Edema Resolution and Clinical Assessment in Poor-Grade Subarachnoid Hemorrhage: Useful Indicators to Predict Delayed Cerebral Infarctions?
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Classification of Edema and CI by CT Scan
2.3. Neurological Evaluation
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. EBI in Severe aSAH Patients
4.2. Biomarkers of EBI and Predictors of CI
4.3. SEBES Scale and GCS During the First Week
4.4. Other Factors Influencing EBI Resolution
4.5. Novel Findings
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rowland, M.J.; Hadjipavlou, G.; Kelly, M.; Westbrook, J.; Pattinson, K.T.S. Delayed cerebral ischaemia after subarachnoid haemorrhage: Looking beyond vasospasm. Br. J. Anaesth. 2012, 109, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Rass, V.; Helbok, R. Early Brain Injury after Poor-Grade Subarachnoid Hemorrhage. Curr. Neurol. Neurosci. 2019, 19, 78. [Google Scholar] [CrossRef] [PubMed]
- Lagares, A.; Gómez, P.A.; Lobato, R.D.; Alén, J.F.; Alday, R.; Campollo, J. Prognostic factors on hospital admission after spontaneous subarachnoid haemorrhage. Acta Neurochir. 2001, 143, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.A.; Bajgur, S.S.; Jones, W.H.; Savarraj, J.P.; Ko, S.B.; Edwards, N.J.; Chang, T.R.; Hergenroeder, G.W.; Dannenbaum, M.J.; Chen, P.R.; et al. Quantification of Cerebral Edema after Subarachnoid Hemorrhage. Neurocritical Care 2015, 25, 64–70. [Google Scholar] [CrossRef]
- Rass, V.; Ianosi, B.A.; Wegmann, A.; Gaasch, M.; Schiefecker, A.J.; Kofler, M.; Lindner, A.; Addis, A.; Almashad, S.S.; Rhomberg, P.; et al. Delayed Resolution of Cerebral Edema Is Associated with Poor Outcome after Nontraumatic Subarachnoid Hemorrhage. Stroke 2019, 50, 828–836. [Google Scholar] [CrossRef]
- Savarraj, J.; Parsha, K.; Hergenroeder, G.; Ahn, S.; Chang, T.R.; Kim, D.H.; Choi, H.A. Early Brain Injury Associated with Systemic Inflammation After Subarachnoid Hemorrhage. Neurocrit. Care 2018, 28, 203–211. [Google Scholar] [CrossRef]
