Prognostic Impact of Percutaneous Coronary Intervention of Chronic Total Occlusion in Acute and Periprocedural Myocardial Infarction
Abstract
:1. Introduction
2. Pathophysiologic Role of CTO and Collateral Supply in AMI Patients
2.1. Pathophysiology of Coronary Collateral Connections
2.2. Pathological Mechanism of CTO in Patients with AMI
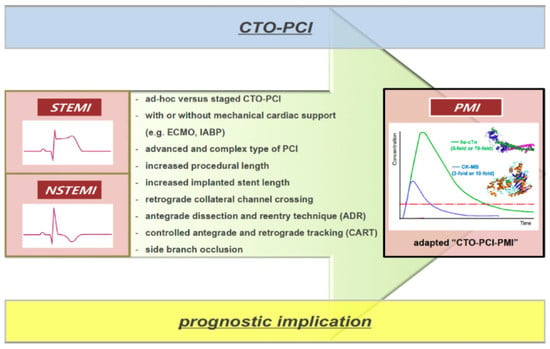
3. CTO-PCI in Patients with AMI
3.1. CTO in STEMI
3.2. CTO in STEMI Complicated by Cardiogenic Shock
3.3. CTO in NSTEMI
4. PMI Caused by CTO-PCI
4.1. Definition and Pathological Mechanism of PMI Caused by CTO-PCI
4.2. Prognostic Impact of PMI Caused by CTO-PCI
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stone, G.W.; Kandzari, D.E.; Mehran, R.; Colombo, A.; Schwartz, R.S.; Bailey, S.; Moussa, I.; Teirstein, P.S.; Dangas, G.; Baim, D.S. Percutaneous Recanalization of Chronically Occluded Coronary Arteries: A Consensus Document: Part I. Circulation 2005, 112, 2364–2372. [Google Scholar] [CrossRef] [PubMed]
- Jaber, W.A. Chronic Total Occlusion Intervention: The Case for More Evidence. JACC Cardiovasc. Interv. 2018, 11, 1992–1994. [Google Scholar] [CrossRef] [PubMed]
- Werner, G.S.; Gitt, A.K.; Zeymer, U.; Juenger, C.; Towae, F.; Wienbergen, H.; Senges, J. Chronic total coronary occlusions in patients with stable angina pectoris: Impact on therapy and outcome in present day clinical practice. Clin. Res. Cardiol. 2009, 98, 435–441. [Google Scholar] [CrossRef]
- Fefer, P.; Knudtson, M.L.; Cheema, A.N.; Galbraith, P.D.; Osherov, A.B.; Yalonetsky, S.; Gannot, S.; Samuel, M.; Weisbrod, M.; Bierstone, D.; et al. Current Perspectives on Coronary Chronic Total Occlusions: The Canadian Multicenter Chronic Total Occlusions Registry. J. Am. Coll. Cardiol. 2012, 59, 991–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toma, M.; Buller, C.E.; Westerhout, C.M.; Fu, Y.; O’Neill, W.W.; Holmes, D.R.; Hamm, C.W.; Granger, C.B.; Armstrong, P.W.; Apex-Ami Investigators. Non-culprit coronary artery percutaneous coronary intervention during acute ST-segment elevation myocardial infarction: Insights from the APEX-AMI trial. Eur. Heart J. 2010, 31, 1701–1707. [Google Scholar] [CrossRef]
- Claessen, B.E.; Dangas, G.D.; Weisz, G.; Witzenbichler, B.; Guagliumi, G.; Möckel, M.; Brener, S.J.; Xu, K.; Henriques, J.P.S.; Mehran, R.; et al. Prognostic impact of a chronic total occlusion in a non-infarct-related artery in patients with ST-segment elevation myocardial infarction: 3-year results from the HORIZONS-AMI trial. Eur. Heart J. 2012, 33, 768–775. [Google Scholar] [CrossRef] [Green Version]
- Moreno, R.; Conde, C.; Perez-Vizcayno, M.-J.; Villarreal, S.; Hernandez-Antolin, R.; Alfonso, F.; Bañuelos, C.; Angiolillo, D.J.; Escaned, J.; Fernández-Ortiz, A.; et al. Prognostic impact of a chronic occlusion in a noninfarct vessel in patients with acute myocardial infarction and multivessel disease undergoing primary percutaneous coronary intervention. J. Invasive Cardiol. 2006, 18, 16–19. [Google Scholar]
- Mozid, A.; MohdNazri, S.; Mannakkara, N.N.; Robinson, N.M.; Jagathesan, R.; Sayer, J.W.; Aggarwal, R.K.; Clesham, G.J.; Gamma, R.A.; Tang, K.H.; et al. Impact of a chronic total occlusion in a non-infarct related artery on clinical outcomes following primary percutaneous intervention in acute ST-elevation myocardial infarction. J. Invasive Cardiol. 2014, 26, 13–16. [Google Scholar]
- O’Connor, S.A.; Garot, P.; Sanguineti, F.; Hoebers, L.P.; Unterseeh, T.; Benamer, H.; Chevalier, B.; Hovasse, T.; Morice, M.-C.; Lefèvre, T.; et al. Meta-Analysis of the Impact on Mortality of Noninfarct-Related Artery Coronary Chronic Total Occlusion in Patients Presenting With ST-Segment Elevation Myocardial Infarction. Am. J. Cardiol. 2015, 116, 8–14. [Google Scholar] [CrossRef]
- Hoebers, L.P.; Vis, M.M.; Claessen, B.E.; Van Der Schaaf, R.J.; Kikkert, W.J.; Baan, J.; De Winter, R.J.; Piek, J.J.; Tijssen, J.G.; Dangas, G.D.; et al. The impact of multivessel disease with and without a co-existing chronic total occlusion on short- and long-term mortality in ST-elevation myocardial infarction patients with and without cardiogenic shock. Eur. J. Heart Fail. 2013, 15, 425–432. [Google Scholar] [CrossRef]
- Henriques, J.P.; Hoebers, L.P.; Ramunddal, T.; Laanmets, P.; Eriksen, E.; Bax, M.; Ioanes, D.; Suttorp, M.J.; Strauss, B.H.; Barbato, E.; et al. Percutaneous Intervention for Concurrent Chronic Total Occlusions in Patients with Stemi: The Explore Trial. J. Am. Coll. Cardiol. 2016, 68, 1622–1632. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Yu, Q.; Li, C.; Shao, X.; Xia, Y. Successful Revascularization of Noninfarct Related Artery with Chronic Total Occlusion among Acute Myocardial Infarction Patients: A Systematic Review and Meta-Analysis. Medicine 2018, 97, e9655. [Google Scholar] [CrossRef] [PubMed]
