MRI Protocol for Pituitary Assessment in Children with Growth or Puberty Disorders—Is Gadolinium Contrast Administration Actually Needed?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Technical Details and Image Analysis
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. The Frequency of Focal Lesions
3.3. Pituitary Dimensions
3.4. Signal Intensity of the Lesion
4. Discussion
- The pituitary gland must not present any focal lesions on the T1 and/or T2 image or the lesion must be in the typical location for RCC, EPP, or MA.
- The AP dimension of the pituitary gland must be smaller than the cut-off value (7.5 mm).
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Derrick, K.M.; Gomes, W.A.; Gensure, R.C. Incidence and Outcomes of Pituitary Microadenomas in Children with Short Stature/Growth Hormone Deficiency. Horm. Res. Paediatr. 2018, 90, 151–160. [Google Scholar] [CrossRef]
- Güneş, A.; Özbal Güneş, S. The neuroimaging features of rathke’s cleft cysts in children with endocrine-related diseases. Diagn. Interv. Radiol. 2020, 26, 61–67. [Google Scholar] [CrossRef]
- Pinker, K.; Ba-Ssalamah, A.; Wolfsberger, S.; Mlynarik, V.; Knosp, E.; Trattnig, S. The value of high-field MRI (3 T) in the assessment of sellar lesions. Eur. J. Radiol. 2005, 54, 327–334. [Google Scholar] [CrossRef]
- Hainc, N.; Stippich, C.; Reinhardt, J.; Stieltjes, B.; Blatow, M.; Mariani, L.; Bink, A. Golden-angle radial sparse parallel (GRASP) MRI in clinical routine detection of pituitary microadenomas: First experience and feasibility. Magn. Reson. Imaging 2019, 60, 38–43. [Google Scholar] [CrossRef]
- Kasaliwal, R.; Sankhe, S.S.; Lila, A.R.; Budyal, S.R.; Jagtap, V.S.; Sarathi, V.; Kakade, H.; Bandgar, T.; Menon, P.S.; Shah, N.S. Volume interpolated 3D-spoiled gradient echo sequence is better than dynamic contrast spin echo sequence for MRI detection of corticotropin secreting pituitary microadenomas. Clin. Endocrinol. 2013, 78, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Fushimi, Y.; Okada, T.; Kanagaki, M.; Yamamoto, A.; Kanda, Y.; Sakamoto, R.; Hojo, M.; Takahashi, J.C.; Miyamoto, S.; Togashi, K. 3D dynamic pituitary MR imaging with CAIPIRINHA: Initial experience and comparison with 2D dynamic MR imaging. Eur. J. Radiol. 2014, 83, 1900–1906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Y.; Wu, Y.; Zhang, H.; Wang, J.; Yao, Z.W. Increased diagnostic confidence in the diagnosis of pituitary micro-lesions with the addition of three-dimensional sampling perfection with application-optimized contrasts using different flip-angle evolutions sequences. Acta Radiol. 2019, 60, 213–220. [Google Scholar] [CrossRef]
- Rossi Espagnet, M.C.; Bangiyev, L.; Haber, M.; Block, K.T.; Babb, J.; Ruggiero, V.; Boada, F.; Gonen, O.; Fatterpekar, G.M. High-Resolution DCE-MRI of the Pituitary Gland Using Radial k -Space Acquisition with Compressed Sensing Reconstruction. Am. J. Neuroradiol. 2015, 36, 1444–1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sen, R.; Sen, C.; Pack, J.; Block, K.T.; Golfinos, J.G.; Prabhu, V.; Boada, F.; Gonen, O.; Kondziolka, D.; Fatterpekar, G. Role of High-Resolution Dynamic Contrast-Enhanced MRI with Golden-Angle Radial Sparse Parallel Reconstruction to Identify the Normal Pituitary Gland in Patients with Macroadenomas. Am. J. Neuroradiol. 2017, 38, 1117–1121. [Google Scholar] [CrossRef]
- Yokota, Y.; Fushimi, Y.; Okada, T.; Fujimoto, K.; Oshima, S.; Nakajima, S.; Fujii, T.; Tanji, M.; Inagaki, N.; Miyamoto, S.; et al. Evaluation of image quality of pituitary dynamic contrast-enhanced MRI using time-resolved angiography with interleaved stochastic trajectories (TWIST) and iterative reconstruction TWIST (IT-TWIST). J. Magn. Reson. Imaging 2020, 51, 1497–1506. [Google Scholar] [CrossRef]
- Karimian-Jazi, K. Pituitary gland tumors. Radiologe 2019, 59, 982–991. [Google Scholar] [CrossRef]
- Holowka, S.; Shroff, M.; Chavhan, G.B. Use and Safety of Gadolinium Based Contrast Agents in Pediatric MR Imaging. Indian J. Pediatr. 2019, 86, 961–966. [Google Scholar] [CrossRef] [PubMed]