- Ahn, S.H.; Savarraj, J.P.; Pervez, M.; Jones, W.; Park, J.; Jeon, S.B.; Kwon, S.U.; Chang, T.R.; Lee, K.; Kim, D.H.; et al. The Subarachnoid Hemorrhage Early Brain Edema Score Predicts Delayed Cerebral Ischemia and Clinical Outcomes. Neurosurgery 2017, 83, 19. [Google Scholar] [CrossRef]
- Foreman, B. The Pathophysiology of Delayed Cerebral Ischemia. J. Clin. Neurophysiol. 2016, 33, 174–182. [Google Scholar] [CrossRef]
- Hoogmoed, J.; de Oliveira Manoel, A.L.; Coert, B.A.; Marotta, T.R.; Macdonald, R.L.; Vandertop, W.P.; Verbaan, D.; Germans, M.R. Why do poor grade subarachnoid hemorrhage patients die? World Neurosurg. 2019, 131, e508–e513. [Google Scholar] [CrossRef]
- Hoogmoed, J.; Coert, B.A.; van den Berg, R.; Roos, Y.B.; Horn, J.; Vandertop, W.P.; Verbaan, D. Early treatment decisions in poor-grade patients with a subarachnoid hemorrhage. World Neurosurg. 2018, 119, e568–e573. [Google Scholar] [CrossRef]
- Zheng, K.; Zhong, M.; Zhao, B.; Chen, S.Y.; Tan, X.X.; Li, Z.Q.; Xiong, Y.; Duan, C.Z. Poor-Grade Aneurysmal Subarachnoid Hemorrhage: Risk Factors Affecting Clinical Outcomes in Intracranial Aneurysm Patients in a Multi-Center Study. Front. Neurol. 2019, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Manoel, A.L.; Mansur, A.; Silva, G.S.; Germans, M.R.; Jaja, B.N.; Kouzmina, E.; Marotta, T.R.; Abrahamson, S.; Schweizer, T.A.; Spears, J.; et al. Functional Outcome After Poor-Grade Subarachnoid Hemorrhage: A Single-Center Study and Systematic Literature Review. Neurocrit. Care 2016, 25, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Akiyama, T.; Horiguchi, T.; Miwa, T.; Takemura, R.; Yoshida, K. Loss of consciousness at ictus and/or poor World Federation of Neurosurgical Societies grade on admission reflects the impact of EBI and predicts poor outcome in patients with SAH. Surg. Neurol. Int. 2020, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, S.R.; Stoffel-Wagner, B.; Kinfe, T.M.; Güresir, E.; Vatter, H.; Dietrich, D.; Lamprecht, A.; Muhammad, S. Elevated Systemic IL-6 Levels in Patients with Aneurysmal Subarachnoid Hemorrhage Is an Unspecific Marker for Post-SAH Complications. Int. J. Mol. Sci. 2017, 18, 2580. [Google Scholar] [CrossRef] [PubMed]