- Dautov, R.; Ybarra, L.F.; Nguyen, C.M.; Gibrat, C.; Joyal, D.; Rinfret, S. Incidence, predictors and longer-term impact of troponin elevation following hybrid chronic total occlusion percutaneous coronary intervention. Catheter. Cardiovasc. Interv. 2018, 92, E308–E316. [Google Scholar] [CrossRef] [PubMed]
- Goliasch, G.; Winter, M.-P.; Ayoub, M.; Bartko, P.E.; Gebhard, C.; Mashayekhi, K.; Ferenc, M.; Buettner, H.J.; Hengstenberg, C.; Neumann, F.-J.; et al. A Contemporary Definition of Periprocedural Myocardial Injury After Percutaneous Coronary Intervention of Chronic Total Occlusions. JACC Cardiovasc. Interv. 2019, 12, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- de Marchi, S.F. Determinants of Human Coronary Collaterals. Curr. Cardiol. Rev. 2014, 10, 24–28. [Google Scholar] [CrossRef] [Green Version]
- Wustmann, K.; Zbinden, S.; Windecker, S.; Meier, B.; Seiler, C. Is There Functional Collateral Flow During Vascular Occlusion in Angiographically Normal Coronary Arteries? Circulation 2003, 107, 2213–2220. [Google Scholar] [CrossRef] [Green Version]
- Rentrop, K.P.; Cohen, M.; Blanke, H.; Phillips, R.A. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J. Am. Coll. Cardiol. 1985, 5, 587–592. [Google Scholar] [CrossRef] [Green Version]
- Werner, G.S.; Ferrari, M.; Heinke, S.; Kuethe, F.; Surber, R.; Richartz, B.M.; Figulla, H.R. Angiographic Assessment of Collateral Connections in Comparison with Invasively Determined Collateral Function in Chronic Coronary Occlusions. Circulation 2003, 107, 1972–1977. [Google Scholar] [CrossRef]
- Huang, C.-C.; Lee, C.-K.; Meng, S.-W.; Hung, C.-S.; Chen, Y.-H.; Lin, M.-S.; Yeh, C.-F.; Kao, H. Collateral Channel Size and Tortuosity Predict Retrograde Percutaneous Coronary Intervention Success for Chronic Total Occlusion. Circ. Cardiovasc. Interv. 2018, 11, e005124. [Google Scholar] [CrossRef]
- Nagamatsu, W.; Tsuchikane, E.; Oikawa, Y.; Sumitsuji, S.; Igarashi, Y.; Yoshikawa, R.; Muto, M.; Okada, H.; Katoh, O. Predicting Successful Guidewire Crossing via Collateral Channel at Retrograde Percutaneous Coronary Intervention for Chronic Total Occlusion: The J-Channel Score as a Difficulty Estimating Tool for Collateral Channel Guidewire Crossing Success from the Japanese CTO-PCI Expert Registry. EuroIntervention 2020, 15, e1624–e1632. [Google Scholar]
- McEntegart, M.B.; Badar, A.A.; Ahmad, F.A.; Shaukat, A.; MacPherson, M.; Irving, J.; Strange, J.; Bagnall, A.; Hanratty, C.; Walsh, S.J.; et al. The collateral circulation of coronary chronic total occlusions. EuroIntervention 2016, 11, e1596–e1603. [Google Scholar] [CrossRef] [PubMed]
- Werner, G.S. The role of coronary collaterals in chronic total occlusions. Curr. Cardiol. Rev. 2014, 10, 57–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaper, W. Collateral Circulation: Past and Present. Basic Res. Cardiol. 2009, 104, 5–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heil, M.; Schaper, W. Insights into pathways of arteriogenesis. Curr. Pharm. Biotechnol. 2007, 8, 35–42. [Google Scholar] [CrossRef]
- Vo, M.N.; Brilakis, E.S.; Kass, M.; Ravandi, A. Physiologic significance of coronary collaterals in chronic total occlusions. Can. J. Physiol. Pharmacol. 2015, 93, 867–871. [Google Scholar] [CrossRef]
- Schaper, W.; Ito, W.D. Molecular Mechanisms of Coronary Collateral Vessel Growth. Circ. Res. 1996, 79, 911–919. [Google Scholar] [CrossRef]
- Mashayekhi, K.; Behnes, M.; Valuckiene, Z.; Bryniarski, L.; Akin, I.; Neuser, H.; Neumann, F.; Reifart, N. Comparison of the ipsi-lateral versus contra-lateral retrograde approach of percutaneous coronary interventions in chronic total occlusions. Catheter. Cardiovasc. Interv. 2017, 89, 649–655. [Google Scholar] [CrossRef]
- Mashayekhi, K.; Behnes, M.; Akin, I.; Kaiser, T.; Neuser, H. Novel retrograde approach for percutaneous treatment of chronic total occlusions of the right coronary artery using ipsilateral collateral connections: A European centre experience. EuroIntervention 2016, 11, e1231–e1236. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.-H.; Chang, S.-A.; Bin Song, Y.; Hahn, J.-Y.; Choi, S.H.; Lee, S.-C.; Oh, J.K.; Choe, Y.; Gwon, H.-C. Frequency of Myocardial Infarction and Its Relationship to Angiographic Collateral Flow in Territories Supplied by Chronically Occluded Coronary Arteries. Circulation 2013, 127, 703–709. [Google Scholar] [CrossRef] [Green Version]
- Antoniucci, D.; Valenti, R.; Moschi, G.; Migliorini, A.; Trapani, M.; Santoro, G.M.; Bolognese, L.; Cerisano, G.; Buonamici, P.; Dovellini, E.V. Relation between preintervention angiographic evidence of coronary collateral circulation and clinical and angiographic outcomes after primary angioplasty or stenting for acute myocardial infarction. Am. J. Cardiol. 2002, 89, 121–125. [Google Scholar] [CrossRef]
