Mildly Reduced Doses of Adrenaline Do Not Affect Key Hemodynamic Parameters during Cardio-Pulmonary Resuscitation in a Pig Model of Cardiac Arrest
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Surgical Preparation
2.3. Experimental Protocol
2.4. Objectives and Endpoints
2.4.1. Main Objective
2.4.2. Secondary Objectives
2.4.3. Primary Endpoint
2.4.4. Secondary Endpoints
2.5. Statistical Analysis
3. Results
4. Discussion
4.1. Coronary Perfusion Pressure and Return of Spontaneous Circulation
4.2. EtCO2 and CPP Interaction
4.3. Cerebral/Peripheral Organ Perfusion and Reduced Doses of Adrenaline
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gueugniaud, P.-Y.; Bertrand, C.; Savary, D.; Hubert, H. L’arrêt Cardiaque En France: Pourquoi Un Registre National? Presse Médicale 2011, 40, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Gräsner, J.-T.; Wnent, J.; Herlitz, J.; Perkins, G.D.; Lefering, R.; Tjelmeland, I.; Koster, R.W.; Masterson, S.; Rossell-Ortiz, F.; Maurer, H.; et al. Survival after Out-of-Hospital Cardiac Arrest in Europe—Results of the EuReCa TWO Study. Resuscitation 2020, 148, 218–226. [Google Scholar] [CrossRef]
- Myat, A.; Song, K.-J.; Rea, T. Out-of-Hospital Cardiac Arrest: Current Concepts. Lancet 2018, 391, 970–979. [Google Scholar] [CrossRef]
- Soar, J.; Böttiger, B.W.; Carli, P.; Couper, K.; Deakin, C.D.; Djärv, T.; Lott, C.; Olasveengen, T.; Paal, P.; Pellis, T.; et al. European Resuscitation Council Guidelines 2021: Adult Advanced Life Support. Resuscitation 2021, 161, 115–151. [Google Scholar] [CrossRef] [PubMed]
- Redding, J.S.; Pearson, J.W. Resuscitation from Asphyxia. JAMA 1962, 182, 283–286. [Google Scholar]
- Perkins, G.D.; Ji, C.; Deakin, C.D.; Quinn, T.; Nolan, J.P.; Scomparin, C.; Regan, S.; Long, J.; Slowther, A.; Pocock, H.; et al. A Randomized Trial of Epinephrine in Out-of-Hospital Cardiac Arrest. N. Engl. J. Med. 2018, 379, 711–721. [Google Scholar] [CrossRef]
- Jacobs, I.G.; Finn, J.C.; Jelinek, G.A.; Oxer, H.F.; Thompson, P.L. Effect of Adrenaline on Survival in Out-of-Hospital Cardiac Arrest: A Randomised Double-Blind Placebo-Controlled Trial. Resuscitation 2011, 82, 1138–1143. [Google Scholar] [CrossRef]
- Paradis, N.A.; Martin, G.B.; Rivers, E.P.; Goetting, M.G.; Appleton, T.J.; Feingold, M.; Nowak, R.M. Coronary Perfusion Pressure and the Return of Spontaneous Circulation in Human Cardiopulmonary Resuscitation. JAMA 1990, 263, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Sanders, A.B.; Ewy, G.A.; Taft, T.V. Prognostic and Therapeutic Importance of the Aortic Diastolic Pressure in Resuscitation from Cardiac Arrest. Crit. Care Med. 1984, 12, 871–873. [Google Scholar] [CrossRef]