- Taub, P.R.; Fields, J.D.; Wu, A.H.; Miss, J.C.; Lawton, M.T.; Smith, W.S.; Young, W.L.; Zaroff, J.G.; Ko, N.U. Elevated BNP is Associated with Vasospasm-Independent Cerebral Infarction Following Aneurysmal Subarachnoid Hemorrhage. Neurocrit. Care 2011, 15, 13–18. [Google Scholar] [CrossRef]
- Lu, G.; Wong, M.S.; Xiong, M.Z.Q.; Leung, C.K.; Su, X.W.; Zhou, J.Y.; Poon, W.S.; Zheng, V.Z.Y.; Chan, W.Y.; Wong, G.K.C. Circulating MicroRNAs in Delayed Cerebral Infarction After Aneurysmal Subarachnoid Hemorrhage. J. Am. Heart Assoc. 2017, 6, e005363. [Google Scholar] [CrossRef]
- Torné, R.; Culebras, D.; Sanchez-Etayo, G.; García-García, S.; Muñoz, G.; Llull, L.; Amaro, S.; Heering, C.; Blasco, J.; Zavala, E.; et al. Double hemispheric Microdialysis study in poor-grade SAH patients. Sci. Rep. 2020, 10, 7466. [Google Scholar] [CrossRef]
- Murphy, A.; Lee, T.Y.; Marotta, T.R.; Spears, J.; Macdonald, R.L.; Aviv, R.I.; Baker, A.; Bharatha, A. Prospective Multicenter Study of Changes in MTT after Aneurysmal SAH and Relationship to Delayed Cerebral Ischemia in Patients with Good- and Poor-Grade Admission Status. Am. J. Neuroradiol. 2018, 39, 2027–2033. [Google Scholar] [CrossRef]
- Foreman, P.M.; Chua, M.H.; Harrigan, M.R.; Fisher, W.S.; Tubbs, R.S.; Shoja, M.M.; Griessenauer, C.J. External validation of the Practical Risk Chart for the prediction of delayed cerebral ischemia following aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2016, 126, 1530–1536. [Google Scholar] [CrossRef]
- Vergouwen, M.D.; Vermeulen, M.; van Gijn, J.; Rinkel, G.J.; Wijdicks, E.F.; Muizelaar, J.P.; Mendelow, A.D.; Juvela, S.; Yonas, H.; Terbrugge, K.G.; et al. Definition of Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Hemorrhage as an Outcome Event in Clinical Trials and Observational Studies. Stroke 2010, 41, 2391–2395. [Google Scholar] [CrossRef]