- Elias, J.; Hoebers, L.P.C.; Van Dongen, I.M.; Claessen, B.E.P.M.; Henriques, J.P.S. Impact of Collateral Circulation on Survival in ST-Segment Elevation Myocardial Infarction Patients Undergoing Primary Percutaneous Coronary Intervention with a Concomitant Chronic Total Occlusion. JACC Cardiovasc. Interv. 2017, 10, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, R.; Agrawal, M.; Flynn, S.E.; Werner, G.S.; Uretsky, B.F. The myocardium supplied by a chronic total occlusion is a persistently ischemic zone. Catheter. Cardiovasc. Interv. 2014, 83, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Werner, G.S.; Surber, R.; Ferrari, M.; Fritzenwanger, M.; Figulla, H.R. The functional reserve of collaterals supplying long-term chronic total coronary occlusions in patients without prior myocardial infarction. Eur. Heart J. 2006, 27, 2406–2412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sachdeva, R.; Agrawal, M.; Flynn, S.E.; Werner, G.S.; Uretsky, B.F. Reversal of ischemia of donor artery myocardium after recanalization of a chronic total occlusion. Catheter. Cardiovasc. Interv. 2013, 82, E453–E458. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, C.-Y.; Kim, N.; Jang, S.Y.; Bae, M.H.; Yang, D.H.; Cho, Y.; Chae, S.C.; Park, H.S. Coronary Collaterals Function and Clinical Outcome Between Patients with Acute and Chronic Total Occlusion. JACC Cardiovasc. Interv. 2017, 10, 585–593. [Google Scholar] [CrossRef]
- Stuijfzand, W.J.; Driessen, R.S.; Raijmakers, P.G.H.M.; Rijnierse, M.T.; Maeremans, J.; Hollander, M.R.; Lammertsma, A.A.; Van Rossum, A.C.; Dens, J.; Nap, A.; et al. Prevalence of ischaemia in patients with a chronic total occlusion and preserved left ventricular ejection fraction. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1025–1033. [Google Scholar] [CrossRef] [Green Version]
- Aboul-Enein, F.; Kar, S.; Hayes, S.W.; Sciammarella, M.; Abidov, A.; Makkar, R.; Friedman, J.D.; Eigler, N.; Berman, D.S. Influence of angiographic collateral circulation on myocardial perfusion in patients with chronic total occlusion of a single coronary artery and no prior myocardial infarction. J. Nucl. Med. 2004, 45, 950–955. [Google Scholar]
- Sen, O.; Sen, F.; Topuz, M.; Allahverdiyev, S.; Baykan, A.O.; Akkuş, O.; Sümbül, Z.; Çaylı, M.; Çayli, M. Defining the prognosis of chronic total occlusions during primary percutaneous coronary intervention. Coron. Artery Dis. 2016, 27, 207–212. [Google Scholar] [CrossRef]
- Van Dongen, I.M.; Elias, J.; Van Houwelingen, K.G.; Agostoni, P.; Claessen, B.E.; Hoebers, L.P.; Ouweneel, D.M.; Scheunhage, E.M.; Delewi, R.; Piek, J.J.; et al. Impact of collateralisation to a concomitant chronic total occlusion in patients with ST-elevation myocardial infarction: A subanalysis of the Explore randomised controlled trial. Open Heart 2018, 5, e000810. [Google Scholar] [CrossRef]
- Ambrosio, G.; Weisman, H.F.; Mannisi, J.A.; Becker, L.C. Progressive impairment of regional myocardial perfusion after initial restoration of postischemic blood flow. Circulation 1989, 80, 1846–1861. [Google Scholar] [CrossRef] [Green Version]
- Habib, G.B.; Heibig, J.; Forman, S.A.; Brown, B.G.; Roberts, R.; Terrin, M.L.; Bolli, R. Influence of coronary collateral vessels on myocardial infarct size in humans. Results of phase I thrombolysis in myocardial infarction (TIMI) trial. The TIMI Investigators. Circulation 1991, 83, 739–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishihara, M.; Inoue, I.; Kawagoe, T.; Shimatani, Y.; Kurisu, S.; Hata, T.; Mitsuba, N.; Kisaka, T.; Nakama, H.; Kijima, Y. Comparison of the cardioprotective effect of prodromal angina pectoris and collateral circulation in patients with a first anterior wall acute myocardial infarction. Am. J. Cardiol. 2005, 95, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Enomoto, D.; Mizobuchi, T.; Kazatani, Y.; Honda, K. Impact of chronic total coronary occlusion on microvascular reperfusion in patients with a first anterior ST-segment elevation myocardial infarction. J. Invasive Cardiol. 2012, 24, 428–432. [Google Scholar]
- Lexis, C.P.H.; Van Der Horst, I.C.; Rahel, B.M.; Lexis, M.A.; Kampinga, M.A.; Gu, Y.L.; De Smet, B.J.; Zijlstra, F. Impact of chronic total occlusions on markers of reperfusion, infarct size, and long-term mortality: A substudy from the TAPAS-trial. Catheter. Cardiovasc. Interv. 2011, 77, 484–491. [Google Scholar] [CrossRef]
- Carnendran, L.; Steinberg, J.S. Does an Open Infarct-Related Artery after Myocardial Infarction Improve Electrical Stability? Prog. Cardiovasc. Dis. 2000, 42, 439–454. [Google Scholar] [CrossRef]
- Tse, G.; Yan, B.P. Traditional and novel electrocardiographic conduction and repolarization markers of sudden cardiac death. Europace 2017, 19, 712–721. [Google Scholar] [CrossRef]
- Behnes, M.; Akin, I.; Kuche, P.; Schupp, T.; Reiser, L.; Bollow, A.; Taton, G.; Reichelt, T.; Ellguth, D.; Engelke, N.; et al. Coronary chronic total occlusions and mortality in patients with ventricular tachyarrhythmias. EuroIntervention 2020, 14, 1278–1285. [Google Scholar] [CrossRef]