- McDonald, R.J.; Levine, D.; Weinreb, J.; Kanal, E.; Davenport, M.S.; Ellis, J.H.; Jacobs, P.M.; Lenkinski, R.E.; Maravilla, K.R.; Prince, M.R.; et al. Gadolinium retention: A research roadmap from the 2018 NIH/ACR/RSNA workshop on gadolinium chelates. Radiology 2018, 289, 517–534. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Fukusato, T.; Matsuda, M.; Toyoda, K.; Oba, H.; Kotoku, J.; Haruyama, T.; Kitajima, K.; Furui, S. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: Evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 2015, 276, 228–232. [Google Scholar] [CrossRef]
- Elbeshlawi, I.; AbdelBaki, M.S. Safety of Gadolinium Administration in Children. Pediatr. Neurol. 2018, 86, 27–32. [Google Scholar] [CrossRef]
- McDonald, J.S.; McDonald, R.J.; Jentoft, M.E.; Paolini, M.A.; Murray, D.L.; Kallmes, D.F.; Eckel, L.J. Intracranial gadolinium deposition following gadodiamide-enhanced magnetic resonance imaging in pediatric patients: A case-control study. JAMA Pediatr. 2017, 171, 705–707. [Google Scholar] [CrossRef] [Green Version]
- Portocarrero-Ortiz, L.; Bonifacio-Delgadillo, D.; Sotomayor-González, A.; Garcia-Marquez, A.; Lopez-Serna, R. A modified protocol using half-dose gadolinium in dynamic 3-Tesla magnetic resonance imaging for detection of ACTH-secreting pituitary tumors. Pituitary 2010, 13, 230–235. [Google Scholar] [CrossRef]
- Rennert, J.; Doerfler, A. Imaging of sellar and parasellar lesions. Clin. Neurol. Neurosurg. 2007, 109, 111–124. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, J.D. Human Studies of Anesthesia-Related Neurotoxicity in Children: A Narrative Review of Recent Additions to the Clinical Literature. Clin. Perinatol. 2019, 46, 637–645. [Google Scholar] [CrossRef]
- Warner, D.O.; Zaccariello, M.J.; Katusic, S.K.; Schroeder, D.R.; Hanson, A.C.; Schulte, P.J.; Buenvenida, S.L.; Gleich, S.J.; Wilder, R.T.; Sprung, J.; et al. Neuropsychological and Behavioral Outcomes after Exposure of Young Children to Procedures Requiring General Anesthesia, The Mayo Anesthesia Safety in Kids (MASK) Study. Anesthesiology 2018, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Bjur, K.A.; Payne, E.T.; Nemergut, M.E.; Hu, D.; Flick, R.P. Anesthetic-Related Neurotoxicity and Neuroimaging in Children: A Call for Conversation. J. Child. Neurol. 2017, 32, 594–602. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.S.; Li, G.; Miller, T.L.K.; Salorio, C.; Byrne, M.W.; Bellinger, D.C.; Ing, C.; Park, R.; Radcliffe, J.; Hays, S.R.; et al. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA J. Am. Med. Assoc. 2016, 315, 2312–2320. [Google Scholar] [CrossRef] [PubMed]
- Barton, K.; Nickerson, J.P.; Higgins, T.; Williams, R.K. Pediatric anesthesia and neurotoxicity: What the radiologist needs to know. Pediatr. Radiol. 2018, 48, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Brambrink, A.M.; Evers, A.S.; Avidan, M.S.; Farber, N.B.; Smith, D.J.; Zhang, X.; Dissen, G.A.; Creeley, C.E.; Olney, J.W. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology 2010, 112, 834–841. [Google Scholar] [CrossRef] [Green Version]
- Dumrongpisutikul, N.; Chuajak, A.; Lerdlum, S. Pituitary height at magnetic resonance imaging in pediatric isolated growth hormone deficiency. Pediatr. Radiol. 2018, 48, 694–700. [Google Scholar] [CrossRef]
- Sharafuddin, M.J.; Luisiri, A.; Garibaldi, L.R.; Fulk, D.L.; Klein, J.B.; Gillespie, K.N.; Graviss, E.R. MR imaging diagnosis of central precocious puberty: Importance of changes in the shape and size of the pituitary gland. AJR Am. J. Roentgenol. 1994, 162, 1167–1173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessler, M.; Tenner, M.; Frey, M.; Noto, R. Pituitary volume in children with growth hormone deficiency, idiopathic short stature and controls. J. Pediatr. Endocrinol. Metab. 2016, 29, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- R Team Core R: A Language and Environment for Statistical Computing Version 4.0.2 2020. Available online: https://www.r-project.org/ (accessed on 10 September 2021).