- Kern, K.B.; Ewy, G.A.; Voorhees, W.D.; Babbs, C.F.; Tacker, W.A. Myocardial Perfusion Pressure: A Predictor of 24-Hour Survival during Prolonged Cardiac Arrest in Dogs. Resuscitation 1988, 16, 241–250. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, J.C.; Salcido, D.D.; Menegazzi, J.J. Coronary Perfusion Pressure and Return of Spontaneous Circulation after Prolonged Cardiac Arrest. Prehospital Emerg. Care 2010, 14, 78–84. [Google Scholar] [CrossRef] [Green Version]
- Michael, J.R.; Guerci, A.D.; Koehler, R.C.; Shi, A.Y.; Tsitlik, J.; Chandra, N.; Niedermeyer, E.; Rogers, M.C.; Traystman, R.J.; Weisfeldt, M.L. Mechanisms by Which Epinephrine Augments Cerebral and Myocardial Perfusion during Cardiopulmonary Resuscitation in Dogs. Circulation 1984, 69, 822–835. [Google Scholar] [CrossRef] [Green Version]
- Pytte, M.; Kramer-Johansen, J.; Eilevstjønn, J.; Eriksen, M.; Strømme, T.A.; Godang, K.; Wik, L.; Steen, P.A.; Sunde, K. Haemodynamic Effects of Adrenaline (Epinephrine) Depend on Chest Compression Quality during Cardiopulmonary Resuscitation in Pigs. Resuscitation 2006, 71, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, L.; Liao, Q.; Steen, S. The Effects of Epinephrine/Norepinephrine on End-Tidal Carbon Dioxide Concentration, Coronary Perfusion Pressure and Pulmonary Arterial Blood Flow during Cardiopulmonary Resuscitation. Resuscitation 2000, 43, 129–140. [Google Scholar] [CrossRef]
- Sandroni, C.; De Santis, P.; D’Arrigo, S. Capnography during Cardiac Arrest. Resuscitation 2018, 132, 73–77. [Google Scholar] [CrossRef]
- Gudipati, C.V.; Weil, M.H.; Bisera, J.; Deshmukh, H.G.; Rackow, E.C. Expired Carbon Dioxide: A Noninvasive Monitor of Cardiopulmonary Resuscitation. Circulation 1988, 77, 234–239. [Google Scholar] [CrossRef] [Green Version]
- Sanders, A.B.; Atlas, M.; Ewy, G.A.; Kern, K.B.; Bragg, S. Expired PCO2 as an Index of Coronary Perfusion Pressure. Am. J. Emerg. Med. 1985, 3, 147–149. [Google Scholar] [CrossRef]