- Behnes, M.; Mashayekhi, K.; Kuche, P.; Kim, S.-H.; Schupp, T.; Von Zworowsky, M.; Reiser, L.; Bollow, A.; Taton, G.; Reichelt, T.; et al. Prognostic impact of coronary chronic total occlusion on recurrences of ventricular tachyarrhythmias and ICD therapies. Clin. Res. Cardiol. 2020, 1–11. [Google Scholar] [CrossRef]
- Chi, W.K.; Gong, M.; Bazoukis, G.; Yan, B.P.; Letsas, K.P.; Liu, T.; Baranchuk, A.; Nombela-Franco, L.; Dong, M.; Tse, G.; et al. Impact of Coronary Artery Chronic Total Occlusion on Arrhythmic and Mortality Outcomes: A Systematic Review and Meta-Analysis. JACC Clin. Electrophysiol. 2018, 4, 1214–1223. [Google Scholar] [CrossRef]
- Di Marco, A.; Anguera, I.; Teruel, L.; Dallaglio, P.; González-Costello, J.; León, V.; Nuñez, E.; Manito, N.; Gómez-Hospital, J.A.; Sabaté, X.; et al. Chronic total occlusion of an infarct-related artery: A new predictor of ventricular arrhythmias in primary prevention implantable cardioverter defibrillator patients. Europace 2017, 19, 267–274. [Google Scholar] [CrossRef]
- Vaidya, Y.; Dhamoon, A.S. Myocardial Stunning and Hibernation; Statpearls: Treasure Island, FL, USA, 2019. [Google Scholar]
- Claessen, B.E.; Van Der Schaaf, R.J.; Verouden, N.J.; Stegenga, N.K.; Engstrom, A.E.; Sjauw, K.D.; Kikkert, W.J.; Vis, M.M.; Baan, J.; Koch, K.T.; et al. Evaluation of the Effect of a Concurrent Chronic Total Occlusion on Long-Term Mortality and Left Ventricular Function in Patients After Primary Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2009, 2, 1128–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, H.; Morimoto, T.; Shiomi, H.; Furukawa, Y.; Nakagawa, Y.; Ando, K.; Kadota, K.; Kimura, T.; Hiroki, W.; Takeshi, M.; et al. Chronic total occlusion in a non-infarct-related artery is closely associated with increased five-year mortality in patients with ST-segment elevation acute myocardial infarction undergoing primary percutaneous coronary intervention (from the CREDO-Kyoto AMI registry). EuroIntervention 2017, 12, e1874–e1882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tajstra, M.; Gąsior, M.; Gierlotka, M.; Pres, D.; Hawranek, M.; Trzeciak, P.; Lekston, A.; Polonski, L.; Zembala, M. Comparison of Five-Year Outcomes of Patients with and Without Chronic Total Occlusion of Noninfarct Coronary Artery After Primary Coronary Intervention for ST-Segment Elevation Acute Myocardial Infarction. Am. J. Cardiol. 2012, 109, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Elias, J.; Van Dongen, I.M.; Råmunddal, T.; Laanmets, P.; Eriksen, E.; Meuwissen, M.; Michels, H.R.; Bax, M.; Ioanes, D.; Suttorp, M.J.; et al. Long-term impact of chronic total occlusion recanalisation in patients with ST-elevation myocardial infarction. Heart 2018, 104, 1432–1438. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, Y.; Kawashima, S.; Mori, T.; Terashima, M.; Ichikawa, S.; Ejiri, J.; Awano, K. Soluble CD40 ligand and interleukin-6 in the coronary circulation after acute myocardial infarction. Int. J. Cardiol. 2006, 112, 52–58. [Google Scholar] [CrossRef]
- Reindl, M.; Reinstadler, S.J.; Feistritzer, H.-J.; Klug, G.; Tiller, C.; Mair, J.; Mayr, A.; Jaschke, W.; Metzler, B. Relation of inflammatory markers with myocardial and microvascular injury in patients with reperfused ST-elevation myocardial infarction. Eur. Heart J. Acute Cardiovasc. Care 2017, 6, 640–649. [Google Scholar] [CrossRef]
- Elias, J.; Van Dongen, I.M.; Hoebers, L.P.; Ouweneel, D.M.; Claessen, B.E.; Råmunddal, T.; Laanmets, P.; Eriksen, E.; Van Der Schaaf, R.J.; Ioanes, D.; et al. Improved Recovery of Regional Left Ventricular Function after PCI of Chronic Total Occlusion in Stemi Patients: A Cardiovascular Magnetic Resonance Study of the Randomized Controlled Explore trial. J. Cardiovasc. Magn. Reson. 2017, 19, 53. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.K.; Zhang, R.Y.; Hu, J.; Zhang, Q.; Ding, F.H.; Shen, W.F. Impact of successful staged revascularization of a chronic total occlusion in the non-infarct-related artery on long-term outcome in patients with acute ST-segment elevation myocardial infarction. Int. J. Cardiol. 2013, 165, 76–79. [Google Scholar] [CrossRef]
- Shi, G.; He, P.; Tan, N.; Lin, Y.; Yang, X.; Chen, J.; Zhou, Y.; Tan, N. Evaluation of the Effect of Concurrent Chronic Total Occlusion and Successful Staged Revascularization on Long-Term Mortality in Patients with ST-Elevation Myocardial Infarction. Sci. World J. 2014, 2014, 756080. [Google Scholar] [CrossRef]
- Valenti, R.; Marrani, M.; Cantini, G.; Migliorini, A.; Antoniucci, D.; Vergara, R.; Cerisano, G.; Parodi, G.; Antoniucci, D. Impact of Chronic Total Occlusion Revascularization in Patients with Acute Myocardial Infarction Treated by Primary Percutaneous Coronary Intervention. Am. J. Cardiol. 2014, 114, 1794–1800. [Google Scholar] [CrossRef]
