An Evaluation of Muscle Repair Techniques: Implications in Musculoskeletal Healing and Corollaries in Oral-Facial Clefting
Abstract
:1. Introduction
2. Methods
2.1. Animal Model
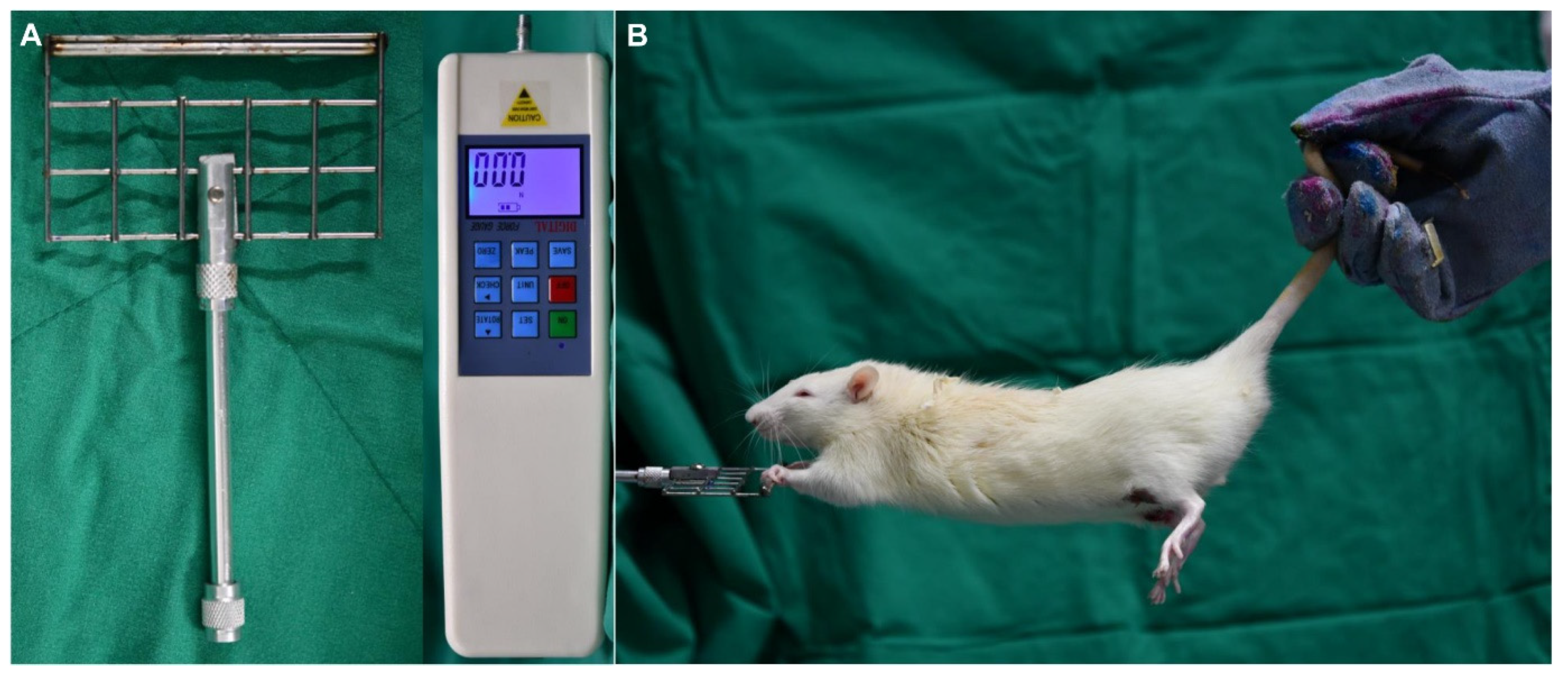
2.2. Grip Strength Test
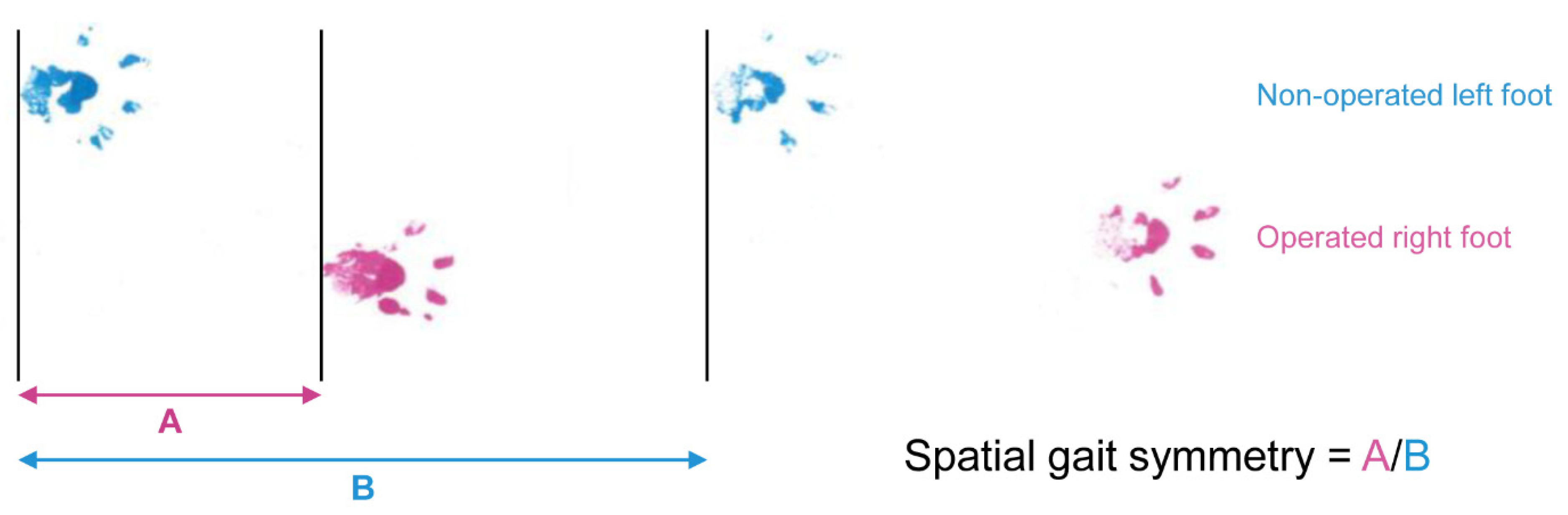
2.3. Spatial Gait Symmetry Test
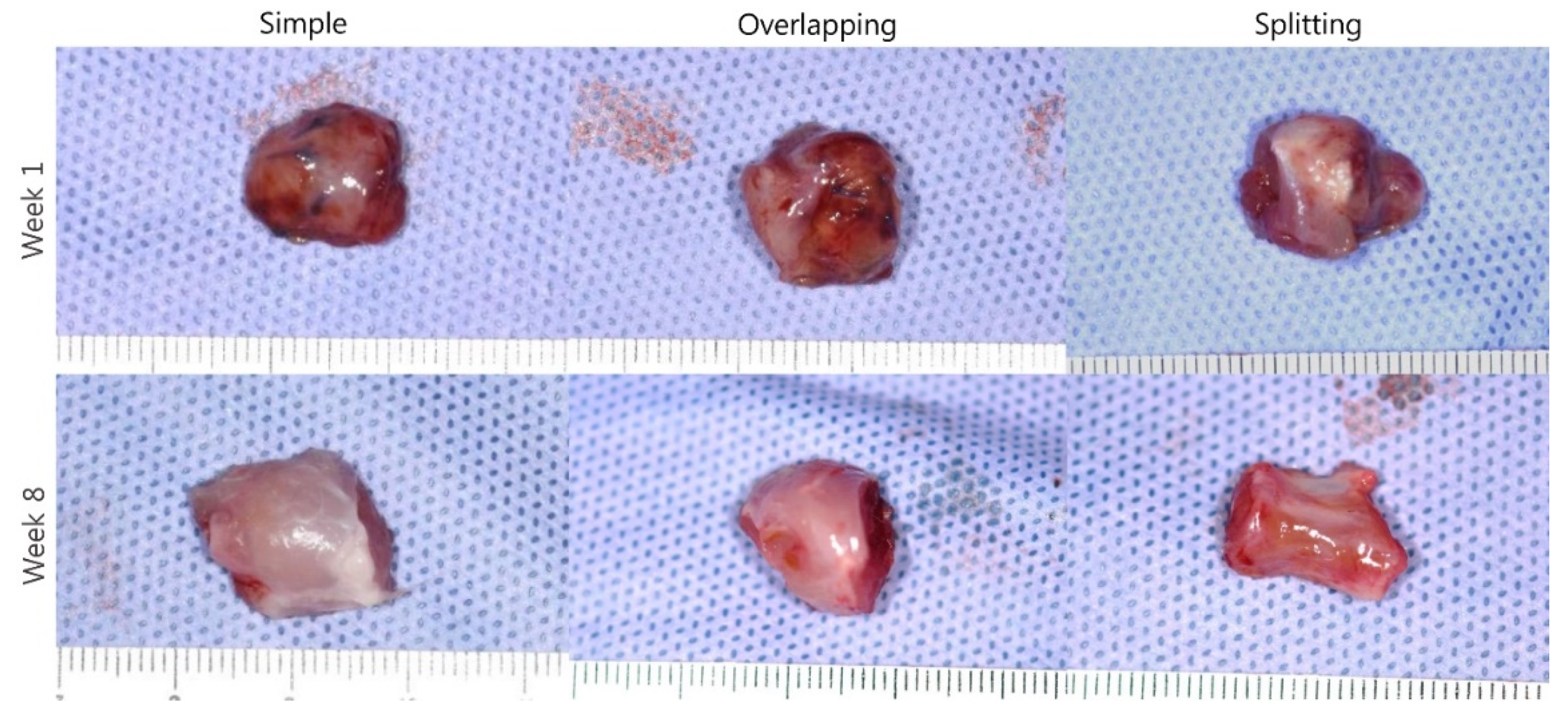
2.4. Histological Analysis
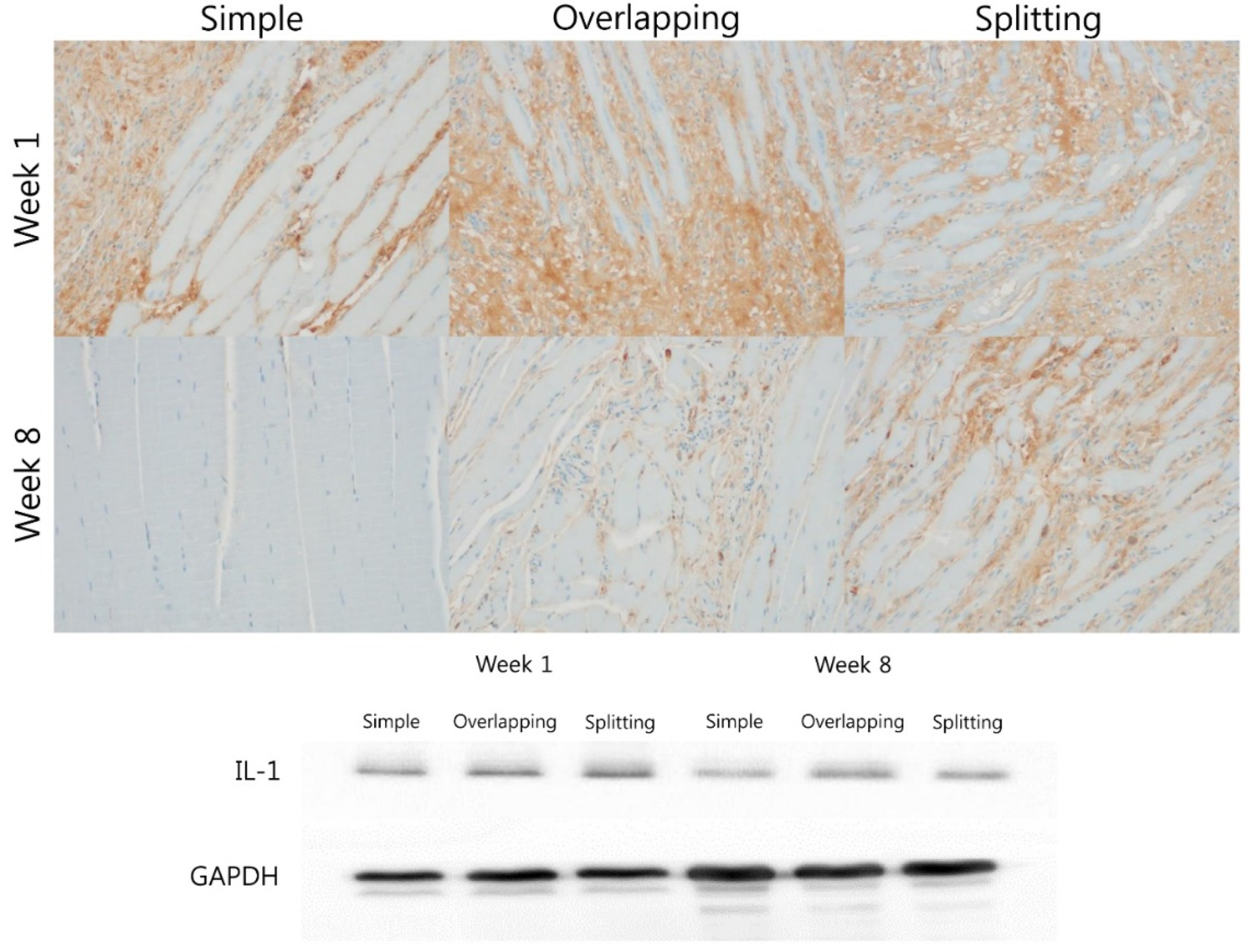
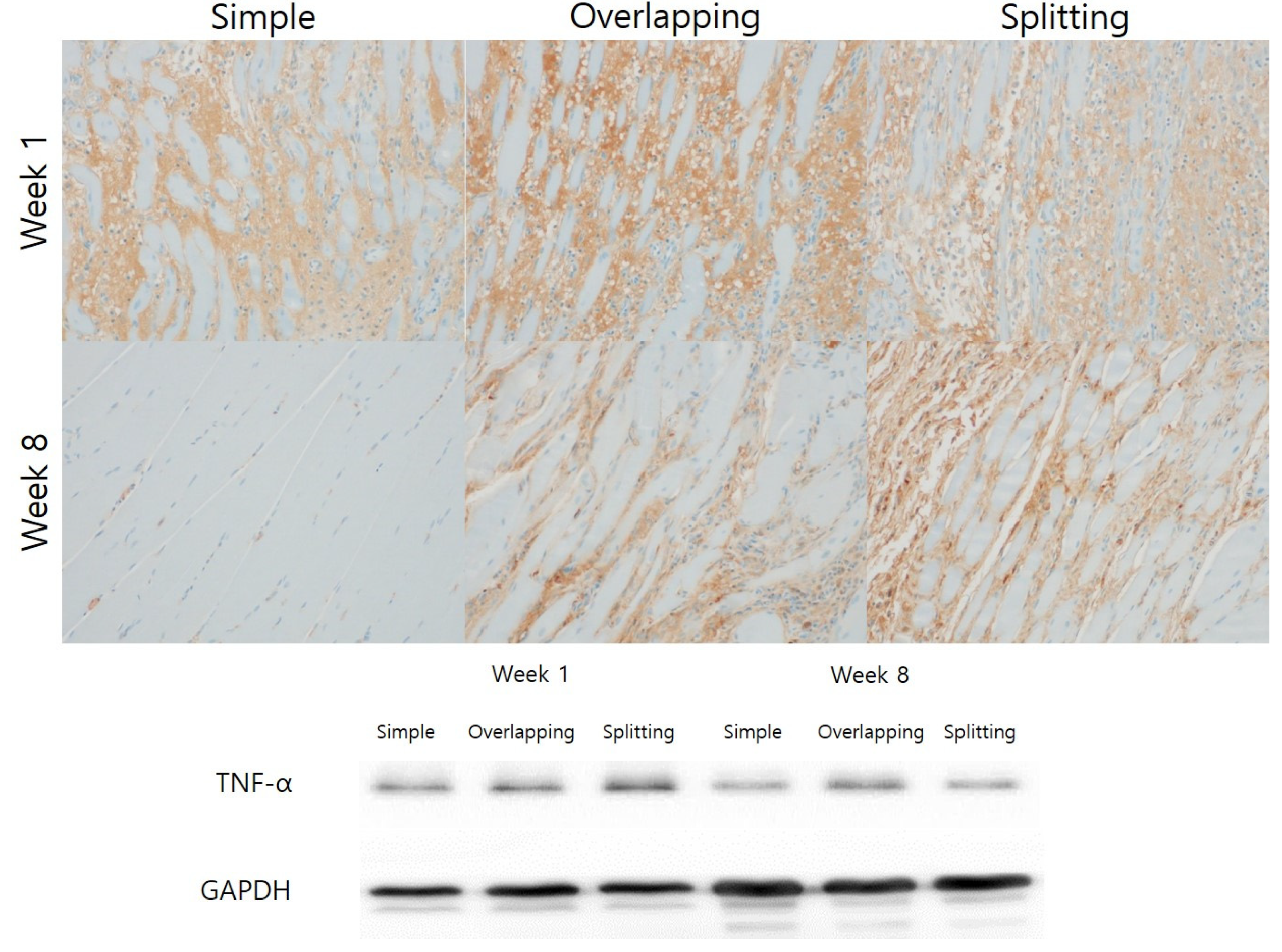
2.5. Western Blot Analysis
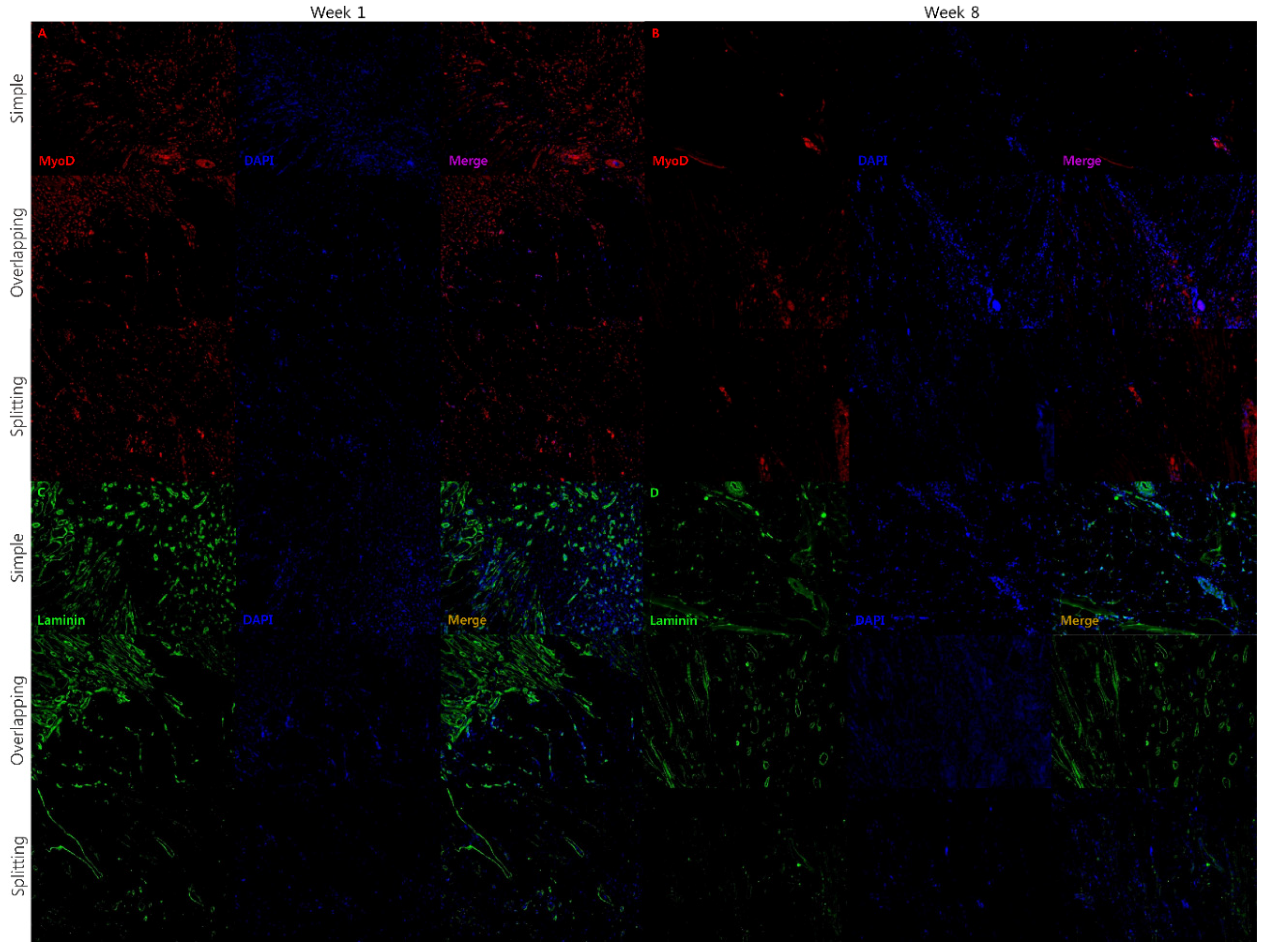
2.6. Immunohistochemistry
2.7. Statistical Analysis
3. Results
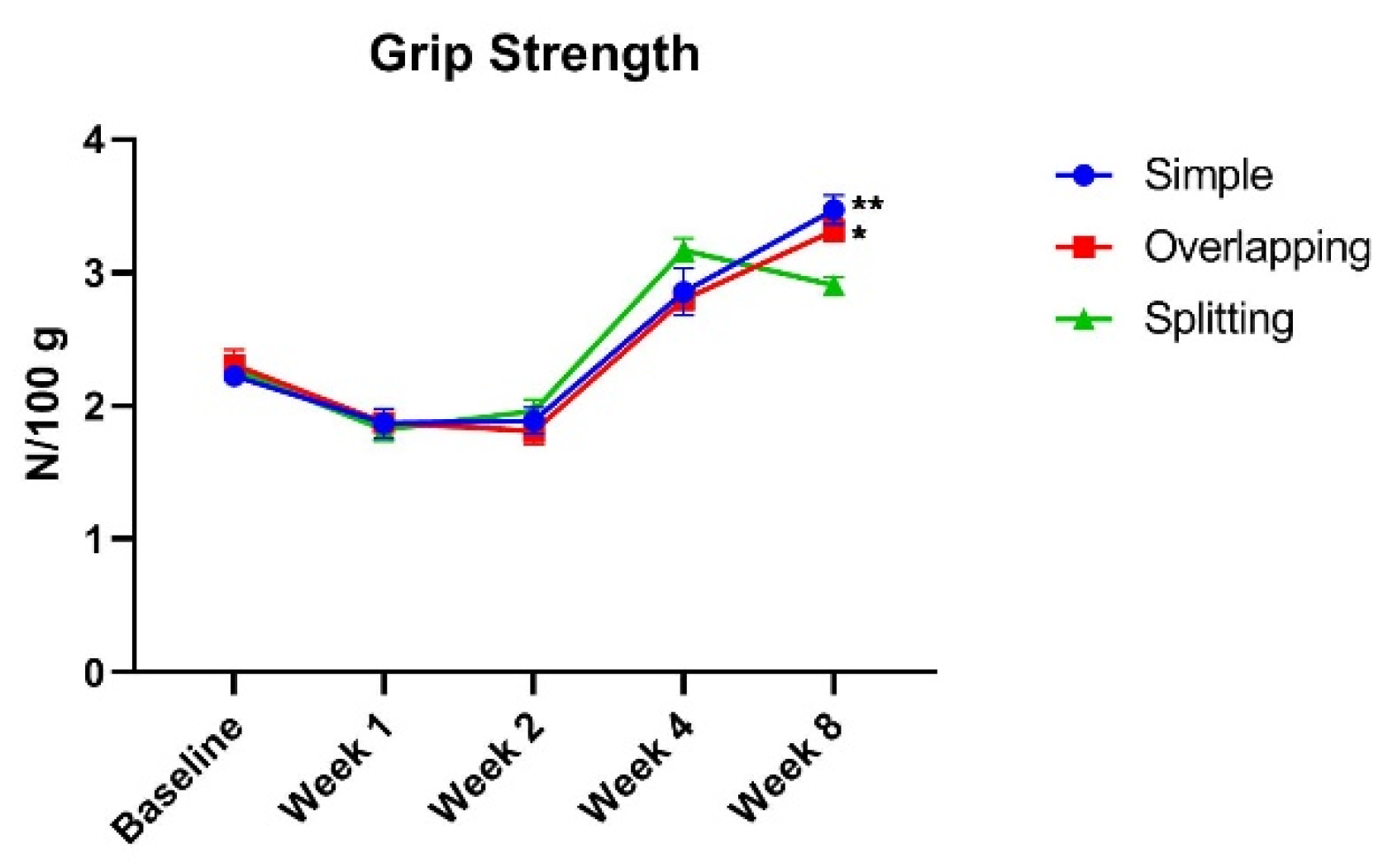
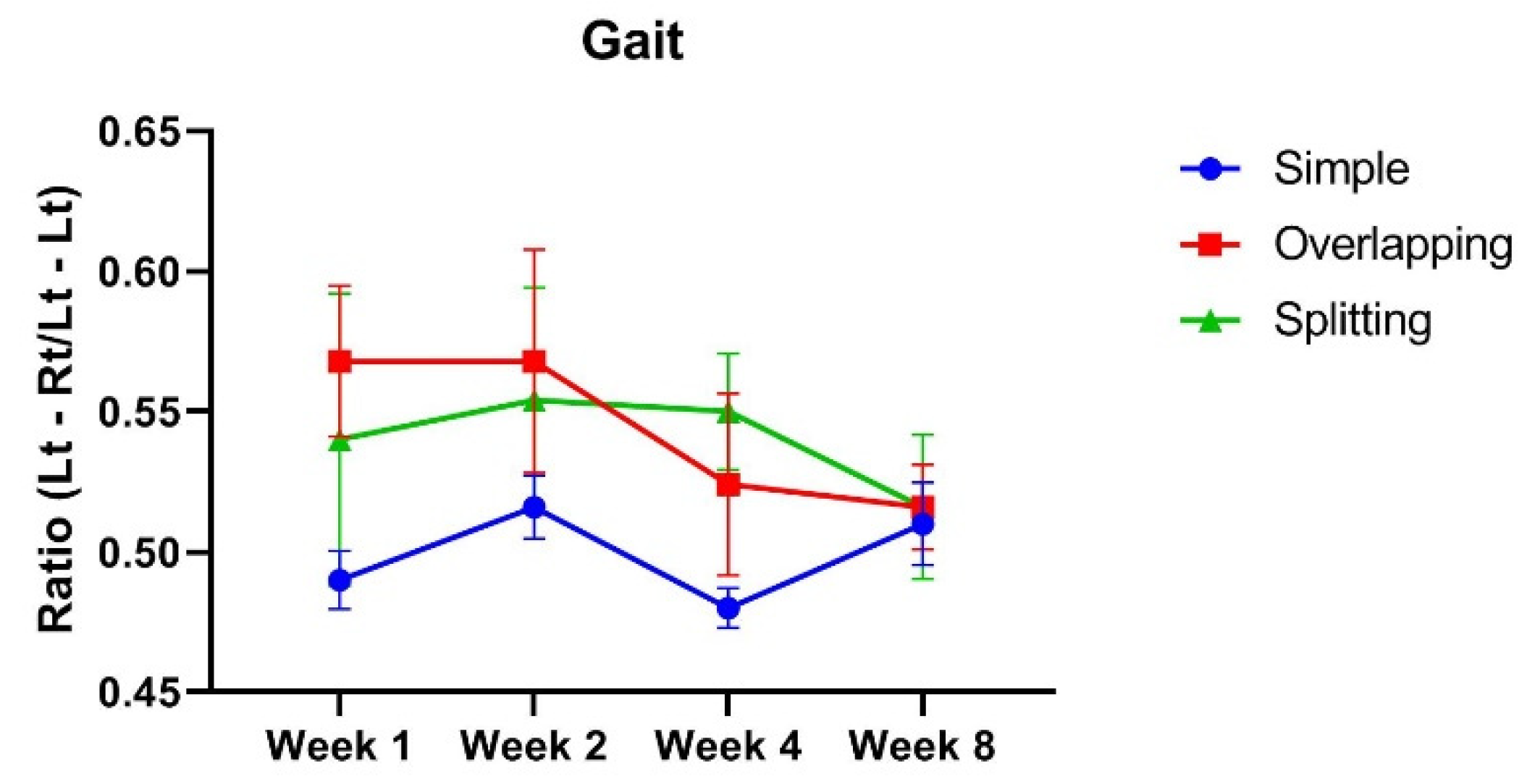
3.1. The Simple Group and Overlapping Group Exhibit Higher Muscle Power Than, but a Similar Range of Motion as the Splitting Group
3.2. Simple Suturing Leads to Earlier Remission of Inflammation Than Overlapping or Splitting Suturing
3.3. Simple and Overlapping Suturing Induced Organized Remodeling of Healing Muscle at Week 8