- Reynolds, J.C.; Salcido, D.; Koller, A.C.; Sundermann, M.L.; Frisch, A.; Suffoletto, B.P.; Menegazzi, J.J. Tissue Oximetry by Near-Infrared Spectroscopy in a Porcine Model of out-of-Hospital Cardiac Arrest and Resuscitation. Resuscitation 2013, 84, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Yagi, T.; Nagao, K.; Kawamorita, T.; Soga, T.; Ishii, M.; Chiba, N.; Watanabe, K.; Tani, S.; Yoshino, A.; Hirayama, A.; et al. Detection of ROSC in Patients with Cardiac Arrest During Chest Compression Using NIRS: A Pilot Study. Adv. Exp. Med. Biol. 2016, 876, 151–157. [Google Scholar] [CrossRef]
- Schnaubelt, S.; Sulzgruber, P.; Menger, J.; Skhirtladze-Dworschak, K.; Sterz, F.; Dworschak, M. Regional Cerebral Oxygen Saturation during Cardiopulmonary Resuscitation as a Predictor of Return of Spontaneous Circulation and Favourable Neurological Outcome—A Review of the Current Literature. Resuscitation 2018, 125, 39–47. [Google Scholar] [CrossRef]
- Soar, J.; Nolan, J.P.; Böttiger, B.W.; Perkins, G.D.; Lott, C.; Carli, P.; Pellis, T.; Sandroni, C.; Skrifvars, M.B.; Smith, G.B.; et al. European Resuscitation Council Guidelines for Resuscitation 2015. Resuscitation 2015, 95, 100–147. [Google Scholar] [CrossRef] [Green Version]
- Ralston, S.H.; Voorhees, W.D.; Babbs, C.F. Intrapulmonary Epinephrine during Prolonged Cardiopulmonary Resuscitation: Improved Regional Blood Flow and Resuscitation in Dogs. Ann. Emerg. Med. 1984, 13, 79–86. [Google Scholar] [CrossRef]
- Ditchey, R.V.; Winkler, J.V.; Rhodes, C.A. Relative Lack of Coronary Blood Flow during Closed-Chest Resuscitation in Dogs. Circulation 1982, 66, 297–302. [Google Scholar] [CrossRef] [Green Version]
- Berg, R.A.; Otto, C.W.; Kern, K.B.; Sanders, A.B.; Hilwig, R.W.; Hansen, K.K.; Ewy, G.A. High-Dose Epinephrine Results in Greater Early Mortality after Resuscitation from Prolonged Cardiac Arrest in Pigs: A Prospective, Randomized Study. Crit. Care Med. 1994, 22, 282–290. [Google Scholar] [CrossRef]
- Hardig, B.M.; Götberg, M.; Rundgren, M.; Götberg, M.; Zughaft, D.; Kopotic, R.; Wagner, H. Physiologic Effect of Repeated Adrenaline (Epinephrine) Doses during Cardiopulmonary Resuscitation in the Cath Lab Setting: A Randomised Porcine Study. Resuscitation 2016, 101, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Wagner, H.; Götberg, M.; Madsen Hardig, B.; Rundgren, M.; Carlson, J.; Götberg, M.; Zughaft, D.; Erlinge, D.; Olivecrona, G.K. Repeated Epinephrine Doses during Prolonged Cardiopulmonary Resuscitation Have Limited Effects on Myocardial Blood Flow: A Randomized Porcine Study. BMC Cardiovasc. Disord. 2014, 14, 199. [Google Scholar] [CrossRef] [Green Version]