- Deng, J.; Wang, X.; Shi, Y.; Zhao, X.; Han, Y. Prognostic value of the age, creatinine, and ejection fraction score for non-infarct-related chronic total occlusion revascularization after primary percutaneous intervention in acute ST-elevation myocardial infarction patients: A retrospective study. J. Interv. Cardiol. 2018, 31, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Babaev, A.; Frederick, P.D.; Pasta, D.J.; Every, N.; Sichrovsky, T.; Hochman, J.S. For the NRMI Investigators Trends in Management and Outcomes of Patients with Acute Myocardial Infarction Complicated by Cardiogenic Shock. JAMA 2005, 294, 448–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Fang, J.; Gillespie, C.; Wang, G.; Hong, Y.; Yoon, P.W. Age-Specific Gender Differences in In-Hospital Mortality by Type of Acute Myocardial Infarction. Am. J. Cardiol. 2012, 109, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Van Der Schaaf, R.J.; Vis, M.M.; Sjauw, K.D.; Koch, K.T.; Baan, J.; Tijssen, J.G.; De Winter, R.J.; Piek, J.J.; Henriques, J.P.S. Impact of Multivessel Coronary Disease on Long-Term Mortality in Patients With ST-Elevation Myocardial Infarction Is Due to the Presence of a Chronic Total Occlusion. Am. J. Cardiol. 2006, 98, 1165–1169. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.; Fuernau, G.; Desch, S.; Eitel, I.; De Waha, S.; Pöss, J.; Ouarrak, T.; Schneider, S.; Zeymer, U.; Thiele, H. Prognostic impact of non-culprit chronic total occlusions in infarct-related cardiogenic shock: Results of the randomised IABP-SHOCK II trial. EuroIntervention 2018, 14, e306–e313. [Google Scholar] [CrossRef]
- Watanabe, H.; Morimoto, T.; Shiomi, H.; Kawaji, T.; Furukawa, Y.; Nakagawa, Y.; Ando, K.; Kadota, K.; Kimura, T.; CREDO-Kyoto AMI investigators. Chronic total occlusion in non-infarct-related artery is associated with increased short-and long-term mortality in patients with ST-segment elevation acute myocardial infarction complicated by cardiogenic shock (from the CREDO-Kyoto AMI registry). Catheter. Cardiovasc. Interv. 2018, 92, 455–463. [Google Scholar] [CrossRef]
- Vallabhajosyula, S.; Prasad, A.; Gulati, R.; Barsness, G.W. Contemporary prevalence, trends, and outcomes of coronary chronic total occlusions in acute myocardial infarction with cardiogenic shock. IJC Heart Vasc. 2019, 24, 100414. [Google Scholar] [CrossRef]
- Bataille, Y.; Déry, J.-P.; LaRose, É.; Déry, U.; Costerousse, O.; Rodés-Cabau, J.; Gleeton, O.; Proulx, G.; Abdelaal, E.; MacHaalany, J.; et al. Deadly association of cardiogenic shock and chronic total occlusion in acute ST-elevation myocardial infarction. Am. Heart J. 2012, 164, 509–515. [Google Scholar] [CrossRef]
- Thiele, H.; Akin, I.; Sandri, M.; Fuernau, G.; De Waha, S.; Meyer-Saraei, R.; Nordbeck, P.; Geisler, T.; Landmesser, U.; Skurk, C.; et al. PCI Strategies in Patients with Acute Myocardial Infarction and Cardiogenic Shock. N. Engl. J. Med. 2017, 377, 2419–2432. [Google Scholar] [CrossRef] [Green Version]
- Braik, N.; Guedeney, P.; Behnes, M.; Desch, S.; Barthélémy, O.; Sandri, M.; De Waha-Thiele, S.; Fuernau, G.; Rouanet, S.; Hauguel-Moreau, M.; et al. Impact of chronic total occlusion and revascularization strategy in patients with infarct-related cardiogenic shock: A subanalysis of the culprit-shock trial. Am. Heart J. 2020, 232, 185–193. [Google Scholar] [CrossRef]
- Henderson, R.A.; Jarvis, C.; Clayton, T.; Pocock, S.J.; Fox, K.A. 10-Year Mortality Outcome of a Routine Invasive Strategy Versus a Selective Invasive Strategy in Non-St-Segment Elevation Acute Coronary Syndrome: The British Heart Foundation Rita-3 Randomized Trial. J. Am. Coll. Cardiol. 2015, 66, 511–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gierlotka, M.; Tajstra, M.; Gąsior, M.; Hawranek, M.; Osadnik, T.; Wilczek, K.; Olszowski, D.; Dyrbuś, K.; Poloński, L. Impact of chronic total occlusion artery on 12-month mortality in patients with non-ST-segment elevation myocardial infarction treated by percutaneous coronary intervention (From the PL-ACS Registry). Int. J. Cardiol. 2013, 168, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.-I.; Sung, S.-H.; Huang, S.-S.; Pan, J.-P.; Lin, S.-J.; Chan, W.-L.; Lee, W.-L.; Lu, T.-M.; Wu, C.-H. The impact of successful revascularization of coronary chronic total occlusions on long-term clinical outcomes in patients with non-ST-segment elevation myocardial infarction. J. Interv. Cardiol. 2018, 31, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Choi, B.G.; Rha, S.W.; Kang, T.S.; Choi, C.U.; Yu, C.W.; Gwon, H.C.; Chae, I.H.; Kim, H.S.; Park, H.S.; et al. Chronic Total Occlusion Intervention of the Non-Infarct-Related Artery in Acute Myocardial Infarction Patients: The Korean Multicenter Chronic Total Occlusion Registry. Coron. Artery. Dis. 2018, 29, 495–501. [Google Scholar] [CrossRef]