- Germans, M.R.; Jaja, B.N.; de Oliviera Manoel, A.L.; Cohen, A.H.; Macdonald, R.L. Sex differences in delayed cerebral ischemia after subarachnoid hemorrhage. J. Neurosurg. 2018, 129, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Oppong, M.D.; Iannaccone, A.; Gembruch, O.; Pierscianek, D.; Chihi, M.; Dammann, P.; Köninger, A.; Müller, O.; Forsting, M.; Sure, U.; et al. Vasospasm-related complications after subarachnoid hemorrhage: The role of patients’ age and sex. Acta Neurochir. 2018, 160, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Dasenbrock, H.H.; Rudy, R.F.; Lai, P.M.R.; Smith, T.R.; Frerichs, K.U.; Gormley, W.B.; Aziz-Sultan, M.A.; Du, R. Cigarette smoking and outcomes after aneurysmal subarachnoid hemorrhage: A nationwide analysis. J. Neurosurg. 2017, 129, 446–457. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Manoel, A.L.; Macdonald, R.L. Neuroinflammation as a Target for Intervention in Subarachnoid Hemorrhage. Front. Neurol. 2018, 9, 292. [Google Scholar] [CrossRef]

| CI n = 11 | No CI n = 36 | p | |
|---|---|---|---|
| mRS (0–3) | 4 (36.36%) | 15 (41.67%) | 0.754 |
| Age | 54.27(SD:12.47) | 60.81 (SD:12.85) | 0.140 |
| Gender (female) | 5 (45.45%) | 28 (77.78%) | 0.040 |
| HBP | 4 (36.36%) | 16 (44.44%) | 0.635 |
| Smoke | 5 (45.45%) | 12 (33.33%) | 0.464 |
| DM | 2 (18.18%) | 2 (5.56%) | 0.189 |
| DLP | 5 (45.45%) | 13 (36.11%) | 0.577 |
| Hydrocephalus | 9 (81.82%) | 29 (80.56%) | 0.926 |
| ICH | 5 (45.45%) | 13 (36.11%) | 0.577 |
| Hijdra Sum Score | 29.4 (SD:5.7) | 23.9 (SD:10.7) | 0.108 |
| Vasospasm | 11 (100%) | 16 (44.4%) | 0.001 |
| Rescue therapy | 5 (45.4%) | 4 (11.1%) | 0.015 |
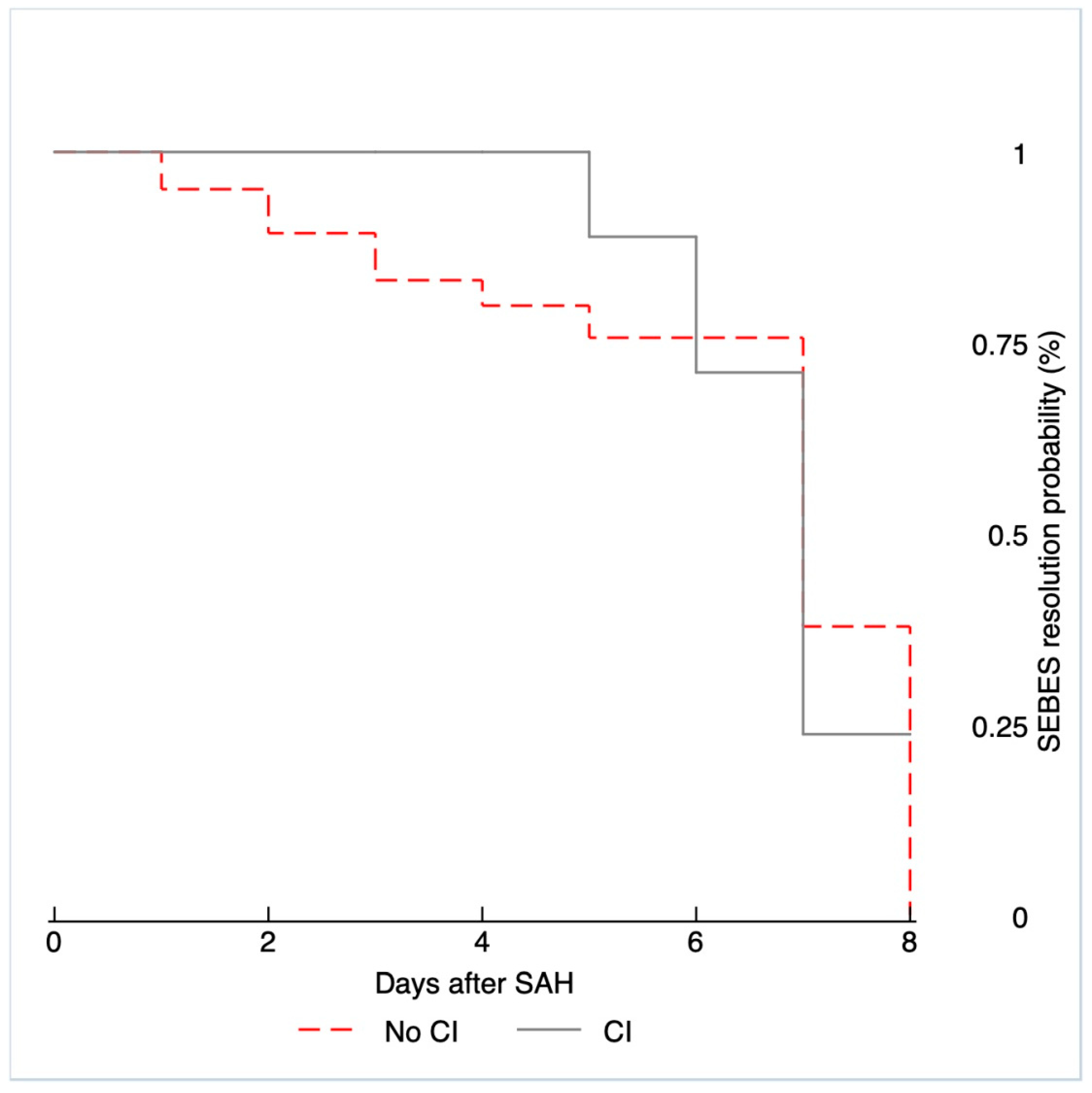
| Early SEBES | |||
| 0 | 0 | 2 (5.56%) | |
| 1 | 1 (9.09%) | 6 (16.67%) | |
| 2 | 0 | 7 (19.44%) | |
| 3 | 4 (36.36%) | 8 (22.22%) | |
| 4 | 6 (54.55%) | 13 (36.11%) | |
| 0.607 | |||
| Delayed SEBES | |||
| 0 | 0 | 6 (16.67%) | |
| 1 | 1 (9.09%) | 7 (19.44%) | |
| 2 | 4 (36.36%) | 8 (22.22%) | |
| 3 | 3 (27.27%) | 7 (19.44%) | |
