The Monitoring Efficacy of Neurogenic Bowel Dysfunction Treatment on Response (MENTOR) in a Non-Hospital Setting
Abstract
1. Introduction
2. Methods
3. Statistical Analysis
3.1. Neurogenic Bowel Dysfunction Score
3.2. Satisfaction with Bowel Function
3.3. Special Attention Symptoms
3.4. The MENTOR Tool
3.5. Effects of Neurogenic Bowel Dysfunction on Daily Life
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ebert, E. Gastrointestinal involvement in spinal cord injury: A clinical perspective. J. Gastrointestin Liver Dis. 2012, 21, 75–82. [Google Scholar] [PubMed]
- Krogh, K.; Nielsen, J.; Djurhuus, J.C.; Mosdal, C.; Sabroe, S.; Laurberg, S. Colorectal function in patients with spinal cord lesions. Dis. Colon Rectum. 1997, 40, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Glickman, S.; Kamm, M.A. Bowel dysfunction in spinal-cord-injury patients. Lancet 1996, 347, 1651–1653. [Google Scholar] [CrossRef]
- Pardee, C.; Bricker, D.; Rundquist, J.; MacRae, C.; Tebben, C. Characteristics of neurogenic bowel in spinal cord injury and perceived quality of life. Rehabil. Nurs. 2012, 37, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, A.V.; Krogh, K.; Bazzocchi, G.; Leroi, A.M.; Bremers, A.; Leder, D.; van Kuppevelt, D.; Mosiello, G.; Vogel, M.; Perrouin-Verbe, B.; et al. Consensus review of best practice of transanal irrigation in adults. Spinal Cord 2013, 51, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Inskip, J.A.; Lucci, V.M.; McGrath, M.S.; Willms, R.; Claydon, V.E. A Community Perspective on Bowel Management and Quality of Life after Spinal Cord Injury: The Influence of Autonomic Dysreflexia. J. Neurotrauma. 2018, 35, 1091–1105. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, A. Managing neurogenic bowel dysfunction. Clin. Rehabil. 2010, 24, 483–488. [Google Scholar] [CrossRef]
- Coggrave, M.; Norton, C. Management of faecal incontinence and constipation in adults with central neurological diseases. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef]
- Krogh, K.; Christensen, P.; Sabroe, S.; Laurberg, S. Neurogenic bowel dysfunction score. Spinal Cord 2006, 44, 625–631. [Google Scholar] [CrossRef]
- Mallek, A.; Elleuch, M.H.; Ghroubi, S. Neurogenic bowel dysfunction (NBD) translation and linguistic validation to classical Arabic. Prog. Urol. 2016, 26, 553–557. [Google Scholar] [CrossRef]
- Erdem, D.; Hava, D.; Keskinoğlu, P.; Bircan, Ç.; Peker, Ö.; Krogh, K.; Gülbahar, S. Reliability, validity and sensitivity to change of neurogenic bowel dysfunction score in patients with spinal cord injury. Spinal Cord 2017, 55, 1084–1087. [Google Scholar] [CrossRef]
- Krause, J.S.; Kjorsvig, J.M. Mortality after spinal cord injury: A four-year prospective study. Arch. Phys. Med. Rehabil. 1992, 73, 558–563. [Google Scholar] [CrossRef]