- Choi, I.J.; Koh, Y.-S.; Lim, S.; Choo, E.H.; Kim, J.J.; Hwang, B.-H.; Kim, T.-H.; Seo, S.M.; Kim, C.J.; Park, M.-W.; et al. Impact of Percutaneous Coronary Intervention for Chronic Total Occlusion in Non–Infarct-Related Arteries in Patients with Acute Myocardial Infarction (from the COREA-AMI Registry). Am. J. Cardiol. 2016, 117, 1039–1046. [Google Scholar] [CrossRef]
- Galassi, A.R.; Tomasello, S.D.; Reifart, N.; Werner, G.S.; Sianos, G.; Bonnier, H.; Sievert, H.; Ehladad, S.; Bufe, A.; Shofer, J.; et al. In-hospital outcomes of percutaneous coronary intervention in patients with chronic total occlusion: Insights from the ERCTO (European Registry of Chronic Total Occlusion) registry. EuroIntervention 2011, 7, 472–479. [Google Scholar] [CrossRef]
- Morino, Y.; Kimura, T.; Hayashi, Y.; Muramatsu, T.; Ochiai, M.; Noguchi, Y.; Kato, K.; Shibata, Y.; Hiasa, Y.; Doi, O.; et al. In-Hospital Outcomes of Contemporary Percutaneous Coronary Intervention in Patients with Chronic Total Occlusion Insights from the J-Cto Registry (Multicenter Cto Registry in Japan). JACC Cardiovasc. Interv. 2010, 3, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Galassi, A.R.; Werner, G.S.; Boukhris, M.; Azzalini, L.; Mashayekhi, K.; Carlino, M.; Avran, A.; Konstantinidis, N.V.; Grancini, L.; Bryniarski, L.; et al. Percutaneous recanalisation of chronic total occlusions: 2019 consensus document from the EuroCTO Club. EuroIntervention 2019, 15, 198–208. [Google Scholar] [CrossRef] [Green Version]
- Brilakis, E.S.; Banerjee, S.; Karmpaliotis, D.; Lombardi, W.L.; Tsai, T.T.; Shunk, K.A.; Kennedy, K.F.; Spertus, J.A.; Holmes, D.R., Jr.; Grantham, J.A. Procedural Outcomes of Chronic Total Occlusion Percutaneous Coronary Intervention: A Report from the Ncdr (National Cardiovascular Data Registry). JACC Cardiovasc. Interv. 2015, 8, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Azzalini, L.; Carlino, M.; Bellini, B.; Marini, C.; Pazzanese, V.; Toscano, E.; Gramegna, M.; Moscardelli, S.; Bognoni, L.; Montorfano, M. Long-Term Outcomes of Chronic Total Occlusion Recanalization Versus Percutaneous Coronary Intervention for Complex Non-Occlusive Coronary Artery Disease. Am. J. Cardiol. 2019, 125, 182–188. [Google Scholar] [CrossRef]
- Azzalini, L.; Agostoni, P.; Benincasa, S.; Knaapen, P.; Schumacher, S.P.; Dens, J.; Maeremans, J.; Kraaijeveld, A.O.; Timmers, L.; Behnes, M.; et al. Retrograde Chronic Total Occlusion Percutaneous Coronary Intervention through Ipsilateral Collateral Channels: A Multicenter Registry. JACC Cardiovasc. Interv. 2017, 10, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Mashayekhi, K.; Nuhrenberg, T.G.; Toma, A.; Gick, M.; Ferenc, M.; Hochholzer, W.; Comberg, T.; Rothe, J.; Valina, C.M.; Loffelhardt, N.; et al. A Randomized Trial to Assess Regional Left Ventricular Function after Stent Implantation in Chronic Total Occlusion: The Revasc Trial. JACC Cardiovasc. Interv. 2018, 11, 1982–1991. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; ESC Scientific Document Group. Fourth universal definition of myocardial infarction. Eur. Heart J. 2019, 40, 237–269. [Google Scholar] [CrossRef] [Green Version]
- Stone, G.W.; Mehran, R.; Dangas, G.; Lansky, A.J.; Kornowski, R.; Leon, M.B. Differential impact on survival of electrocardiographic Q-wave versus enzymatic myocardial infarction after percutaneous intervention: A device-specific analysis of 7147 patients. Circulation 2001, 104, 642–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brener, S.; Ellis, S.; Schneider, J.; Topol, E.J. Frequency and long-term impact of myonecrosis after coronary stenting. Eur. Heart J. 2002, 23, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, J.B.; Kennedy, K.F.; Stolker, J.M.; Gilchrist, I.C.; Mukherjee, D.; Marso, S.P.; Pencina, M.J.; Kleiman, N.S.; Cohen, D.J. Prognostic Implications of Creatine Kinase-Mb Elevation after Percutaneous Coronary Intervention: Results from the Evaluation of Drug-Eluting Stents and Ischemic Events (Event) Registry. Circ. Cardiovasc. Interv. 2011, 4, 474–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, G.W.; Rizvi, A.; Newman, W.; Mastali, K.; Wang, J.C.; Caputo, R.; Doostzadeh, J.; Cao, S.; Simonton, C.A.; Sudhir, K.; et al. Everolimus-Eluting versus Paclitaxel-Eluting Stents in Coronary Artery Disease. N. Engl. J. Med. 2010, 362, 1663–1674. [Google Scholar] [CrossRef]
- Herrmann, J. Peri-procedural myocardial injury: 2005 update. Eur. Heart J. 2005, 26, 2493–2519. [Google Scholar] [CrossRef]
- Paizis, I.A.; Manginas, A.; Voudris, V.; Pavlides, G.; Spargias, K.; Cokkinos, D.V. Percutaneous coronary intervention for chronic total occlusions: The role of side-branch obstruction. EuroIntervention 2009, 4, 600–606. [Google Scholar] [CrossRef]
- Lee, S.; Lee, P.H.; Kang, S.H.; Choi, H.; Chang, M.; Roh, J.-H.; Yoon, S.-H.; Ahn, J.-M.; Park, D.; Kang, S.-J.; et al. Determinants and Prognostic Significance of Periprocedural Myocardial Injury in Patients with Successful Percutaneous Chronic Total Occlusion Interventions. JACC Cardiovasc. Interv. 2016, 9, 2220–2228. [Google Scholar] [CrossRef]