- Chang, W.-T.; Ma, M.H.-M.; Chien, K.-L.; Huang, C.-H.; Tsai, M.-S.; Shih, F.-Y.; Yuan, A.; Tsai, K.-C.; Lin, F.-Y.; Lee, Y.-T.; et al. Postresuscitation Myocardial Dysfunction: Correlated Factors and Prognostic Implications. Intensive Care Med. 2007, 33, 88–95. [Google Scholar] [CrossRef]
- Tang, W.; Weil, M.H.; Sun, S.; Noc, M.; Yang, L.; Gazmuri, R.J. Epinephrine Increases the Severity of Postresuscitation Myocardial Dysfunction. Circulation 1995, 92, 3089–3093. [Google Scholar] [CrossRef]
- Livesay, J.J.; Follette, D.M.; Fey, K.H.; Nelson, R.L.; DeLand, E.C.; Barnard, R.J.; Buckberg, G.D. Optimizing Myocardial Supply/Demand Balance with Alpha-Adrenergic Drugs during Cardiopulmonary Resuscitation. J. Thorac. Cardiovasc. Surg. 1978, 76, 244–251. [Google Scholar] [CrossRef]
- Garnett, A.R. End-Tidal Carbon Dioxide Monitoring During Cardiopulmonary Resuscitation. JAMA J. Am. Med. Assoc. 1987, 257, 512–515. [Google Scholar] [CrossRef]
- Murphy, R.A.; Bobrow, B.J.; Spaite, D.W.; Hu, C.; McDannold, R.; Vadeboncoeur, T.F. Association between Prehospital CPR Quality and End-Tidal Carbon Dioxide Levels in Out-of-Hospital Cardiac Arrest. Prehosp. Emerg. Care 2016, 20, 369–377. [Google Scholar] [CrossRef]
- Martin, G.B.; Gentile, N.T.; Paradis, N.A.; Moeggenberg, J.; Appleton, T.J.; Nowak, R.M. Effect of Epinephrine on End-Tidal Carbon Dioxide Monitoring during CPR. Ann. Emerg. Med. 1990, 19, 396–398. [Google Scholar] [CrossRef]
- Tang, W.; Weil, M.H.; Gazmuri, R.J.; Sun, S.; Duggal, C.; Bisera, J. Pulmonary Ventilation/Perfusion Defects Induced by Epinephrine during Cardiopulmonary Resuscitation. Circulation 1991, 84, 2101–2107. [Google Scholar] [CrossRef] [Green Version]
- Burnett, A.M.; Segal, N.; Salzman, J.G.; McKnite, M.S.; Frascone, R.J. Potential Negative Effects of Epinephrine on Carotid Blood Flow and ETCO2 during Active Compression–Decompression CPR Utilizing an Impedance Threshold Device. Resuscitation 2012, 83, 1021–1024. [Google Scholar] [CrossRef]
- Murkin, J.M.; Arango, M. Near-Infrared Spectroscopy as an Index of Brain and Tissue Oxygenation. Br. J. Anaesth. 2009, 103 (Suppl. S1), i3–i13. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, M.; Giannini, I.; Sideri, G.; Zanette, E. Continuous Non Invasive Monitoring of Human Brain by Near Infrared Spectroscopy. In Oxygen Transport to Tissue VII; Kreuzer, F., Cain, S.M., Turek, Z., Goldstick, T.K., Eds.; Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 1985; Volume 191, pp. 873–882. ISBN 978-1-4684-3293-0. [Google Scholar]