- Emmanuel, A.; Krogh, K.; Kirshblum, S.; Christensen, P.; Spinelli, M.; van Kuppevelt, D.; Abel, R.; Leder, D.; Santacruz, B.G.; Bain, K.; et al. Creation and validation of a new tool for the monitoring efficacy of neurogenic bowel dysfunction treatment on response: The MENTOR tool. Spinal Cord. 2020, 58, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Krogh, K.; Mosdal, C.; Gregersen, H.; Laurberg, S. Rectal wall properties in patients with acute and chronic spinal cord lesions. Dis. Colon Rectum. 2002, 45, 641–649. [Google Scholar] [CrossRef]
- Krogh, K.; Olsen, N.; Christensen, P.; Madsen, J.L.; Laurberg, S. Colorectal transport during defecation in patients with lesions of the sacral spinal cord. Neurogastroenterol. Motil. 2003, 15, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.M.; Krogh, K.; Clemmensen, D.; Bluhme, H.; Rawashdeh, Y.; Christensen, P. Colorectal transport during defecation in subjects with supraconal spinal cord injury. Spinal Cord 2013, 51, 683–687. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tate, D.G.; Wheeler, T.; Lane, G.I.; Forchheimer, M.; Anderson, K.D.; Biering-Sorensen, F.; Cameron, A.P.; Santacruz, B.G.; Jakeman, L.B.; Kennelly, M.J.; et al. Recommendations for evaluation of neurogenic bladder and bowel dysfunction after spinal cord injury and/or disease. J. Spinal Cord Med. 2020, 43, 141–164. [Google Scholar] [CrossRef]
- Krassioukov, A.; Eng, J.J.; Claxton, G.; Sakakibara, B.M.; Shum, S. Neurogenic bowel management after spinal cord injury: A systematic review of the evidence. Spinal Cord 2010, 48, 718–733. [Google Scholar] [CrossRef]
- Krogh, K.; Emmanuel, A.; Perrouin-Verbe, B.; Korsten, M.A.; Mulcahey, M.J.; Biering-Sørensen, F. International spinal cord injury bowel function basic data set (Version 2.0). Spinal Cord 2017, 55, 692–698. [Google Scholar] [CrossRef]
- Christensen, P.; Bazzocchi, G.; Coggrave, M.; Abel, R.; Hultling, C.; Krogh, K.; Media, S.; Laurberg, S. A randomized, controlled trial of transanal irrigation versus conservative bowel management in spinal cord-injured patients. Gastroenterology 2006, 131, 738–747. [Google Scholar] [CrossRef]
- Burns, A.S.; St-Germain, D.; Connolly, M.; Delparte, J.J.; Guindon, A.; Hitzig, S.L.; Craven, B.C. Phenomenological study of neurogenic bowel from the perspective of individuals living with spinal cord injury. Arch. Phys. Med. Rehabil. 2015, 96, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, A.; Kumar, G.; Christensen, P.; Mealing, S.; Størling, Z.M.; Andersen, F.; Kirshblum, S. Long-Term Cost-Effectiveness of Transanal Irrigation in Patients with Neurogenic Bowel Dysfunction. PLoS ONE 2016, 11, e0159394. [Google Scholar] [CrossRef] [PubMed]
- Fynne, L.; Worsøe, J.; Gregersen, T.; Schlageter, V.; Laurberg, S.; Krogh, K. Gastric and small intestinal dysfunction in spinal cord injury patients. Acta Neurol. Scand. 2012, 125, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Faaborg, P.M.; Christensen, P.; Krassioukov, A.; Laurberg, S.; Frandsen, E.; Krogh, K. Autonomic dysreflexia during bowel evacuation procedures and bladder filling in subjects with spinal cord injury. Spinal Cord 2014, 52, 494–498. [Google Scholar] [CrossRef]