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12. [Google Scholar] [CrossRef]
- Bunin, G.R.; Surawicz, T.S.; Witman, P.A.; Preston-Martin, S.; Davis, F.; Bruner, J.M. The descriptive epidemiology of craniopharyngioma. J. Neurosurg. 1998, 89, 547–551. [Google Scholar] [CrossRef]
- Jagannathan, J.; Dumont, A.S.; Jane, J.A. Diagnosis and management of pediatric sellar lesions. Front. Horm. Res. 2006, 34, 83–104. [Google Scholar] [CrossRef]
- Poussaint, T.Y. Magnetic resonance imaging of pediatric brain tumors: State of the art. Top. Magn. Reson. Imaging 2001, 12, 411–433. [Google Scholar] [CrossRef]
- Rossi, A.; Cama, A.; Consales, A.; Gandolfo, C.; Garrè, M.L.; Milanaccio, C.; Pavanello, M.; Piatelli, G.; Ravegnani, M.; Tortori-Donati, P. Neuroimaging of pediatric craniopharyngiomas: A pictorial essay. J. Pediatr. Endocrinol. Metab. 2006, 19 (Suppl. 1), 299–319. [Google Scholar]
- Souteiro, P.; Maia, R.; Santos-Silva, R.; Figueiredo, R.; Costa, C.; Belo, S.; Castro-Correia, C.; Carvalho, D.; Fontoura, M. Pituitary incidentalomas in paediatric age are different from those described in adulthood. Pituitary 2019, 22, 124–128. [Google Scholar] [CrossRef]
- Takanashi, J.I.; Tada, H.; Barkovich, A.J.; Saeki, N.; Kohno, Y. Pituitary cysts in childhood evaluated by MR imaging. Am. J. Neuroradiol. 2005, 26, 2144–2147. [Google Scholar]
- Giannattasio, G.; Bassetti, M. Human pituitary adenomas. Recent advances in morphological studies. J. Endocrinol. Investig. Off. J. Ital. Soc. Endocrinol. 1990, 13, 435–454. [Google Scholar] [CrossRef]
- Teramoto, A.; Hirakawa, K.; Sanno, N.; Osamura, Y. Incidental pituitary lesions in 1,000 unselected autopsy specimens. Radiology 1994, 193, 161–164. [Google Scholar] [CrossRef]
- Sumida, M.; Uozumi, T.; Mukada, K.; Arita, K.; Kurisu, K.; Eguchi, K. Rathke cleft cysts: Correlation of enhanced MR and surgical findings. AJNR Am. J. Neuroradiol. 1994, 15, 525–532. [Google Scholar]
- Guaraldi, F.; Storr, H.L.; Ghizzoni, L.; Ghigo, E.; Savage, M.O. Paediatric pituitary adenomas: A decade of change. Horm. Res. Paediatr. 2014, 81, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Kao, S.C.S.; Cook, J.S.; Hansen, J.R.; Simonson, T.M. MR imaging of the pituitary gland in central precocious puberty. Pediatr. Radiol. 1992, 22, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Quattrocchi, C.C.; Errante, Y.; Mallio, C.A.; Marinelli, L.; Lovullo, G.; Giannotti, G.; Della Sala, S.W.; Van Der Molen, A.J.; Beomonte Zobel, B. Effect of Age on High T1 Signal Intensity of the Dentate Nucleus and Globus Pallidus in a Large Population Exposed to Gadodiamide. Invest. Radiol. 2018, 53, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Osawa, M.; Oba, H.; Toyoda, K.; Kotoku, J.; Haruyama, T.; Takeshita, K.; Furui, S. High signal intensity in dentate nucleus on unenhanced T1-weighted MR images: Association with linear versus macrocyclic gadolinium chelate administration. Radiology 2015, 275, 803–809. [Google Scholar] [CrossRef] [Green Version]
- Rossi Espagnet, M.C.; Bernardi, B.; Pasquini, L.; Figà-Talamanca, L.; Tomà, P.; Napolitano, A. Signal intensity at unenhanced T1-weighted magnetic resonance in the globus pallidus and dentate nucleus after serial administrations of a macrocyclic gadolinium-based contrast agent in children. Pediatr. Radiol. 2017, 47, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gulani, V.; Calamante, F.; Shellock, F.G.; Kanal, E.; Reeder, S.B. Gadolinium deposition in the brain: Summary of evidence and recommendations. Lancet Neurol. 2017, 16, 564–570. [Google Scholar] [CrossRef]
- Mallio, C.A.; Rovira, À.; Parizel, P.M.; Quattrocchi, C.C. Exposure to gadolinium and neurotoxicity: Current status of preclinical and clinical studies. Neuroradiology 2020, 62, 925–934. [Google Scholar] [CrossRef]
- European Medicines Agency. EMA’s Final Opinion Confirms Restrictions on Use of Linear Gadolinium Agents in Body Scans. 2017. Available online: https://www.ema.europa.eu/en/documents/press-release/emas-final-opinion-confirms-restrictions-use-linear-gadolinium-agents-body-scans_en.pdf (accessed on 10 September 2021).
- Fingerhut, S.; Sperling, M.; Holling, M.; Niederstadt, T.; Allkemper, T.; Radbruch, A.; Heindel, W.; Paulus, W.; Jeibmann, A.; Karst, U. Gadolinium-based contrast agents induce gadolinium deposits in cerebral vessel walls, while the neuropil is not affected: An autopsy study. Acta Neuropathol. 2018, 136, 127–138. [Google Scholar] [CrossRef]
- FDA. FDA Drug Safety Communication: FDA Warns that Gadolinium-Based Contrast Agents (GBCAs) Are Retained in the Body; Requires New Class Warnings. 2018. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-warns-gadolinium-based-contrast-agents-gbcas-are-retained-body (accessed on 10 September 2021).
- Dünger, D.; Krause, M.; Gräfe, D.; Merkenschlager, A.; Roth, C.; Sorge, I. Do we need gadolinium-based contrast medium for brain magnetic resonance imaging in children? Pediatr. Radiol. 2018, 48, 858–864. [Google Scholar] [CrossRef]
- Bonneville, J.F. A plea for the T2W MR sequence for pituitary imaging. Pituitary 2019, 22, 195–197. [Google Scholar] [CrossRef]
- Bonneville, J.F. Long term MRI surveillance of pituitary macroadenomas: Gadolinium is not obligatory. Pituitary 2019, 22, 100–102. [Google Scholar] [CrossRef] [PubMed]





| Characteristic | Value |
|---|---|
| No. of patients | 567 |
| Mean age [years] (± SD) | 6.99 ± 2.59 |
| Range of age [years] | 0.9–17.4 |
| No. of male patients | 294 |
| No. of female patients | 259 |
| Clinical diagnosis (M/F): | |
| - Short stature - Gigantism - Precocious puberty | 509 (294/215) 5 (3/2) 54 (11/43) |
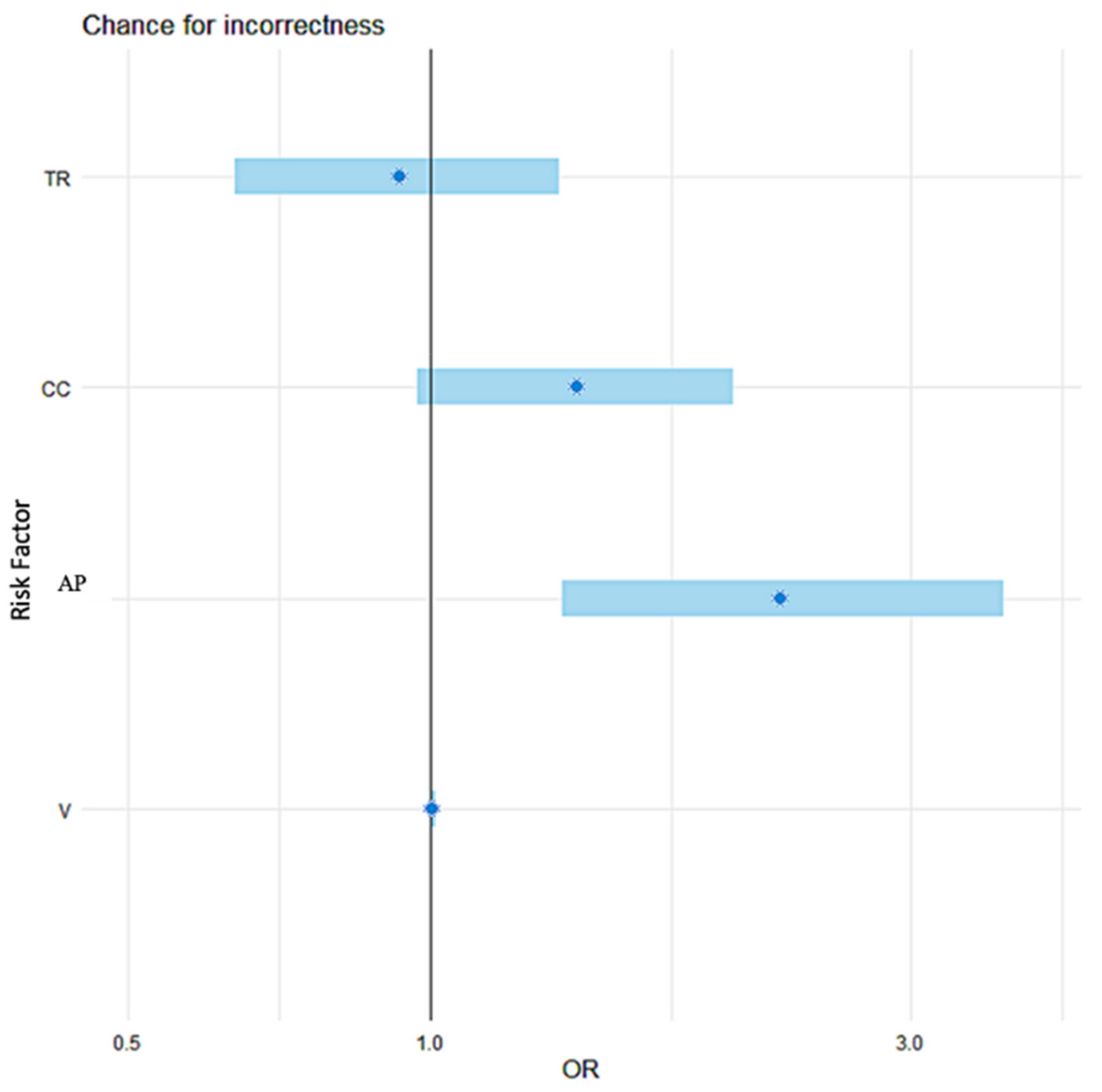
| Risk Factor | OR | CI 95% | p-Value |
|---|---|---|---|
| AP [mm] | 2.23 | (1.35, 3.71) | 0.002 |
| CC [mm] | 1.40 | (0.97, 2.00) | 0.071 |
| V [mm3] | 1.005 | (0.999, 1.011) | 0.055 |
| TR [mm] | 0.93 | (0.64, 1.34) | 0.679 |
| Risk Factor | AUC | p-Value | Cut-Off | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| AP [mm] | 0.729 (0.580 − 0.878) | 0.002 | 7.5 | 69.2 | 73.5 |
| CC [mm] | 0.66 (0.512 − 0.808) | 0.071 | 4.5 | 46.2 | 80.1 |
| V [mm3] | 0.686 (0.564 − 0.808) | 0.055 | 123.3 | 100% | 36.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michali-Stolarska, M.; Tukiendorf, A.; Zacharzewska-Gondek, A.; Jacków-Nowicka, J.; Chrzanowska, J.; Trybek, G.; Bladowska, J. MRI Protocol for Pituitary Assessment in Children with Growth or Puberty Disorders—Is Gadolinium Contrast Administration Actually Needed? J. Clin. Med. 2021, 10, 4598. https://doi.org/10.3390/jcm10194598
Michali-Stolarska M, Tukiendorf A, Zacharzewska-Gondek A, Jacków-Nowicka J, Chrzanowska J, Trybek G, Bladowska J. MRI Protocol for Pituitary Assessment in Children with Growth or Puberty Disorders—Is Gadolinium Contrast Administration Actually Needed? Journal of Clinical Medicine. 2021; 10(19):4598. https://doi.org/10.3390/jcm10194598
Chicago/Turabian StyleMichali-Stolarska, Marta, Andrzej Tukiendorf, Anna Zacharzewska-Gondek, Jagoda Jacków-Nowicka, Joanna Chrzanowska, Grzegorz Trybek, and Joanna Bladowska. 2021. "MRI Protocol for Pituitary Assessment in Children with Growth or Puberty Disorders—Is Gadolinium Contrast Administration Actually Needed?" Journal of Clinical Medicine 10, no. 19: 4598. https://doi.org/10.3390/jcm10194598
APA StyleMichali-Stolarska, M., Tukiendorf, A., Zacharzewska-Gondek, A., Jacków-Nowicka, J., Chrzanowska, J., Trybek, G., & Bladowska, J. (2021). MRI Protocol for Pituitary Assessment in Children with Growth or Puberty Disorders—Is Gadolinium Contrast Administration Actually Needed? Journal of Clinical Medicine, 10(19), 4598. https://doi.org/10.3390/jcm10194598