- Tobias, J.D. Cerebral Oxygenation Monitoring: Near-Infrared Spectroscopy. Expert Rev. Med. Devices 2006, 3, 235–243. [Google Scholar] [CrossRef]
- Pellicer, A.; del Bravo, M.C. Near-Infrared Spectroscopy: A Methodology-Focused Review. Semin. Fetal Neonatal Med. 2011, 16, 42–49. [Google Scholar] [CrossRef]
- Parnia, S.; Nasir, A.; Shah, C.; Patel, R.; Mani, A.; Richman, P. A Feasibility Study Evaluating the Role of Cerebral Oximetry in Predicting Return of Spontaneous Circulation in Cardiac Arrest. Resuscitation 2012, 83, 982–985. [Google Scholar] [CrossRef]
- Müllner, M.; Sterz, F.; Binder, M.; Hirschl, M.M.; Janata, K.; Laggner, A.N. Near Infrared Spectroscopy during and after Cardiac Arrest—Preliminary Results. Clin. Intensive Care Int. J. Crit. Coron. Care Med. 1995, 6, 107–111. [Google Scholar]
- Prosen, G.; Strnad, M.; Doniger, S.J.; Markota, A.; Stožer, A.; Borovnik-Lesjak, V.; Mekiš, D. Cerebral Tissue Oximetry Levels during Prehospital Management of Cardiac Arrest—A Prospective Observational Study. Resuscitation 2018, 129, 141–145. [Google Scholar] [CrossRef]
- Nosrati, R.; Lin, S.; Mohindra, R.; Ramadeen, A.; Toronov, V.; Dorian, P. Study of the Effects of Epinephrine on Cerebral Oxygenation and Metabolism during Cardiac Arrest and Resuscitation by Hyperspectral Near-Infrared Spectroscopy. Crit. Care Med. 2019, 47, e349–e357. [Google Scholar] [CrossRef]
- Johansson, J.; Gedeborg, R.; Basu, S.; Rubertsson, S. Increased Cortical Cerebral Blood Flow by Continuous Infusion of Adrenaline (Epinephrine) during Experimental Cardiopulmonary Resuscitation. Resuscitation 2003, 57, 299–307. [Google Scholar] [CrossRef]
- Overgaard, C.B.; Dzavík, V. Inotropes and Vasopressors: Review of Physiology and Clinical Use in Cardiovascular Disease. Circulation 2008, 118, 1047–1056. [Google Scholar] [CrossRef] [Green Version]
- Ristagno, G.; Tang, W.; Huang, L.; Fymat, A.; Chang, Y.-T.; Sun, S.; Castillo, C.; Weil, M.H. Epinephrine Reduces Cerebral Perfusion during Cardiopulmonary Resuscitation. Crit. Care Med. 2009, 37, 1408–1415. [Google Scholar] [CrossRef]
- Ristagno, G.; Sun, S.; Tang, W.; Castillo, C.; Weil, M.H. Effects of Epinephrine and Vasopressin on Cerebral Microcirculatory Flows during and after Cardiopulmonary Resuscitation. Crit. Care Med. 2007, 35, 2145–2149. [Google Scholar] [CrossRef]