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Lonic, D.; Morris, D.E.; Lo, L.J. Primary overcorrection of the unilateral cleft nasal deformity: Quantifying the results. Ann. Plast. Surg. 2016, 77 (Suppl. 1), S25–S29. [Google Scholar] [CrossRef]
- Kim, S.; Choi, T.H.; Park, J.U.; Kwon, G.; Kim, J.C. Influence of modified furlow double opposing z-plasty on mandibular growth in oriental patients with cleft palate and/or lip. Ann. Plast. Surg. 2014, 73, 311–314. [Google Scholar] [CrossRef]
- Wong, L.S.; Lu, T.C.; Hang, D.T.D.; Chen, P.K. The impact of facial growth in unilateral cleft lip and palate treated with 2 different protocols. Ann. Plast. Surg. 2020, 84, 541–544. [Google Scholar] [CrossRef]
- Stal, S.; Brown, R.H.; Higuera, S.; Hollier, L.H., Jr.; Byrd, H.S.; Cutting, C.B.; Mulliken, J.B. Fifty years of the millard rotation-advancement: Looking back and moving forward. Plast. Reconstr. Surg. 2009, 123, 1364–1377. [Google Scholar] [CrossRef] [PubMed]
- Cutting, C.B.; Dayan, J.H. Lip height and lip width after extended mohler unilateral cleft lip repair. Plast. Reconstr. Surg. 2003, 111, 17–23, discussion 24–26. [Google Scholar] [CrossRef]