- Selvanayagam, J.B.; Porto, I.; Channon, K.; Petersen, S.E.; Francis, J.M.; Neubauer, S.; Banning, A.P. Troponin Elevation after Percutaneous Coronary Intervention Directly Represents the Extent of Irreversible Myocardial Injury: Insights from Cardiovascular Magnetic Resonance Imaging. Circulation 2005, 111, 1027–1032. [Google Scholar] [CrossRef] [Green Version]
- Kumagai, S.; Ishii, H.; Amano, T.; Uetani, T.; Kato, B.; Yoshida, T.; Ando, H.; Kunimura, A.; Shimbo, Y.; Kitagawa, K.; et al. Impact of chronic kidney disease on the incidence of peri-procedural myocardial injury in patients undergoing elective stent implantation. Nephrol. Dial. Transplant. 2012, 27, 1059–1063. [Google Scholar] [CrossRef] [Green Version]
- Werner, G.S.; Coenen, A.; Tischer, K.-H. Periprocedural ischaemia during recanalisation of chronic total coronary occlusions: The influence of the transcollateral retrograde approach. EuroIntervention 2014, 10, 799–805. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, J.; Yang, Z.K.; Ding, F.H.; Zhang, J.S.; Du, R.; Zhu, T.Q.; Shen, W.F.; Kirtane, A.J.; Zhang, R.Y. Correlates and outcomes related to periprocedural myocardial injury during percutaneous coronary intervention for chronic total occlusion: Results from a prospective, single center PCI registry. Catheter. Cardiovasc. Interv. 2016, 87 (Suppl. S1), 616–623. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, B.-K.; Kim, S.; Ahn, C.-M.; Kim, J.-S.; Ko, Y.-G.; Choi, D.; Hong, M.-K.; Jang, Y. Incidence, predicting factors, and clinical outcomes of periprocedural myocardial infarction after percutaneous coronary intervention for chronic total occlusion in the era of new-generation drug-eluting stents. Catheter. Cardiovasc. Interv. 2018, 92, 477–485. [Google Scholar] [CrossRef]
- Lo, N.; Michael, T.T.; Moin, D.; Patel, V.G.; Alomar, M.; Papayannis, A.; Cipher, D.; Abdullah, S.M.; Banerjee, S.; Brilakis, E.S. Periprocedural Myocardial Injury in Chronic Total Occlusion Percutaneous Interventions: A Systematic Cardiac Biomarker Evaluation Study. JACC Cardiovasc. Interv. 2014, 7, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.J.; Chen, C.F.; Gao, X.F.; Liu, X.H.; Xu, Y.Z. Impact of Periprocedural Myocardial Injury on Long-Term Clinical Outcomes of Chronic Total Occlusion Patients Undergoing Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis. Coron. Artery Dis. 2020, 3, 208–214. [Google Scholar] [CrossRef]
- Park, D.W.; Kim, Y.H.; Yun, S.C.; Ahn, J.M.; Lee, J.Y.; Kim, W.J.; Kang, S.J.; Lee, S.W.; Lee, C.W.; Park, S.W.; et al. Frequency, Causes, Predictors, and Clinical Significance of Peri-Procedural Myocardial Infarction Following Percutaneous Coronary Intervention. Eur. Heart J. 2013, 34, 1662–1669. [Google Scholar] [CrossRef] [Green Version]
- Jang, W.J.; Kim, W.S.; Lee, Y.T.; Kim, B.S.; Gwon, H.-C.; Yang, J.H.; Choi, S.-H.; Bin Song, Y.; Hahn, J.-Y. Association of periprocedural myocardial infarction with long-term survival in patients treated with coronary revascularization therapy of chronic total occlusion. Catheter. Cardiovasc. Interv. 2016, 87, 1042–1049. [Google Scholar] [CrossRef] [Green Version]
- Di Serafino, L.; Borgia, F.; Maeremans, J.; Pyxaras, S.A.; De Bruyne, B.; Wijns, W.; Heyndrickx, G.R.; Dens, J.; Di Mario, C.; Barbato, E. Periprocedural Myocardial Injury and Long-Term Clinical Outcome in Patients Undergoing Percutaneous Coronary Interventions of Coronary Chronic Total Occlusion. J. Invasive Cardiol. 2016, 28, 410–414. [Google Scholar]
- Jaguszewski, M.; Gilis-Malinowska, N.; Gutiérrez-Chico, J.L.; Chmielecki, M.; Skarzynski, P.; Burakowski, S.; Drewla, P.; Targonski, R.; Lewicki, L.; Dubaniewicz, W.; et al. Periprocedural Myocardial Injury After Recanalization of Single Chronic Coronary Occlusion—A Propensity Score Analysis Comparing Long-Term Clinical Outcomes. J. Invasive Cardiol. 2017, 29, 63–67. [Google Scholar]
- Cottens, D.; Maeremans, J.; McCutcheon, K.; Lamers, S.; Roux, L.; Duponselle, J.; Bennett, J.; Dens, J. Prognostic value of the high-sensitivity troponin T assay after percutaneous intervention of chronic total occlusions. J. Cardiovasc. Med. 2018, 19, 366–372. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Infarction Executive Group on behalf of the Joint European Society of Cardiology/American College of Cardiology/American Heart Association/World Heart Federation Task Force for the Universal Definition of Myocardial. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef]
- Khatri, J. Clinically Meaningful Definition of Myocardial Injury After Chronic Total Occlusion Intervention. JACC Cardiovasc. Interv. 2019, 12, 1924–1926. [Google Scholar] [CrossRef]
