.; Díez Villanueva, P.; García Del Blanco, B.; et al. Invasive versus Conservative Strategy in Frail Patients with NSTEMI: The MOSCA-FRAIL Clinical Trial Study Design. Rev. Esp. Cardiol. 2019, 72, 154–159. [Google Scholar] [CrossRef]
- Alonso Salinas, G.L.; Sanmartín Fernández, M.; Pascual Izco, M.; Marco Del Castillo, Á.; Rincón Díaz, L.M.; Lozano Granero, C.; Valverde Gómez, M.; Pastor Pueyo, P.; Del Val Martín, D.; Pardo Sanz, A.; et al. Frailty predicts major bleeding within 30 days in elderly patients with acute coronary syndrome. Int. J. Cardiol. 2016, 222, 590–593. [Google Scholar] [CrossRef] [PubMed]
- White, H.D.; Westerhout, C.M.; Alexander, K.P.; Roe, M.T.; Winters, K.J.; Cyr, D.D.; Fox, K.A.; Prabhakaran, D.; Hochman, J.S.; Armstrong, P.W.; et al. TRILOGY ACS investigators. Frailty is associated with worse outcomes in non-ST segment elevation acute coronary syndromes: Insights from the TaRgeted platelet Inhibition to cLarify the Optimal strateGy to medicallY manage Acute Coronary Syndromes (TRILOGY ACS) trial. Eur. Heart J. Acute Cardiovasc. Care 2016, 5, 231–242. [Google Scholar]
- Sanchis, J.; Sastre, C.; Ruescas, A.; Ruiz, V.; Valero, E.; Bonanad, C.; García-Blas, S.; Fernández-Cisnal, A.; González, J.; Miñana, G.; et al. Randomized comparison of exercise intervention versus usual care in older adult patients with frailty after acute myocardial infarction. Am. J. Med. 2021, 134, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, J.; Bonanad, C.; García-Blas, S.; Ruiz, V.; Fernández-Cisnal, A.; Sastre, C.; Ruescas, A.; Valero, E.; González, J.; Mollar, A.; et al. Long-term prognostic value of cognitive impairment on top of frailty in older adults after acute coronary syndrome. J. Clin. Med. 2021, 10, 444. [Google Scholar] [CrossRef]
- Calabrò, P.; Moscarella, E.; Gragnano, F.; Cesaro, A.; Pafundi, P.C.; Patti, G.; Cavallari, I.; Antonucci, E.; Cirillo, P.; Pignatelli, P.; et al. Effect of Body Mass Index on Ischemic and Bleeding Events in Patients Presenting with Acute Coronary Syndromes (from the START-ANTIPLATELET Registry). Am. J. Cardiol. 2019, 124, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, J.; Núñez, J.; Bodí, V.; Núñez, E.; García-Alvarez, A.; Bonanad, C.; Regueiro, A.; Bosch, X.; Heras, M.; Sala, J.; et al. Influence of comorbid conditions on one-year outcomes in non-ST-segment elevation acute coronary syndrome. Mayo Clin. Proc. 2011, 86, 291–296. [Google Scholar] [CrossRef] [Green Version]
- Núñez, J.E.; Núñez, E.; Fácila, L.; Bertomeu, V.; Llàcer, A.; Bodí, V.; Sanchis, J.; Sanjuán, R.; Blasco, M.L.; Consuegra, L.; et al. Prognostic value of Charlson comorbidity index at 30 days and 1 year after acute myocardial infarction. Rev. Esp. Cardiol. 2004, 57, 842–849. [Google Scholar] [CrossRef]
- Sanchis, J.; Soler, M.; Núñez, J.; Ruiz, V.; Bonanad, C.; Formiga, F.; Valero, E.; Martínez-Sellés, M.; Marín, F.; Ruescas, A.; et al. Comorbidity assessment for mortality risk stratification in elderly patients with acute coronary syndrome. Eur. J. Intern. Med. 2019, 62, 48–53. [Google Scholar] [CrossRef]
- Kwok, C.S.; Martinez, S.C.; Pancholy, S.; Ahmed, W.; Al-Shaibi, K.; Potts, J.; Mohamed, M.; Kontopantelis, E.; Curzen, N.; Mamas, M.A. Effect of comorbidity on unplanned readmissions after percutaneous coronary intervention (from the nationwide readmission database). Sci. Rep. 2018, 8, 11156. [Google Scholar] [CrossRef]
