The Role of Prostate Combination Biopsy Consisting of Targeted and Additional Systematic Biopsy
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Study Population
2.3. Study Design
2.4. mpMRI and Biopsy Protocol
2.5. Statistical Analysis
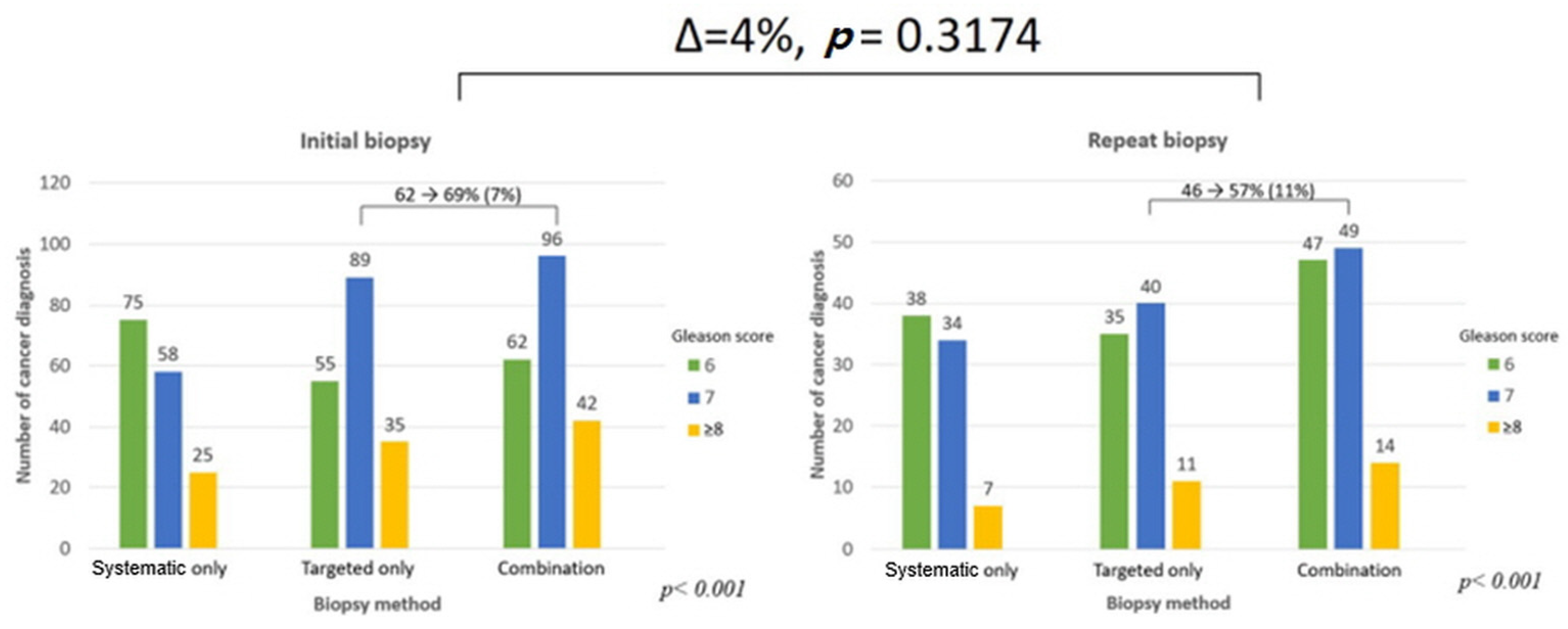
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Jung, K.-W.; Won, Y.-J.; Kong, H.-J.; Lee, E.S. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2016. Cancer Res. Treat. 2019, 51, 417–430. [Google Scholar] [CrossRef]
- Kim, E.H.; Andriole, G.L. Prostate-specific antigen-based screening: Controversy and guidelines. BMC Med. 2015, 13, 61. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.; Warde, P.; Pickles, T.; Crook, J.; Brundage, M.; Souhami, L.; Lukka, H. Pre-treatment risk stratification of prostate cancer patients: A critical review. Can. Urol. Assoc. J. 2012, 6, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Ryznarova, Z.; Keller, J.; Záleský, M.; Zachoval, R.; Čapek, V.; Malikova, H. Comparison of Prostate Imaging Reporting and Data System (PI-RADS) version 1 and version 2 and combination with apparent diffusion coefficient as a predictor of biopsy outcome. Neuroendocrinol. Lett. 2019, 40, 41–50. [Google Scholar] [PubMed]
- Washino, S.; Okochi, T.; Saito, K.; Konishi, T.; Hirai, M.; Kobayashi, Y.; Miyagawa, T. Combination of prostate imaging reporting and data system (PI-RADS) score and prostate-specific antigen (PSA) density predicts biopsy outcome in prostate biopsy naïve patients. BJU Int. 2017, 119, 225–233. [Google Scholar] [CrossRef]
- King, C.R.; McNeal, J.E.; Gill, H.; Presti, J.C. Extended prostate biopsy scheme improves reliability of Gleason grading: Implications for radiotherapy patients. Int. J. Radiat. Oncol. 2004, 59, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Filson, C.P.; Natarajan, S.; Margolis, D.J.; Huang, J.; Lieu, P.; Dorey, F.J.; Reiter, R.E.; Marks, L.S. Prostate cancer detection with magnetic resonance-ultrasound fusion biopsy: The role of systematic and targeted biopsies. Cancer 2016, 122, 884–892. [Google Scholar] [CrossRef]
- Cash, H.; Günzel, K.; Maxeiner, A.; Stephan, C.; Fischer, T.; Durmus, T.; Miller, K.; Asbach, P.; Haas, M.; Kempkensteffen, C. Prostate cancer detection on transrectal ultrasonography-guided random biopsy despite negative real-time magnetic resonance imaging/ultrasonography fusion-guided targeted biopsy: Reasons for targeted biopsy failure. BJU Int. 2015, 118, 35–43. [Google Scholar] [CrossRef]
- Kim, H.S.; Jeon, S.S.; Choi, J.D.; Kim, W.; Han, D.H.; Jeong, B.C.; Seo, S.I.; Lee, K.S.; Lee, S.W.; Lee, H.M.; et al. Detection Rates of Nonpalpable Prostate Cancer in Korean Men with Prostate-specific Antigen Levels Between 2.5 and 4.0 ng/mL. Urology 2010, 76, 919–922. [Google Scholar] [CrossRef]
- Lee, H.W.; Jeon, H.G.; Jeong, B.C.; Seo, S.I.; Jeon, S.S.; Choi, H.Y.; Lee, H.M. Comparison of Pathological and Biochemical Outcomes after Radical Prostatectomy in Korean Patients with Serum PSA Ranges. J. Korean Med. Sci. 2015, 30, 317–322. [Google Scholar] [CrossRef]
- Das, C.; Razik, A.; Sharma, S.; Verma, S. Prostate biopsy: When and how to perform. Clin. Radiol. 2019, 74, 853–864. [Google Scholar] [CrossRef]