- Chang, F.-S.; Wallace, C.; Hsiao, Y.-C.; Huang, J.-J.; Liu, C.-W.; Chen, Z.-C.; Chen, P.-T.; Chen, J.-P.; Chen, Y.-R. Long-term comparison study of philtral ridge morphology with two different techniques of philtral reconstruction. Int. J. Oral Maxillofac. Surg. 2020, 49, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kwon, J.; Kwon, G.Y.; Choi, T.H. Dynamic reconstruction of the philtrum using coronal muscle splitting technique in microform cleft lip. J. Craniofacial Surg. 2014, 25, 742–745. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.C. New technique for correction of the microform cleft lip using vertical interdigitation of the orbicularis oris muscle through the intraoral incision. Plast. Reconstr. Surg. 2004, 114, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xie, F.; Ma, T.; Zhang, Z. Reconstruction of philtrum using partial splitting and folding of orbicularis oris muscle in secondary unilateral cleft lip. Plast. Reconstr. Surg. 2015, 136, 1274–1278. [Google Scholar] [CrossRef]
- Nguyen, D.C.; Patel, K.B.; Skolnick, G.B.; Skladman, R.; Grames, L.M.; Stahl, M.B.; Marsh, J.L.; Woo, A.S. Progressive tightening of the levator veli palatini muscle improves velopharyngeal dysfunction in early outcomes of primary palatoplasty. Plast. Reconstr. Surg. 2015, 136, 131–141. [Google Scholar] [CrossRef]
- Jeong, W.S.; Lee, S.S.; Park, E.J.; Han, J.J.; Choi, J.W.; Koh, K.S.; Oh, T.S. Comparison of biomechanical and histological outcomes of different suture techniques in rat rectus abdominis muscle repair. Ann. Plast. Surg. 2017, 78, 78–82. [Google Scholar] [CrossRef]
- Wang, Y.X.; Rudnicki, M.A. Satellite cells, the engines of muscle repair. Nat. Rev. Mol. Cell Biol. 2011, 13, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Boldrin, L.; Knopp, P.; Morgan, J.E.; Zammit, P.S. Muscle satellite cells are a functionally heterogeneous population in both somite-derived and branchiomeric muscles. Dev. Biol. 2010, 337, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Bertelli, J.A.; Mira, J.C. The grasping test: A simple behavioral method for objective quantitative assessment of peripheral nerve regeneration in the rat. J. Neurosci. Methods 1995, 58, 151–155. [Google Scholar] [CrossRef]