- Coggrave, M.; Norton, C.; Wilson-Barnett, J. Management of neurogenic bowel dysfunction in the community after spinal cord injury: A postal survey in the United Kingdom. Spinal Cord 2009, 47, 323–330, quiz 331-323. [Google Scholar] [CrossRef]
- Alexander, M.S.; Biering-Sorensen, F.; Bodner, D.; Brackett, N.L.; Cardenas, D.; Charlifue, S.; Creasey, G.; Dietz, V.; Ditunno, J.; Donovan, W.; et al. International standards to document remaining autonomic function after spinal cord injury. Spinal Cord 2009, 47, 36–43. [Google Scholar] [CrossRef]
- Faaborg, P.M.; Christensen, P.; Finnerup, N.; Laurberg, S.; Krogh, K. The pattern of colorectal dysfunction changes with time since spinal cord injury. Spinal Cord 2008, 46, 234–238. [Google Scholar] [CrossRef]
- Faaborg, P.M.; Christensen, P.; Rosenkilde, M.; Laurberg, S.; Krogh, K. Do gastrointestinal transit times and colonic dimensions change with time since spinal cord injury? Spinal Cord 2011, 49, 549–553. [Google Scholar] [CrossRef][Green Version]
- Nielsen, S.D.; Faaborg, P.M.; Finnerup, N.B.; Christensen, P.; Krogh, K. Ageing with neurogenic bowel dysfunction. Spinal Cord 2017, 55, 769–773. [Google Scholar] [CrossRef]
- Burns, A.S.; St-Germain, D.; Connolly, M.; Delparte, J.J.; Guindon, A.; Hitzig, S.L.; Craven, B.C. Neurogenic bowel after spinal cord injury from the perspective of support providers: A phenomenological study. Phys. Med. Rehabil. Clin. 2015, 7, 407–416. [Google Scholar] [CrossRef]
| When Have You Last Seen a Doctor/Nurse Because of SCI? | When Have You Last Discussed Your Bowel Function with a Doctor/Nurse? | |
|---|---|---|
| Less than one year ago | 166 (28%) | 156 (26%) |
| 1–2 years | 200 (34%) | 156 (26%) |
| >2–5 years | 147 (25%) | 113 (19%) |
| More than 5 years | 63 (11%) | 78 (13%) |
| Never | 20 (3%) | 105 (17%) |
| Missing values | 34 (5%) | 22 (3%) |
| NBD Score | n (%) |
|---|---|
| 1. How often do you defacate? | |
| Daily | 335 (53.3%) |
| 2–6 times per week | 281 (44.7%) |
| Less than once per week | 12 (1.9%) |
| 2. How much times do you spend on each defaecation? | |
| Less than 30 min. | 402 (64.1%) |
| 31–60 min. | 190 (30.3%) |
| More than an hour | 35 (5.6%) |
| 3. Do you experience uneasiness, sweating or headaches during or after defaecation? | |
| Yes | 150 (23.9%) |
| No | 478 (76.1%) |
| 4. Do you take medication (tablets) to treat constipation? | |
| Yes | 281 (45.0%) |
| No | 344 (55.0%) |
| 5. Do you take medication (drops or liquid) to treat constipation? | |
| Yes | 170 (27.2%) |
| No | 455 (72.8%) |
| 6. How often do you use digital evacuation? | |
| Less than once per week (score 0) | 329 (52.6%) |
| Once or more per week (score 6) | 297 (47.4%) |
| 7. How often do you have involuntary defaecation? | |