| 4 | 3 (27.27%) | 8 (22.22%) | |
| 0.500 | |||
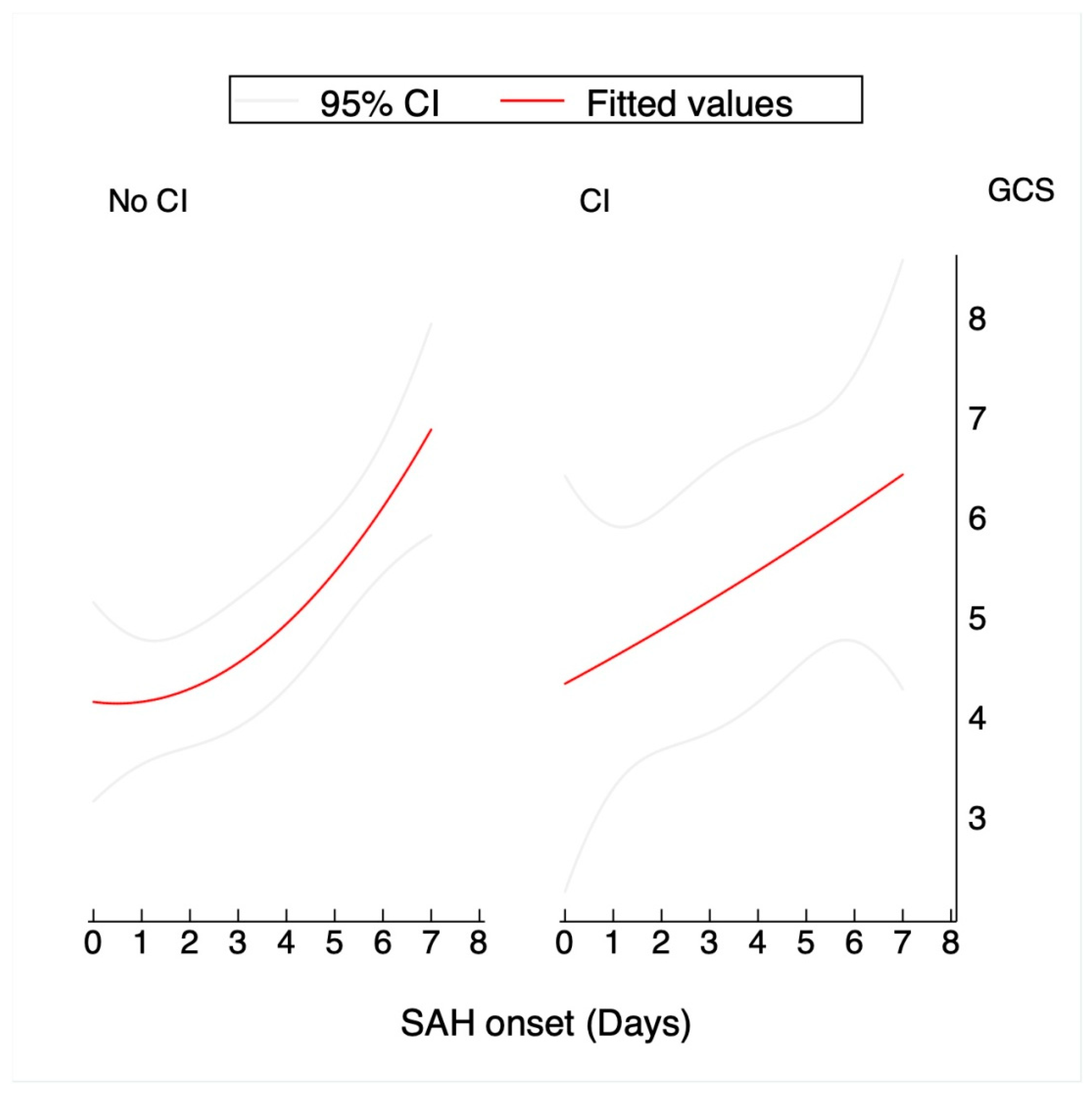
| GCS (day) | |||
| 0 | 6.2 (SD:1.29) | 5.48 (SD:0.59) | |
| 1 | 3.7 (SD:0.7%) | 3.26 (SD:0.26) | |
| 2 | 4.9 (SD:1.32) | 3.84 (SD:0.49) | |
| 3 | 5 (SD:1.31) | 4.39 (SD:0.65) | |
| 4 | 6.1 (SD:1.64) | 5.1 (SD:0.71) | |
| 5 | 6.1 (SD:1.63) | 5.64 (SD:0.75) | |
| 6 | 6.1 (SD:1.63) | 6.32 (SD:0.79) | |
| 7 | 6.1 (SD:1.63) | 6.35 (SD:0.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torné, R.; Hoyos, J.; Llull, L.; Rodríguez-Hernández, A.; Muñoz, G.; Mellado-Artigas, R.; Santana, D.; Pedrosa, L.; Di Somma, A.; San Roman, L.; et al. Edema Resolution and Clinical Assessment in Poor-Grade Subarachnoid Hemorrhage: Useful Indicators to Predict Delayed Cerebral Infarctions? J. Clin. Med. 2021, 10, 321. https://doi.org/10.3390/jcm10020321
Torné R, Hoyos J, Llull L, Rodríguez-Hernández A, Muñoz G, Mellado-Artigas R, Santana D, Pedrosa L, Di Somma A, San Roman L, et al. Edema Resolution and Clinical Assessment in Poor-Grade Subarachnoid Hemorrhage: Useful Indicators to Predict Delayed Cerebral Infarctions? Journal of Clinical Medicine. 2021; 10(2):321. https://doi.org/10.3390/jcm10020321
Chicago/Turabian StyleTorné, Ramon, Jhon Hoyos, Laura Llull, Ana Rodríguez-Hernández, Guido Muñoz, Ricard Mellado-Artigas, Daniel Santana, Leire Pedrosa, Alberto Di Somma, Luis San Roman, and et al. 2021. "Edema Resolution and Clinical Assessment in Poor-Grade Subarachnoid Hemorrhage: Useful Indicators to Predict Delayed Cerebral Infarctions?" Journal of Clinical Medicine 10, no. 2: 321. https://doi.org/10.3390/jcm10020321
APA StyleTorné, R., Hoyos, J., Llull, L., Rodríguez-Hernández, A., Muñoz, G., Mellado-Artigas, R., Santana, D., Pedrosa, L., Di Somma, A., San Roman, L., Amaro, S., & Enseñat, J. (2021). Edema Resolution and Clinical Assessment in Poor-Grade Subarachnoid Hemorrhage: Useful Indicators to Predict Delayed Cerebral Infarctions? Journal of Clinical Medicine, 10(2), 321. https://doi.org/10.3390/jcm10020321