- Forcina, L.; Cosentino, M.; Musarò, A. Mechanisms regulating muscle regeneration: Insights into the interrelated and time-dependent phases of tissue healing. Cells 2020, 9, 1297. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jiang, G.; Zhang, P.; Fan, J. Programmed cell death and its role in inflammation. Mil. Med. Res. 2015, 2, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Hu, P. Skeletal muscle regeneration is modulated by inflammation. J. Orthop. Transl. 2018, 13, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Mauro, A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. 1961, 9, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Scharner, J.; Zammit, P.S. The muscle satellite cell at 50: The formative years. Skelet. Muscle 2011, 1, 28. [Google Scholar] [CrossRef] [Green Version]
- Creuzet, S.; Lescaudron, L.; Li, Z.; Fontaine-Pérus, J. Myod, myogenin, and desmin-nls-lacz transgene emphasize the distinct patterns of satellite cell activation in growth and regeneration. Exp. Cell Res. 1998, 243, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Sanes, J.R. The basement membrane/basal lamina of skeletal muscle. J. Biol. Chem. 2003, 278, 12601–12604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Q.; Li, Y.; Danning, Z.; Zhang, B.; Chen, S.; Wang, J. “Three-unit” muscle reconstruction in secondary cleft lip repair. Cleft Palate-Craniofacial J. 2015, 52, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, J.; Uhm, K.-I.; Shin, D.; Choi, H. Formation of the philtral column using a dermal graft in secondary unilateral cleft lip. J. Craniofacial Surg. 2017, 28, 1661–1663. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.A.; Allam, K.A.; Taneja, R.; Kawamoto, H.K. Constructing the philtral column in the secondary cleft lip deformity: Utilizing the palmaris longus graft. Ann. Plast. Surg. 2013, 70, 296–300. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, J.; Herrler, T.; Zhang, Y.; Li, Q.; Hua, C.; Dai, C. Philtrum reconstruction using a triangular-frame conchae cartilage graft in secondary cleft lip deformities. J. Craniofacial Surg. 2020, 31, 1556–1559. [Google Scholar] [CrossRef]