| Daily | 5 (0.8%) |
| 1–6 times a week | 19 (3.0%) |
| 3–4 times a month | 83 (13.3%) |
| A few times a year or less | 518 (82.9%) |
| 8. Do you take medication to treat faecal incontinence? | |
| Yes | 23 (3.7%) |
| No | 605 (96.3%) |
| 9. Do you experience uncontrollable flatus? | |
| Yes | 379 (60.4%) |
| No | 248 (39.6%) |
| 10. Do you have peri-anal skin problems? | |
| Yes | 118 (19.0%) |
| No | 508 (81.2%) |
| NBD Score | Patient Satisfaction | |||
|---|---|---|---|---|
| Good | Acceptable | Poor | Very Poor | |
| 14 or more | 9 (1.5%) | 44 (7.1%) | 51 (8.3%) | 17 (2.8%) |
| 10–13 | 27 (4.4%) | 75 (12.2%) | 36 (5.8%) | 3 (0.5%) |
| 0–9 | 96 (15.6%) | 205 (33.3%) | 49 (7.9%) | 5 (0.8%) |
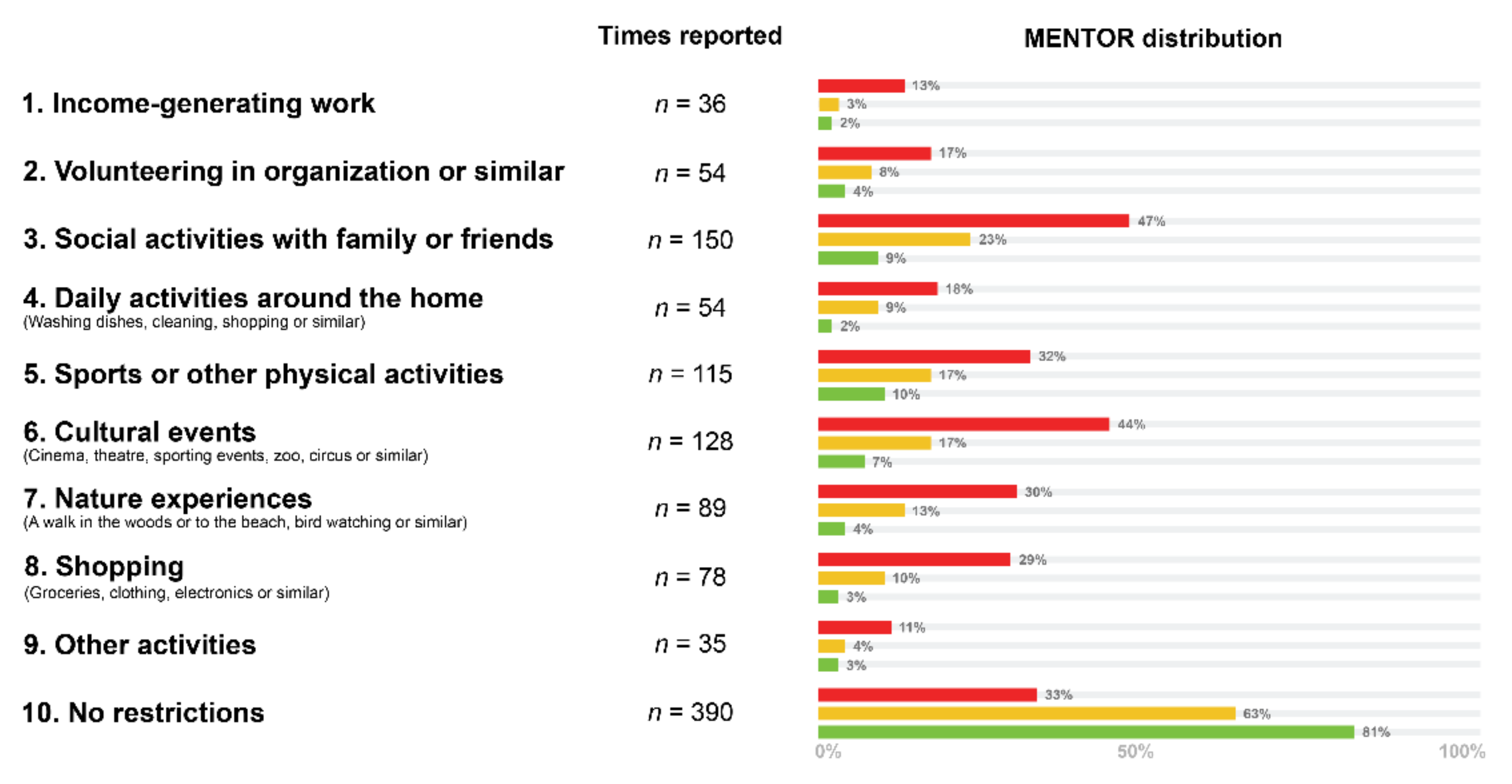
| Green n = 281 | Yellow n = 175 | Red n = 181 | |
|---|---|---|---|
| Income-generating work | 6 (2.14%) | 6 (3.43%) | 24 (13.26%) |
| Volunteering in organization or similar | 10 (3.56%) | 14 (8%) | 30 (16.57%) |
| Social activities with family or friends | 24 (8.54%) | 41 (23.43%) | 85 (46.96%) |
| Daily activities around the home (washing dishes, cleaning, shopping or similar) | 5 (1.78%) | 16 (9.14%) | 33 (18.23%) |
| Sports or other physical activities | 28 (9.96%) | 29 (16.57%) | 58 (32.04%) |
| Cultural events (cinema, theatre, sporting events, zoo, circus or similar) | 19 (6.76%) | 29 (16.57%) | 80 (44.2%) |
| Nature experiences (a walk in the woods or to the beach, bird watching or similar) | 12 (4.27%) | 22 (12.57%) | 55 (30.39%) |
| Shopping (groceries, clothing, electronics or similar) | 8 (2.85%) | 18 (10.29%) | 52 (28.73%) |
| Other activities | 9 (3.2%) | 7 (4%) | 19 (10.5%) |
| No restrictions | 228 (81.14%) | 110 (62.86%) | 59 (32.6%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Studsgaard Slot, S.D.; Baunwall, S.M.D.; Emmanuel, A.; Christensen, P.; Krogh, K. The Monitoring Efficacy of Neurogenic Bowel Dysfunction Treatment on Response (MENTOR) in a Non-Hospital Setting. J. Clin. Med. 2021, 10, 263. https://doi.org/10.3390/jcm10020263
Studsgaard Slot SD, Baunwall SMD, Emmanuel A, Christensen P, Krogh K. The Monitoring Efficacy of Neurogenic Bowel Dysfunction Treatment on Response (MENTOR) in a Non-Hospital Setting. Journal of Clinical Medicine. 2021; 10(2):263. https://doi.org/10.3390/jcm10020263
Chicago/Turabian StyleStudsgaard Slot, Sofie Dagmar, Simon Mark Dahl Baunwall, Anton Emmanuel, Peter Christensen, and Klaus Krogh. 2021. "The Monitoring Efficacy of Neurogenic Bowel Dysfunction Treatment on Response (MENTOR) in a Non-Hospital Setting" Journal of Clinical Medicine 10, no. 2: 263. https://doi.org/10.3390/jcm10020263
APA StyleStudsgaard Slot, S. D., Baunwall, S. M. D., Emmanuel, A., Christensen, P., & Krogh, K. (2021). The Monitoring Efficacy of Neurogenic Bowel Dysfunction Treatment on Response (MENTOR) in a Non-Hospital Setting. Journal of Clinical Medicine, 10(2), 263. https://doi.org/10.3390/jcm10020263