- Sanchis, J.; Núñez, E.; Barrabés, J.A.; Marín, F.; Consuegra-Sánchez, L.; Ventura, S.; Valero, E.; Roqué, M.; Bayés-Genís, A.; Del Blanco, B.G.; et al. Randomized comparison between the invasive and conservative strategies in comorbid elderly patients with non-ST elevation myocardial infarction. Eur. J. Intern. Med. 2016, 35, 8. [Google Scholar] [CrossRef]
- Martínez-Sellés, D.; Martínez-Sellés, H.; Martinez-Sellés, M. Ethical Issues in Decision-making Regarding the Elderly Affected by Coronavirus Disease 2019: An Expert Opinion. Eur. Cardiol. 2020, 15, e48. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.S.; Kannoth, S.; Levy, S.; Wang, S.Y.; Lee, J.E.; Levy, B.R. Global reach of ageism on older persons’ health: A systematic review. PLoS ONE 2020, 15, e0220857. [Google Scholar] [CrossRef] [Green Version]
- Torjesen, I. European doctors worry about the care they will receive when they are old. BMJ 2012, 344, e77. [Google Scholar] [CrossRef]
- Gorodeski, E.Z.; Goyal, P.; Hummel, S.L.; Krishnaswami, A.; Goodlin, S.J.; Hart, L.L.; Forman, D.E.; Wenger, N.K.; Kirkpatrick, J.N.; Alexander, K.P.; et al. Domain management approach to heart failure in the geriatric patient: Present and future. J. Am. Coll. Cardiol. 2018, 71, 1921–1936. [Google Scholar] [CrossRef]
- Alexander, K.P.; Newby, L.K.; Cannon, C.P.; Armstrong, P.W.; Gibler, W.B.; Rich, M.W.; Van de Werf, F.; White, H.D.; Weaver, W.D.; Naylor, M.D.; et al. Acute coronary care in the elderly, part I: Non-ST-segment-elevation acute coronary syndromes: A scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: In collaboration with the Society of Geriatric Cardiology. Circulation 2007, 115, 2549–2569. [Google Scholar] [PubMed] [Green Version]
- Mannelli, C. Whose life to save? Scarce resources allocation in the COVID-19 outbreak. J. Med. Ethics 2020, 46, 364–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emanuel, E.J.; Persad, G.; Upshur, R.; Thome, B.; Parker, M.; Glickman, A.; Zhang, C.; Boyle, C.; Smith, M.; Phillips, J.P. Fair allocation of scarce medical resources in the time of Covid-19. N. Engl. J. Med. 2020, 382, 2049–2055. [Google Scholar] [CrossRef] [PubMed]
- Bonanad, C.; García-Blas, S.; Tarazona-Santabalbina, F.J.; Díez-Villanueva, P.; Ayesta, A.; Sanchis Forés, J.; Vidán-Austiz, M.T.; Formiga, F.; Ariza-Solé, A.; Martínez-Sellés, M. Coronavirus: The geriatric emergency of 2020. Joint document of the Section on Geriatric Cardiology of the Spanish Society of Cardiology and the Spanish Society of Geriatrics and Gerontology. Rev. Esp. Cardiol. 2020, 73, 569–576. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Miura, S.I.; Katsuda, Y.; Sugihara, M.; Ike, A.; Nishikawa, H.; Kawamura, A. A strict target for low-density lipoprotein cholesterol may not be necessary for secondary prevention of cardiovascular disease in all elderly patients with dyslipidemia. Cardiovasc. Res. 2020, 11, 366–369. [Google Scholar] [CrossRef]
- Afilalo, J.; Duque, G.; Steele, R.; Jukema, J.W.; de Craen, A.J.; Eisenberg, M.J. Statins for secondary prevention in elderly patients: A hierarchical bayesian meta-analysis. J. Am. Coll. Cardiol. 2008, 51, 37–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of statin therapy in older people: A meta-analysis of individual participant data from 28 randomised controlled trials. Lancet 2019, 393, 407–415. [Google Scholar] [CrossRef] [Green Version]
- Lionakis, N.; Mendrinos, D.; Sanidas, E.; Favatas, G.; Georgopoulou, M. Hypertension in the elderly. World J. Cardiol. 2012, 4, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Denardo, S.J.; Gong, Y.; Nichols, W.W.; Messerli, F.H.; Bavry, A.A.; Cooper-Dehoff, R.M.; Handberg, E.M.; Champion, A.; Pepine, C.J. Blood pressure and outcomes in very old hypertensive coronary artery disease patients: An INVEST substudy. Am. J. Med. 2010, 123, 719–726. [Google Scholar] [CrossRef] [Green Version]
| Topic | Authors | Year | Study Type | Main Results |
|---|---|---|---|---|
| Troponin in the elderly | Boeddinghaus et al. [11] | 2018 | Observational | 0/1 h troponin algorithm has similar rule-out safety but lower rule-in accuracy in ≥70 years. Specific thresholds may increase performance. |
| Welsh et al. [8] | 2018 | Observational | Higher 99th percentile for troponin increases after the age of 60. | |
| NSTEMI | Tegn et al. [32] (After-Eighty) | 2016 | Randomized trial | >80 years NSTEMI patients. Invasive vs. conservative strategy reduced combined endpoint of myocardial infarction, urgent revascularization, stroke, or death. |
| Kaura et al. [33] (SENIOR-NSTEMI) | 2020 | Randomized trial | >80 years NSTEMI patients. Revascularization within the first 3 days of admission was associated to 32% reduction in all-cause mortality. | |
| Antithrombotic treatment | Husted et al. [54] (PLATO substudy) | 2012 | Randomized trial | No increase in overall major bleeding with ticagrelor versus clopidogrel was observed in patients aged ≥ 75 years. |
| Cayla et al. [57] (TRITON substudy) | 2016 | Randomized trial | No clinical benefit of prasugrel was observed in patients >75 years due to their higher rates of bleeding. | |
| Gimbel et al. [58] (POPULAR AGE) | 2020 | Randomized trial | >75 years NSTEMI patients. Lower bleeding rate with clopidogrel compared to ticagrelor or prasugrel, without an increase in the combined endpoint of all cause of death, myocardial infarction, stroke and bleeding. | |
| Geriatric conditions | Llao et al. [81] (LONGEVO registry) | 2018 | Observational | Conservative treatment is associated with worse prognosis in older NSTEMI patients only if non-frail. |
| Sanchis et al. [82] (MOSCA) | 2016 | Randomized trial | Older patient with comorbidities. Invasive strategy did not improve outcomes in terms of mortality or ischemic events at long-term follow-up. | |
| Sanchis et al. | 2021 | Randomized trial | Frailty status improved significantly after myocardial infarction if a cardiac rehabilitation program was followed. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Blas, S.; Cordero, A.; Diez-Villanueva, P.; Martinez-Avial, M.; Ayesta, A.; Ariza-Solé, A.; Mateus-Porta, G.; Martínez-Sellés, M.; Escribano, D.; Gabaldon-Perez, A.; et al. Acute Coronary Syndrome in the Older Patient. J. Clin. Med. 2021, 10, 4132. https://doi.org/10.3390/jcm10184132
García-Blas S, Cordero A, Diez-Villanueva P, Martinez-Avial M, Ayesta A, Ariza-Solé A, Mateus-Porta G, Martínez-Sellés M, Escribano D, Gabaldon-Perez A, et al. Acute Coronary Syndrome in the Older Patient. Journal of Clinical Medicine. 2021; 10(18):4132. https://doi.org/10.3390/jcm10184132
Chicago/Turabian StyleGarcía-Blas, Sergio, Alberto Cordero, Pablo Diez-Villanueva, Maria Martinez-Avial, Ana Ayesta, Albert Ariza-Solé, Gemma Mateus-Porta, Manuel Martínez-Sellés, David Escribano, Ana Gabaldon-Perez, and et al. 2021. "Acute Coronary Syndrome in the Older Patient" Journal of Clinical Medicine 10, no. 18: 4132. https://doi.org/10.3390/jcm10184132
APA StyleGarcía-Blas, S., Cordero, A., Diez-Villanueva, P., Martinez-Avial, M., Ayesta, A., Ariza-Solé, A., Mateus-Porta, G., Martínez-Sellés, M., Escribano, D., Gabaldon-Perez, A., Bodi, V., & Bonanad, C. (2021). Acute Coronary Syndrome in the Older Patient. Journal of Clinical Medicine, 10(18), 4132. https://doi.org/10.3390/jcm10184132