- Harel, I.; Nathan, E.; Tirosh-Finkel, L.; Zigdon, H.; Guimarães-Camboa, N.; Evans, S.M.; Tzahor, E. Distinct origins and genetic programs of head muscle satellite cells. Dev. Cell 2009, 16, 822–832. [Google Scholar] [CrossRef] [Green Version]
- Monroy, P.L.C.; Grefte, S.; Kuijpers-Jagtman, A.M.; Helmich, M.P.A.C.; Ulrich, D.J.O.; von den Hoff, J.W.; Wagener, F.A.D.T.G. A rat model for muscle regeneration in the soft palate. PLoS ONE 2013, 8, e59193. [Google Scholar]
- Pavlath, G.K.; Thaloor, D.; Rando, T.A.; Cheong, M.; English, A.W.; Zheng, B. Heterogeneity among muscle precursor cells in adult skeletal muscles with differing regenerative capacities. Dev. Dyn 1998, 212, 495–508. [Google Scholar] [CrossRef]
- Monroy, P.L.C.; Grefte, S.; Kuijpers-Jagtman, A.M.; Helmich, M.P.A.C.; Wagener, F.A.D.T.G.; von den Hoff, J.W. Fibrosis impairs the formation of new myofibers in the soft palate after injury. Wound Repair Regen. 2015, 23, 866–873. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Choi, J.; Kim, J.; Jo, T.; Hwang, I.; Han, K.; Jeong, W. An Evaluation of Muscle Repair Techniques: Implications in Musculoskeletal Healing and Corollaries in Oral-Facial Clefting. J. Clin. Med. 2021, 10, 4803. https://doi.org/10.3390/jcm10214803
Kim J, Choi J, Kim J, Jo T, Hwang I, Han K, Jeong W. An Evaluation of Muscle Repair Techniques: Implications in Musculoskeletal Healing and Corollaries in Oral-Facial Clefting. Journal of Clinical Medicine. 2021; 10(21):4803. https://doi.org/10.3390/jcm10214803
Chicago/Turabian StyleKim, Jaehoon, Jaehoon Choi, Junhyung Kim, Taehee Jo, Ilseon Hwang, Kihwan Han, and Woonhyeok Jeong. 2021. "An Evaluation of Muscle Repair Techniques: Implications in Musculoskeletal Healing and Corollaries in Oral-Facial Clefting" Journal of Clinical Medicine 10, no. 21: 4803. https://doi.org/10.3390/jcm10214803
APA StyleKim, J., Choi, J., Kim, J., Jo, T., Hwang, I., Han, K., & Jeong, W. (2021). An Evaluation of Muscle Repair Techniques: Implications in Musculoskeletal Healing and Corollaries in Oral-Facial Clefting. Journal of Clinical Medicine, 10(21), 4803. https://doi.org/10.3390/jcm10214803