- Das, C.J.; Razik, A.; Netaji, A.; Verma, S. Prostate MRI–TRUS fusion biopsy: A review of the state of the art procedure. Abdom. Radiol. 2020, 45, 2176–2183. [Google Scholar] [CrossRef] [PubMed]
- Lancet, T. To screen or not to screen for prostate cancer? Lancet 2012, 379, 2024. [Google Scholar] [CrossRef]
- Moyer, V.A. Screening for Prostate Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann. Intern. Med. 2012, 157, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Abrate, A.; Lazzeri, M.; Lughezzani, G.; Buffi, N.; Bini, V.; Haese, A.; De La Taille, A.; McNicholas, T.; Redorta, J.P.; Gadda, G.M.; et al. Clinical performance of the Prostate Health Index (PHI) for the prediction of prostate cancer in obese men: Data from the PROMEtheuS project, a multicentre European prospective study. BJU Int. 2015, 115, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Chiu, P.K.-F.; Ng, C.-F.; Semjonow, A.; Zhu, Y.; Vincendeau, S.; Houlgatte, A.; Lazzeri, M.; Guazzoni, G.; Stephan, C.; Haese, A.; et al. A Multicentre Evaluation of the Role of the Prostate Health Index (PHI) in Regions with Differing Prevalence of Prostate Cancer: Adjustment of PHI Reference Ranges is Needed for European and Asian Settings. Eur. Urol. 2019, 75, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Ferro, M.; Lucarelli, G.; Bruzzese, D.; Perdonà, S.; Mazzarella, C.; Perruolo, G.; Marino, A.; Cosimato, V.; Giorgio, E.; Tagliamonte, V.; et al. Improving the prediction of pathologic outcomes in patients undergoing radical prostatectomy: The value of prostate cancer antigen 3 (PCA3), prostate health index (phi) and sarcosine. Anticancer Res. 2015, 35, 1017–1023. [Google Scholar]
- Na, R.; Ye, D.; Liu, F.; Chen, H.; Qi, J.; Wu, Y.; Zhang, G.; Wang, M.; Wang, W.; Sun, J.; et al. Performance of serum prostate-specific antigen isoform [-2] proPSA (p2PSA) and the prostate health index (PHI) in a Chinese hospital-based biopsy population. Prostate 2014, 74, 1569–1575. [Google Scholar] [CrossRef]
- Kim, H.; Filson, C.; Joski, P.; von Esenwein, S.; Lipscomb, J. Association Between Online Information-Seeking and Adherence to Guidelines for Breast and Prostate Cancer Screening. Prev. Chronic Dis. 2018, 15, E45. [Google Scholar] [CrossRef]
- Kasivisvanathan, V.; Stabile, A.; Neves, J.B.; Giganti, F.; Valerio, M.; Shanmugabavan, Y.; Clement, K.D.; Sarkar, D.; Philippou, Y.; Thurtle, D.; et al. Magnetic Resonance Imaging-targeted Biopsy Versus Systematic Biopsy in the Detection of Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. 2019, 76, 284–303. [Google Scholar] [CrossRef] [PubMed]
- Miah, S.; Hosking-Jervis, F.; Connor, M.; Eldred-Evans, D.; Shah, T.T.; Arya, M.; Barber, N.; Bhardwa, J.; Bott, S.; Burke, D.; et al. A Multicentre Analysis of the Detection of Clinically Significant Prostate Cancer Following Transperineal Image-fusion Targeted and Nontargeted Systematic Prostate Biopsy in Men at Risk. Eur. Urol. Oncol. 2020, 3, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Labanaris, A.P.; Engelhard, K.; Zugor, V.; Nützel, R.; Kühn, R. Prostate cancer detection using an extended prostate biopsy schema in combination with additional targeted cores from suspicious images in conventional and functional endorectal magnetic resonance imaging of the prostate. Prostate Cancer Prostatic Dis. 2009, 13, 65–70. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rouviere, O.; Puech, P.; Renard-Penna, R.; Claudon, M.; Roy, C.; Mège-Lechevallier, F.; Decaussin-Petrucci, M.; Dubreuil-Chambardel, M.; Magaud, L.; Remontet, L.; et al. Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): A prospective, multicentre, paired diagnostic study. Lancet Oncol. 2019, 20, 100–109. [Google Scholar] [CrossRef]
- Stone, N.N.; Crawford, E.D.; Skouteris, V.M.; Arangua, P.; Metsinis, P.-M.; Lucia, M.S.; La Rosa, F.G.; Werahera, P.N. The Ratio of the Number of Biopsy Specimens to Prostate Volume (Biopsy Density) Greater Than 1.5 Improves the Prostate Cancer Detection Rate in Men Undergoing Transperineal Biopsy of the Prostate. J. Urol. 2019, 202, 264–271. [Google Scholar] [CrossRef]
- Gomez-Gomez, E.; Sorribas, S.M.; Valero-Rosa, J.; Blanca, A.; Mesa, J.; Salguero, J.; Carrasco-Valiente, J.; López-Ruiz, D.; Anglada-Curado, F. Does Adding Standard Systematic Biopsy to Targeted Prostate Biopsy in PI-RADS 3 to 5 Lesions Enhance the Detection of Clinically Significant Prostate Cancer? Should All Patients with PI-RADS 3 Undergo Targeted Biopsy? Diagnostics 2021, 11, 1335. [Google Scholar] [CrossRef]
- Mottet, N.; Bergh, R.C.N.V.D.; Briers, E.; Bourke, L.; Cornford, P.; Santis, M.D.; Gillessen, S.; Govorov, A.; Grummet, J.; Henry, A.M.; et al. EAU-ESUR-ESTRO-SIOG Guidelines on Prostate Cancer; European Association of Urology: Arnhem, The Netherlands, 2018. [Google Scholar]
- Macleod, L.C.; Yabes, J.G.; Fam, M.M.; Bandari, J.; Yu, M.; Maganty, A.; Furlan, A.; Filson, C.P.; Davies, B.J.; Jacobs, B.L. Multiparametric Magnetic Resonance Imaging Is Associated with Increased Medicare Spending in Prostate Cancer Active Surveillance. Eur. Urol. Focus 2020, 6, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Omri, N.; Kamil, M.; Alexander, K.; Alexander, K.; Edmond, S.; Ariel, Z.; David, K.; Gilad, A.E.; Azik, H. Association between PSA density and pathologically significant prostate cancer: The impact of prostate volume. Prostate 2020, 80, 1444–1449. [Google Scholar] [CrossRef]
- Massanova, M.; Robertson, S.; Barone, B.; Dutto, L.; Caputo, V.F.; Bhatt, J.R.; Ahmad, I.; Bada, M.; Obeidallah, A.; Crocetto, F. The Comparison of Imaging and Clinical Methods to Estimate Prostate Volume: A Single-Centre Retrospective Study. Urol. Int. 2021, 105, 804–810. [Google Scholar] [CrossRef]
- Xu, G.; Xiang, L.; Wu, J.; Shao, H.; Liu, H.; Ding, S.; Wu, R. The accuracy of prostate lesion localization in cognitive fusion. Clin. Hemorheol. Microcirc. 2020, 74, 223–229. [Google Scholar] [CrossRef]
- Turkay, R.; Inci, E.; Yildiz, O.; Ozgur, E.; Taşci, A.I. Cognitive Versus Magnetic Resonance-Ultrasound Fusion Prostate Biopsy: Which One Is Worthier to Perform? Ultrasound Q. 2020, 36, 345–349. [Google Scholar] [CrossRef]
- Yamada, Y.; Shiraishi, T.; Ueno, A.; Ueda, T.; Fujihara, A.; Naitoh, Y.; Hongo, F.; Ukimura, O. Magnetic resonance imaging-guided targeted prostate biopsy: Comparison between computer-software-based fusion versus cognitive fusion technique in biopsy-naïve patients. Int. J. