Biomechanical Value of a Protective Proximal Humeral Cerclage in Reverse Total Shoulder Arthroplasty
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimens
2.2. Cortical Thickness Measurement
2.3. Cerclage Fixation
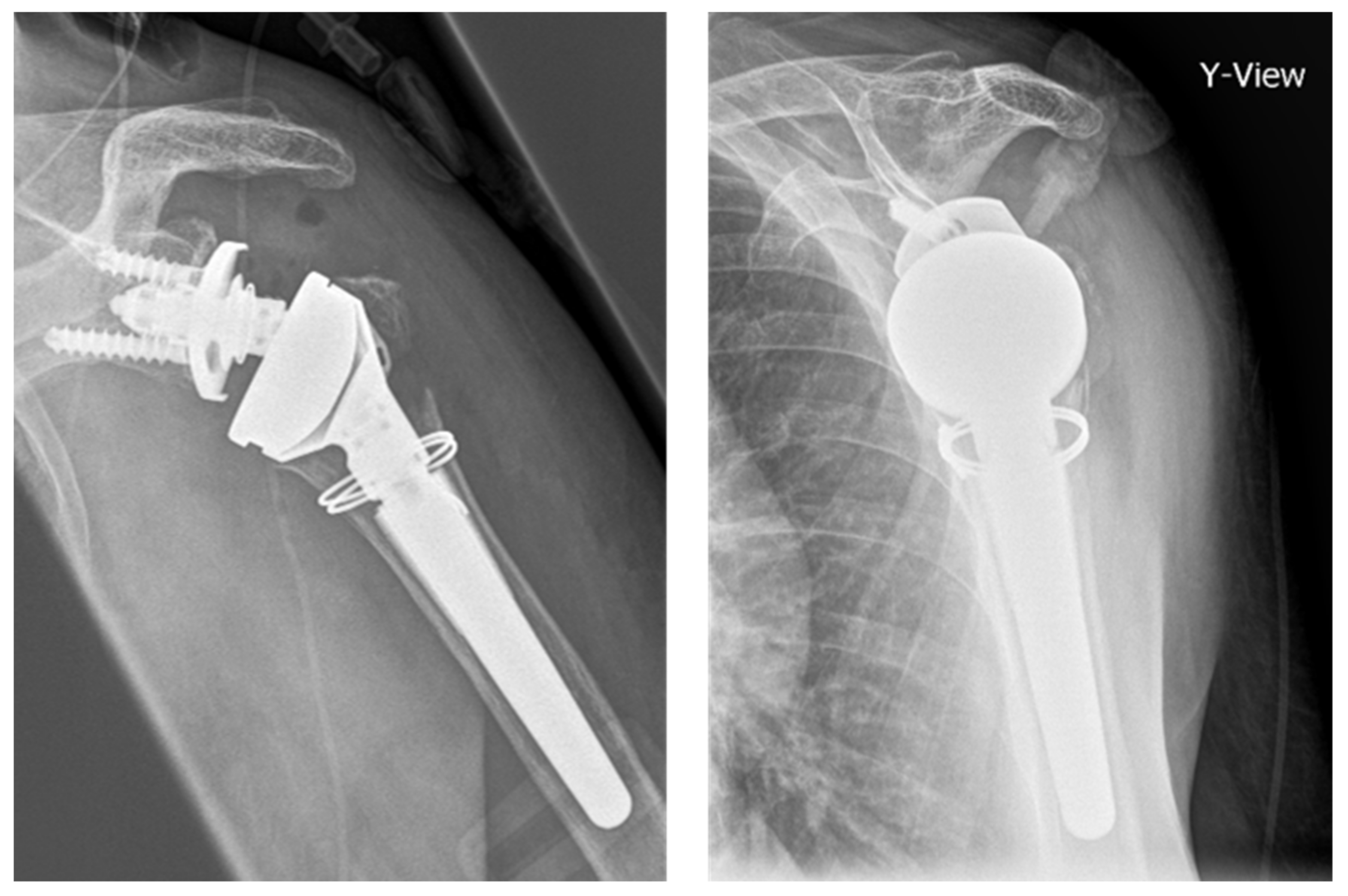
2.4. Biomechanical Testing
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sumrein, B.O.; Huttunen, T.; Launonen, A.P.; Berg, H.E.; Felländer-Tsai, L.; Mattila, V.M. Proximal humeral fractures in Sweden—A registry-based study. Osteoporos. Int. 2017, 28, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Launonen, A.P.; Lepola, V.; Saranko, A.; Flinkkilä, T.; Laitinen, M.; Mattila, V.M. Epidemiology of proximal humerus fractures. Arch. Osteoporos. 2015, 10, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kannus, P.; Palvanen, M.; Niemi, S.; Sievänen, H.; Parkkari, J. Rate of proximal humeral fractures in older Finnish women between 1970 and 2007. Bone 2009, 44, 656–659. [Google Scholar] [CrossRef]
- Hemmann, P.; Ziegler, P.; Konrads, C.; Ellmerer, A.; Klopfer, T.; Schreiner, A.J.; Bahrs, C. Trends in fracture development of the upper extremity in Germany—a population-based description of the past 15 years. J. Orthop. Surg. Res. 2020, 15, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Beks, R.B.; Ochen, Y.; Frima, H.; Smeeing, D.P.; van der Meijden, O.; Timmers, T.K.; van der Velde, D.; van Heijl, M.; Leenen, L.P.; Groenwold, R.; et al. Operative versus nonoperative treatment of proximal humeral fractures: A systematic review, meta-analysis, and comparison of observational studies and randomized controlled trials. J. Shoulder Elb. Surg. 2018, 27, 1526–1534. [Google Scholar] [CrossRef]
- Du, S.; Ye, J.; Chen, H.; Li, X.; Lin, Q. Interventions for Treating 3- or 4-part proximal humeral fractures in elderly patient: A network meta-analysis of randomized controlled trials. Int. J. Surg. 2017, 48, 240–246. [Google Scholar] [CrossRef]
- Fraser, A.N.; Bjørdal, J.; Wagle, T.M.; Karlberg, A.C.; Lien, O.A.; Eilertsen, L.; Mader, K.; Apold, H.; Larsen, L.B.; Madsen, J.E.; et al. Reverse Shoulder Arthroplasty Is Superior to Plate Fixation at 2 Years for Displaced Proximal Humeral Fractures in the Elderly. J. Bone Jt. Surg.-Am. Vol. 2020, 102, 477–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastor, M.-F.; Kieckbusch, M.; Kaufmann, M.; Ettinger, M.; Wellmann, M.; Smith, T. Reverse shoulder arthroplasty for fracture sequelae: Clinical outcome and prognostic factors. J. Orthop. Sci. 2019, 24, 237–242. [Google Scholar] [CrossRef]
- Schliemann, B.; Theisen, C.; Kösters, C.; Raschke, M.J.; Weimann, A. Reverse total shoulder arthroplasty for type I fracture sequelae after internal fixation of proximal humerus fractures. Arch. Orthop. Trauma Surg. 2017, 137, 1677–1683. [Google Scholar] [CrossRef]
- Grubhofer, F.; Wieser, K.; Meyer, D.C.; Catanzaro, S.; Schürholz, K.; Gerber, C. Reverse total shoulder arthroplasty for failed open reduction and internal fixation of fractures of the proximal humerus. J. Shoulder Elb. Surg. 2017, 26, 92–100. [Google Scholar] [CrossRef]
- Shannon, S.F.; Wagner, E.; Houdek, M.T.; Cross, W.W.; Sánchez-Sotelo, J. Reverse shoulder arthroplasty for proximal humeral fractures: Outcomes comparing primary reverse arthroplasty for fracture versus reverse arthroplasty after failed osteosynthesis. J. Shoulder Elb. Surg. 2016, 25, 1655–1660. [Google Scholar] [CrossRef]
- Holschen, M.; Pallmann, J.; Schorn, D.; Witt, K.-A.; Steinbeck, J. Simultaneous removal of a locking plate and implantation of a reversed shoulder prosthesis in elderly patients suffering from fracture sequelae of the proximal humerus. Musculoskelet. Surg. 2020, 104, 295–301. [Google Scholar] [CrossRef]
- Nowak, L.L.; Hall, J.; McKee, M.D.; Schemitsch, E.H. A higher reoperation rate following arthroplasty for failed fixation versus primary arthroplasty for the treatment of proximal humeral fractures. Bone Jt. J. 2019, 101-B, 1272–1279. [Google Scholar] [CrossRef]
- Bois, A.J.; Knight, P.; Alhojailan, K.; Bohsali, K.I. Clinical outcomes and complications of reverse shoulder arthroplasty used for failed prior shoulder surgery: A systematic review and meta-analysis. JSES Int. 2020, 4, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Katthagen, J.C.; Hesse, E.; Lill, H.; Schliemann, B.; Ellwein, A.; Raschke, M.J.; Imrecke, J. Outcomes and revision rates of primary vs. secondary reverse total shoulder arthroplasty for proximal humeral fractures. Obere Extrem. 2020, 15, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Ingoe, H.M.; Holland, P.; Cowling, P.; Kottam, L.; Baker, P.N.; Rangan, A. Intraoperative complications during revision shoulder arthroplasty: A study using the National Joint Registry dataset. Shoulder Elb. 2017, 9, 92–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowling, P.D.; Holland, P.; Kottam, L.; Baker, P.; Rangan, A. Risk factors associated with intraoperative complications in primary shoulder arthroplasty. Acta Orthop. 2017, 88, 587–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Athwal, G.S.; Sperling, J.W.; Rispoli, D.M.; Cofield, R.H. Periprosthetic Humeral Fractures During Shoulder Arthroplasty. J. Bone Jt. Surg.-Am. Vol. 2009, 91, 594–603. [Google Scholar] [CrossRef]
- Wagner, E.; Houdek, M.T.; Elhassan, B.T.; Sanchez-Sotelo, J.; Cofield, R.H.; Sperling, J.W. What Are Risk Factors for Intraoperative Humerus Fractures During Revision Reverse Shoulder Arthroplasty and Do They Influence Outcomes? Clin. Orthop. Relat. Res. 2015, 473, 3228–3234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eyberg, B.A.; Walker, J.B.; Harmsen, S.M.; Gobezie, R.; Denard, P.J.; Lederman, E.S. Suture cerclage for stabilizing the humeral shaft during shoulder arthroplasty. JSES Int. 2020, 4, 688–693. [Google Scholar] [CrossRef]
- Kriechling, P.; Hasler, A.; Passaplan, C.; Wieser, K. Is suture cerclage fixation a valid treatment for intraoperative nondisplaced calcar fractures in reverse total shoulder arthroplasties? JSES Int. 2021, 5, 673–678. [Google Scholar] [CrossRef]
- Denard, P.J.; Nolte, P.-C.; Millett, P.J.; Adams, C.R.; Liebler, S.A.; Rego, G.; Higgins, L.D. A Tensionable Suture-based Cerclage Is an Alternative to Stainless Steel Cerclage Fixation for Stabilization of a Humeral Osteotomy during Shoulder Arthroplasty. J. Am. Acad. Orthop. Surg. 2021, 29, e609–e617. [Google Scholar] [CrossRef]
- Waligora, A.C.; Owen, J.R.; Wayne, J.S.; Hess, S.R.; Golladay, G.J.; Jiranek, W.A. The Effect of Prophylactic Cerclage Wires in Primary Total Hip Arthroplasty: A Biomechanical Study. J. Arthroplast. 2017, 32, 2023–2027. [Google Scholar] [CrossRef]
- Kuster, L.S.; Marshall, D.; Fässler, C.; Duff, S.J.; Hayes, A.; Kuster, M.S. Can Prophylactic Cables Stop Crack Propagation in Revision Arthroplasty: A Biomechanical Study. J. Arthroplast. 2019, 34, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Kralinger, F.; Blauth, M.; Goldhahn, J.; Käch, K.; Voigt, C.; Platz, A.; Hanson, B. The Influence of Local Bone Density on the Outcome of One Hundred and Fifty Proximal Humeral Fractures Treated with a Locking Plate. J. Bone Jt. Surg.-Am. Vol. 2014, 96, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Krappinger, D.; Roth, T.; Gschwentner, M.; Suckert, A.; Blauth, M.; Hengg, C.; Kralinger, F. Preoperative assessment of the cancellous bone mineral density of the proximal humerus using CT data. Skelet. Radiol. 2012, 41, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Lill, H.; Hepp, P.; Gowin, W.; Oestmann, J.W.; Korner, J.; Haas, N.P.; Josten, C.; Duda, G.-N. Alters- und geschlechtsabhängige Knochenmineraldichteverteilung und mechanische Eigenschaften des proximalen Humerus. RöFo-Fortschr. Geb. Röntgenstrahlen Bildgeb. Verfahr. 2002, 174, 1544–1550. [Google Scholar] [CrossRef]
- Katthagen, J.C.; Michel, P.; Raschke, M.J.; Sußiek, J.; Frank, A.; Wermers, J.; Dyrna, F.; Schliemann, B. The forgotten fragment: Additional lesser tuberosity fixation of 4-part proximal humeral fractures—A biomechanical investigation. J. Shoulder Elb. Surg. 2021. [Google Scholar] [CrossRef]
- Tan, J.S.; Uppuganti, S. Cumulative Multiple Freeze-Thaw Cycles and Testing Does Not Affect Subsequent Within-Day Variation in Intervertebral Flexibility of Human Cadaveric Lumbosacral Spine. Spine 2012, 37, E1238–E1242. [Google Scholar] [CrossRef]
- Spross, C.; Kaestle, N.; Benninger, E.; Fornaro, J.; Erhardt, J.; Zdravkovic, V.; Jost, B. Deltoid Tuberosity Index: A Simple Radiographic Tool to Assess Local Bone Quality in Proximal Humerus Fractures. Clin. Orthop. Relat. Res. 2015, 473, 3038–3045. [Google Scholar] [CrossRef]
- Wähnert, D.; Lenz, M.; Schlegel, U.; Perren, S.; Windolf, M. Cerclage handling for improved fracture treatment. A biomechanical study on the twisting procedure. Acta Chir. Orthop. Traumatol. Cechoslov. 2011, 78, 78. [Google Scholar]
- Lo, I.K.; Burkhart, S.S.; Chan, K.; Athanasiou, K. Arthroscopic knots: Determining the optimal balance of loop security and knot security. Arthrosc. J. Arthrosc. Relat. Surg. 2004, 20, 489–502. [Google Scholar] [CrossRef] [PubMed]
- DiFazio, F.; Incavo, S.; Howe, J. Prevention of longitudinal crack propagation around a femoral prosthesis: A study of cerclage wire fixation. Clin. Biomech. 1993, 8, 274–276. [Google Scholar] [CrossRef]
- Herzwurm, P.J.; Walsh, J.; A Pettine, K.; Ebert, F.R. Prophylactic cerclage: A method of preventing femur fracture in uncemented total hip arthroplasty. Orthopedics 1992, 15, 143–146. [Google Scholar] [CrossRef]
- Mont, M.; Maar, D.; Krackow, K.; Hungerford, D. Hoop-stress fractures of the proximal femur during hip arthroplasty. Management and results in 19 cases. J. Bone Jt. Surg. Br. Vol. 1992, 74-B, 257–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pechon, P.H.M.; Pullin, R.; Eaton, M.J.; Jones, S.A.; Evans, S. Acoustic emission technology can warn of impending iatrogenic femur fracture during femoral canal preparation for uncemented hip replacement. A cadaveric animal bone study. J. Med. Eng. Technol. 2018, 42, 72–87. [Google Scholar] [CrossRef]
- Sheth, U.; Saltzman, M. Reverse Total Shoulder Arthroplasty: Implant Design Considerations. Curr. Rev. Musculoskelet. Med. 2019, 12, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Renner, N.; Wieser, K.; Lajtai, G.; Morrey, M.E.; Meyer, D.C. Stainless steel wire versus FiberWire suture cerclage fixation to stabilize the humerus in total shoulder arthroplasty. J. Shoulder Elb. Surg. 2014, 23, 1568–1574. [Google Scholar] [CrossRef]
- Peeters, I.; Depover, A.; Van Tongel, A.; De Wilde, L. A review of metallic and non-metallic cerclage in orthopaedic surgery: Is there still a place for metallic cerclage? Injury 2019, 50, 1627–1633. [Google Scholar] [CrossRef]







| Groups | Steel Wire | FT-Tension | FT-Hand | Native |
|---|---|---|---|---|
| BMD (mg/cm3) | 75.6 ± 30.3 | 83.8 ± 24.7 | 90.0 ± 38.8 | 89.5 ± 32.2 |
| DTI | 1.32 ± 0.08 | 1.33 ± 0.1 | 1.33 ± 0.06 | 1.32 ± 0.1 |
| Step 1 | Step 2 | Step 3 | Step 4 | Step 5 | |
|---|---|---|---|---|---|
| Weight (g) | 250 | 250 | 250 | 500 | 500 |
| Height (cm) | 20 | 27.5 | 35 | 35 | 42.5 |
| Strikes | 5 | 10 | 10 | 10 | 10 |
| Joules per strike | 0.5 | 0.7 | 0.9 | 1.7 | 2.1 |
| Joules per step | 2.5 | 7 | 9 | 17 | 21 |
| Joules in total | 2.5 | 9.5 | 18.5 | 35.5 | 56.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michel, P.A.; Katthagen, J.C.; Schliemann, B.; Wilkens, S.; Frank, A.; Heilmann, L.F.; Dyrna, F.; Raschke, M.J. Biomechanical Value of a Protective Proximal Humeral Cerclage in Reverse Total Shoulder Arthroplasty. J. Clin. Med. 2021, 10, 4600. https://doi.org/10.3390/jcm10194600
Michel PA, Katthagen JC, Schliemann B, Wilkens S, Frank A, Heilmann LF, Dyrna F, Raschke MJ. Biomechanical Value of a Protective Proximal Humeral Cerclage in Reverse Total Shoulder Arthroplasty. Journal of Clinical Medicine. 2021; 10(19):4600. https://doi.org/10.3390/jcm10194600
Chicago/Turabian StyleMichel, Philipp A., J. Christoph Katthagen, Benedikt Schliemann, Sina Wilkens, Andre Frank, Lukas F. Heilmann, Felix Dyrna, and Michael J. Raschke. 2021. "Biomechanical Value of a Protective Proximal Humeral Cerclage in Reverse Total Shoulder Arthroplasty" Journal of Clinical Medicine 10, no. 19: 4600. https://doi.org/10.3390/jcm10194600
APA StyleMichel, P. A., Katthagen, J. C., Schliemann, B., Wilkens, S., Frank, A., Heilmann, L. F., Dyrna, F., & Raschke, M. J. (2021). Biomechanical Value of a Protective Proximal Humeral Cerclage in Reverse Total Shoulder Arthroplasty. Journal of Clinical Medicine, 10(19), 4600. https://doi.org/10.3390/jcm10194600