- Moussa, I.D.; Klein, L.W.; Shah, B.; Mehran, R.; Mack, M.J.; Brilakis, E.S.; Reilly, J.P.; Zoghbi, G.; Holper, E.; Stone, G.W. Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization: An expert consensus document from the society for cardiovascular angiography and interventions (SCAI). J. Am. Coll. Cardiol. 2013, 62, 1563–1570. [Google Scholar] [CrossRef] [Green Version]
- Koskinas, K.C.; Ndrepepa, G.; Räber, L.; Karagiannis, A.; Kufner, S.; Zanchin, T.; Hieber, J.; Hunziker, L.; Mayer, K.; Byrne, R.A.; et al. Prognostic Impact of Periprocedural Myocardial Infarction in Patients Undergoing Elective Percutaneous Coronary Interventions. Circ. Cardiovasc. Interv. 2018, 11, e006752. [Google Scholar] [CrossRef]
- Toma, A.; Stähli, B.; Gebhard, C.; Gick, M.; Minners, J.; Mashayekhi, K.; Avran, A.; Ferenc, M.; Buettner, H.J.; Neumann, F.-J. Clinical implications of periprocedural myocardial injury in patients undergoing percutaneous coronary intervention for chronic total occlusion: Role of antegrade and retrograde crossing techniques. EuroIntervention 2018, 13, 2051–2059. [Google Scholar] [CrossRef]
| Elias et al. [55] | Yang et al. [59] | Shi et al. [60] | Valenti et al. [61] | Deng et al. [62] | |
|---|---|---|---|---|---|
| Study design | Randomized controlled trial | Retrospective cohort study | Retrospective cohort study | Retrospective cohort study | Retrospective cohort study |
| Number of CTO patients | 302 | 136 | 152 | 169 | 377 |
| Duration of follow up (median) | 48 months | 24 months | 36 months | 36 months | 12 months |
| Success rate of CTO-PCI (%) | 73 | 64 | 68 | 78 | 79 |
| Time interval between primary PCI and staged PCI (days) | 5 ± 2 | 7–10 | 7–10 | Up to 30 | 7–28 |
| All-cause Mortality (%) (CTO-PCI vs. control) | 12.9 vs. 6.2, p = 0.11 | — | — | 3.4 vs. 15.0, p = 0.02 | 4.1 vs. 15.4, p < 0.001 |
| Cardiac Mortality (%) (CTO-PCI vs. control) | 6.0 vs. 1.0, p = 0.02 | 8.0 vs. 20.4, p = 0.036 | 9.0 vs. 22.9, p = 0.02 | 1.7 vs. 12, p = 0.025 | 3.6 vs. 15.0, p < 0.001 |
| MACE (%) (CTO-PCI vs. control) | 13.5 vs. 12.3, p = 0.93 | 21.8 vs. 38.8, p = 0.042 | 28.0 vs. 50.0, p = 0.009 | — | 4.6 vs. 19.5, p < 0.001 |
| Lee et al. [55] | Zhang et al. [61] | Kim et al. [59] | Lo et al. [60] | |
|---|---|---|---|---|
| Study design | Retrospective cohort study | Prospective cohort study | Prospective cohort study | Retrospective cohort study |
| Number of CTO patients | 1058 | 629 | 337 | 325 |
| Duration of follow up (median) | 52 months | 12 months | 30 months | 28 months |
| Definition of PMI | CK-MB ≥ 3xURL | CK-MB ≥ 3xURL | CK-MB ≥ 3xURL | CK-MB ≥ 3xURL |
| Rate of PMI (%) | 11.4 | 18.3 | 6.8 | 8.6 |
| Association of retrograde approach with PMI | OR: 2.27, p = 0.002 | OR: 1.35, p = 0.04 | No association found | 13.8% vs. 6.7%, p = 0.044 |
| All-cause mortality (PMI vs. non-PMI) | HR: 1.96, p = 0.01 | 2.6% vs. 1.0%, p = 0.16 | HR: 4.35, p = 0.048 | — |
| Cardiac Mortality (PMI vs. non-PMI) | HR: 1.92, p = 0.04 | 2.6% vs. 0.8%, p = 0.09 | HR: 13.3, p = 0.001 | — |
| MACE (PMI vs. non-PMI) | — | 12.2% vs. 4.1%, p = 0.001 | HR: 5.2, p = 0.002 | HR: 2.25, p = 0.006 |
| Jang et al. [55] | Jaguszewski et al. [59] | Cottens et al. [60] | |
|---|---|---|---|
| Study design | Retrospective cohort study | Prospective cohort study | Retrospective cohort study |
| Number of CTO patients | 927 | 1110 | 409 |
| Duration of follow up (median) | 42 months | 65 months | 12 months |
| Definition of PMI | hs-TnI ≥ 5xURL | hs-TnI ≥ 5xURL | hs-TnT ≥ 5xURL |
| Rate of PMI (%) | 12.7 | 4.7 | 42 |
| Association of retrograde approach with PMI | No association found | No association found | OR: 1.2,p = 0.04 |
| All-cause mortality (PMI vs. non-PMI) | HR: 1.58, p = 0.31 | HR: 1.45, p = 0.33 | — |
| Cardiac Mortality (PMI vs. non-PMI) | HR: 1.72, p = 0.39 | HR: 2.51, p = 0.05 | — |
| MACE (PMI vs. non-PMI) | HR: 1.10, p = 0.75 | HR: 1.19, p = 0.49 | HR: 1.37, p = 0.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-H.; Behnes, M.; Mashayekhi, K.; Bufe, A.; Meyer-Gessner, M.; El-Battrawy, I.; Akin, I. Prognostic Impact of Percutaneous Coronary Intervention of Chronic Total Occlusion in Acute and Periprocedural Myocardial Infarction. J. Clin. Med. 2021, 10, 258. https://doi.org/10.3390/jcm10020258
Kim S-H, Behnes M, Mashayekhi K, Bufe A, Meyer-Gessner M, El-Battrawy I, Akin I. Prognostic Impact of Percutaneous Coronary Intervention of Chronic Total Occlusion in Acute and Periprocedural Myocardial Infarction. Journal of Clinical Medicine. 2021; 10(2):258. https://doi.org/10.3390/jcm10020258
Chicago/Turabian StyleKim, Seung-Hyun, Michael Behnes, Kambis Mashayekhi, Alexander Bufe, Markus Meyer-Gessner, Ibrahim El-Battrawy, and Ibrahim Akin. 2021. "Prognostic Impact of Percutaneous Coronary Intervention of Chronic Total Occlusion in Acute and Periprocedural Myocardial Infarction" Journal of Clinical Medicine 10, no. 2: 258. https://doi.org/10.3390/jcm10020258