- Cherry, B.H.; Nguyen, A.Q.; Hollrah, R.A.; Olivencia-Yurvati, A.H.; Mallet, R.T. Modeling Cardiac Arrest and Resuscitation in the Domestic Pig. World J. Crit. Care Med. 2015, 4, 1–12. [Google Scholar] [CrossRef]
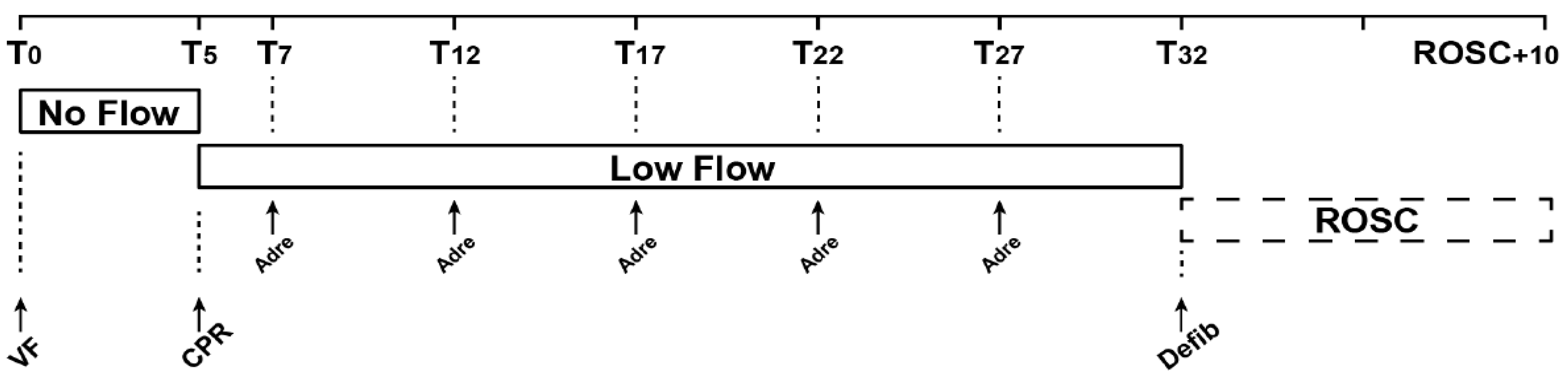

| Baseline Characteristics | 1 mg Adrenaline (n = 5) | 0.50 mg Adrenaline (n = 5) | 0.25 mg Adrenaline (n = 5) |
|---|---|---|---|
| Weight (kg) | 61.1 (46.4; 65.7) | 53.9 (50; 63.5) | 55 (45.4; 66.3) |
| ETCO2 (mmHg) | 44 (37; 51) | 44 (41; 48) | 47 (34; 55) |
| SpO2 (%) | 99 (95; 100) | 97 (93; 97) | 96 (94; 97) |
| HR (/min) | 93 (81; 106) | 94 (81; 118) | 101 (83; 126) |
| Temperature (Celsius) | 38.8 (38.2; 40.8) | 39.3 (38.9; 40.6) | 39.4 (39.1; 39.6) |
| pH | 7.39 (7.35; 7.49) | 7.42 (7.38; 7.43) | 7.38 (7.31; 7.47) |
| pO2 (mmHg) | 102 (96; 143) | 119 (99; 160) | 96 (88; 194) |
| pCO2 (mmHg) | 49 (42; 53) | 49 (47; 51) | 52 (40; 60) |
| Lactate (mmol/L) | 1.7 (1.2; 2.4) | 1.4 (1; 1.8) | 1 (0.75; 2.3) |
| AP systolic (mmHg) | 109 (82; 140) | 108 (100; 114) | 109 (89.5; 141) |
| RAP systolic (mmHg) | 7.7 (3.12; 9.28) | 7.18 (2.32; 8.88) | 8.3 (4.79; 15) |
| AP diastolic (mmHg) | 81.1 (58.4; 104) | 74.1 (61.5; 83.7) | 83.4 (52.1; 104) |
| RAP diastolic (mmHg) | 3.83 (−1.49; 4.18) | 3.06 (2.25; 5.17) | 3.66 (0.902; 9.94) |
| CPP (mmHg) | 76.9 (54.4; 106) | 71 (58.5; 80.4) | 79.8 (51.2; 100) |
| NIRSc Baseline (%) | 54 (47; 59) | 54.5 (46.5; 65.5) | 57.5 (53.5; 69.5) |
| NIRSt Baseline (%) | 41 (38; 62) | 31 (20; 70) | 39 (25; 43) |
| ROSC | |||
| No | 1 (20.0 %) | 1 (20.0 %) | 1 (20.0 %) |
| Yes | 4 (80.0 %) | 4 (80.0 %) | 4 (80.0 %) |
| Parameter Measured | 1 mg Adrenaline (n = 5) | 0.50 mg Adrenaline (n = 5) | 0.25 mg Adrenaline (n = 5) | p-Value 0.50 mg vs. 1 mg * | p-Value 0.25 mg vs. 1 mg * | p-Value Overall ** |
|---|---|---|---|---|---|---|
| CPP | ||||||
| T7 | 27.8 (24.3; 42.2) | 28.6 (18.6; 33.5) | 24.5 (12; 29.8) | 0.55 | 0.15 | 0.25 |
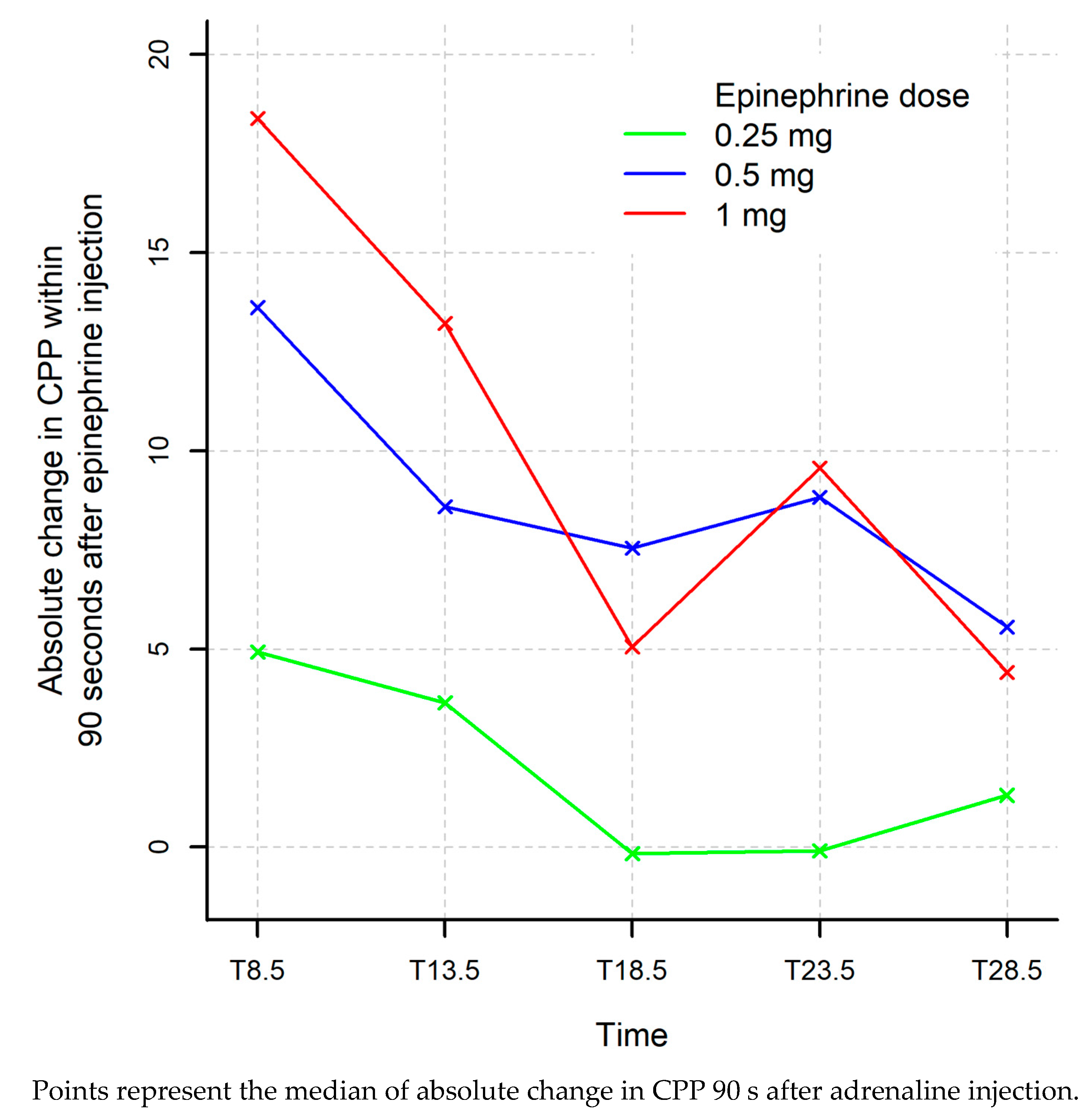
| T8.5 | 53.8 (37.8; 58.2) | 39.6 (32.7; 52.5) | 28.9 (21.2; 35.4) | 0.056 | 0.008 ‡ | 0.0007 ‡ |
| Absolute Δ T7–T8.5 | 18.4 (13.4; 30.4) | 13.6 (11; 19) | 4.93 (3.31; 15.2) | 0.22 | 0.032 ‡ | 0.030 ‡ |
| T12 | 36.5 (15.8; 40.4) | 23.3 (12.1; 42) | 22 (9.25; 29.6) | 0.69 | 0.095 | 0.25 |
| T13.5 | 48.3 (30.2; 64.1) | 39.2 (23.5; 49.4) | 22.6 (13; 36.7) | 0.31 | 0.032 ‡ | 0.026 ‡ |
| Absolute Δ T12–T13.5 | 13.2 (−6.28; 32.5) | 8.59 (5.03; 22.1) | 3.64 (−0.06; 14.7) | 0.55 | 0.31 | 0.19 |
| T17 | 33 (8.14; 59.7) | 25.5 (19.5; 38.1) | 24.3 (16.3; 51.8) | 1.00 | 0.69 | 0.86 |
| T18.5 | 38.2 (24.2; 69.6) | 31.9 (27; 55.6) | 24.1 (19.1; 28.3) | 1.00 | 0.032 ‡ | 0.029 ‡ |
| Absolute Δ T17–T18.5 | 5.05 (−3.2; 30.1) | 7.54 (4.99; 17.5) | −0.159 (−0.342; 7.73) | 0.69 | 0.41 | 0.27 |
| T22 | 15.1 (5.38; 28.8) | 21.6 (20.2; 33.8) | 21.9 (6.01; 24.7) | 0.22 | 0.73 | 0.36 |
| T23.5 | 38.3 (20.3; 66.8) | 29.7 (26.8; 45.1) | 19.6 (10.5; 25.6) | 1.00 | 0.063 | 0.028 ‡ |
| Absolute Δ T22–T23.5 | 9.56 (3.22; 61.4) | 8.82 (5.86; 11.3) | −0.0938 (−3.38; 4.45) | 0.84 | 0.032 ‡ | 0.017 ‡ |
| T27 | 23.3 (9.56; 57.7) | 23.2 (18.9; 31.3) | 22.2 (6.59; 31.3) | 0.84 | 0.69 | 0.72 |
| T28.5 | 34.1 (10.9; 62.1) | 25.3 (21.8; 45.1) | 23.5 (5.41; 48.7) | 0.55 | 0.42 | 0.55 |
| Absolute Δ T27–T28.5 | 4.41 (−0.577; 26.3) | 5.55 (−1.39; 13.8) | 1.31 (−1.18; 17.4) | 1.00 | 0.55 | 0.86 |
| ROSC10 | 52.5 (44.6; 58.4) | 43.3 (3.59; 66) | 54.9 (25.6; 86.1) | 0.86 | 0.86 | 0.83 |
| NIRSc | ||||||
| Baseline | 54 (47; 59) | 54.5 (46.5; 65.5) | 57.5 (53.5; 69.5 | 1.00 | 0.29 | 0.36 |
| T32 | 32 (29.5; 43.5) | 36 (32; 49) | 42 (39.5; 59.5) | 0.46 | 0.39 | 0.34 |
| ROSC10 | 51.8 (42.5; 58.5) | 47 (42; 50) | 46.8 (46; 49) | 0.69 | 1.00 | 0.92 |
| NIRS T | ||||||
| Baseline | 41 (38; 62) | 31 (20; 70) | 39 (25; 43) | 0.38 | 0.52 | 0.64 |
| T32 | 0 (0; 0) | 6 (0; 22) | 9 (0; 12) | 0.048 ‡ | 0.048 ‡ | 0.030 ‡ |
| ROSC10 | 30 (23; 37) | 38.5 (28; 57) | 29 (0; 43) | 0.23 | 1.00 | 0.47 |
| CPP >35 mmHg | 1 mg Adrenaline (n = 5) | 0.50 mg Adrenaline (n = 5) | 0.25 mg Adrenaline (n = 5) | p-Value 0.50 mg vs. 1 mg | p-Value 0.25 mg vs. 1 mg | p-Value * Overall | |||
|---|---|---|---|---|---|---|---|---|---|
| N | n (%) | N | n (%) | N | n (%) | ||||
| T8.5 | 5 | 5 (100 %) | 5 | 3 (60 %) | 5 | 1 (20 %) | 0.44 | 0.048 ‡ | 0.066 ‡ |
| T13.5 | 5 | 4 (80 %) | 5 | 3 (60 %) | 5 | 1 (20 %) | 1.00 | 0.21 | 0.30 |
| T18.5 | 5 | 3 (60 %) | 5 | 2 (40 %) | 4 | 0 (0 %) | 1.00 | 0.17 | 0.30 |
| T23.5 | 5 | 3 (60 %) | 5 | 2 (40 %) | 4 | 0 (0 %) | 1.00 | 0.17 | 0.30 |
| T28.5 | 5 | 2 (40 %) | 5 | 1 (20 %) | 5 | 1 (20 %) | 1.00 | 1.00 | 1.00 |
| ROSC10 | 3 | 3 (100 %) | 4 | 2 (50 %) | 4 | 3 (75 %) | 0.43 | 1.00 | 0.71 |
| ETCO2 (mmHg) | 1 mg Adrenaline (n = 5) | 0.50 mg Adrenaline (n = 5) | 0.25 mg Adrenaline (n = 5) | p-Value 0.50 mg vs. 1 mg * | p-Value 0.25 mg vs. 1 mg * | p-Value Overall ** | |||
|---|---|---|---|---|---|---|---|---|---|
| n | Median (Min–Max) | n | Median (Min–Max) | n | Median (Min–Max) | ||||
| T7 | 5 | 28 (23; 36) | 5 | 32 (23; 37) | 5 | 29 (9; 40) | 0.71 | 0.88 | 0.79 |
| T9 | 5 | 24 (18; 31) | 5 | 26 (17; 30) | 5 | 26 (16; 28) | 0.84 | 0.73 | 0.91 |
| Absolute Δ T7–T9 | 5 | −5 (−11; −2) | 5 | −6 (−9; −4) | 5 | −3 (−13; 7) | 1.00 | 0.55 | 0.59 |
| T12 | 5 | 19 (16; 26) | 5 | 32 (23; 35) | 5 | 21 (16; 34) | 0.016 ‡ | 0.45 | 0.032 ‡ |
| T14 | 5 | 19 (12; 22) | 5 | 26 (20; 34) | 5 | 22 (18; 27) | 0.032 ‡ | 0.21 | 0.052 |
| Absolute Δ T12–T14 | 5 | 0 (−6; 1) | 5 | −3 (−9; −1) | 5 | 0 (−8; 3) | 0.29 | 0.75 | 0.30 |
| T17 | 5 | 19 (15; 23) | 5 | 29 (22; 37) | 5 | 23 (12; 34) | 0.016 ‡ | 0.48 | 0.049 ‡ |
| T19 | 5 | 19 (16; 24) | 5 | 22 (15; 31) | 5 | 18 (10; 27) | 0.69 | 1.00 | 0.84 |
| Absolute Δ T17–T19 | 5 | 0 (−3; 9) | 5 | −7 (−10; −5) | 5 | −3 (−7; 0) | 0.008 ‡ | 0.17 | 0.005 ‡ |
| T22 | 5 | 17 (14; 23) | 5 | 24 (13; 29) | 5 | 20 (7; 33) | 0.29 | 0.61 | 0.49 |
| T24 | 5 | 23 (12; 26) | 5 | 15 (12; 29) | 5 | 17 (8; 29) | 0.72 | 0.84 | 1.00 |
| Absolute Δ T22–T24 | 5 | 0 (−2; 7) | 5 | −2 (−9; 0) | 5 | −4 (−5; 1) | 0.13 | 0.071 | 0.11 |
| T27 | 5 | 19 (11; 29) | 5 | 17 (10; 32) | 5 | 16 (7; 34) | 0.89 | 0.81 | 0.95 |
| T29 | 5 | 18 (11; 28) | 4 | 19 (14; 26) | 5 | 17 (6; 20) | 0.78 | 0.41 | 0.66 |
| Absolute Δ T27–T29 | 5 | 0 (−1; 2) | 4 | −2 (−6; 0) | 5 | −1 (−14; 2) | 0.19 | 0.49 | 0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaeger, D.; Koger, J.; Duhem, H.; Fritz, C.; Jeangeorges, V.; Duarte, K.; Levy, B.; Debaty, G.; Chouihed, T. Mildly Reduced Doses of Adrenaline Do Not Affect Key Hemodynamic Parameters during Cardio-Pulmonary Resuscitation in a Pig Model of Cardiac Arrest. J. Clin. Med. 2021, 10, 4674. https://doi.org/10.3390/jcm10204674
Jaeger D, Koger J, Duhem H, Fritz C, Jeangeorges V, Duarte K, Levy B, Debaty G, Chouihed T. Mildly Reduced Doses of Adrenaline Do Not Affect Key Hemodynamic Parameters during Cardio-Pulmonary Resuscitation in a Pig Model of Cardiac Arrest. Journal of Clinical Medicine. 2021; 10(20):4674. https://doi.org/10.3390/jcm10204674
Chicago/Turabian StyleJaeger, Deborah, Jonathan Koger, Helene Duhem, Caroline Fritz, Victor Jeangeorges, Kevin Duarte, Bruno Levy, Guillaume Debaty, and Tahar Chouihed. 2021. "Mildly Reduced Doses of Adrenaline Do Not Affect Key Hemodynamic Parameters during Cardio-Pulmonary Resuscitation in a Pig Model of Cardiac Arrest" Journal of Clinical Medicine 10, no. 20: 4674. https://doi.org/10.3390/jcm10204674






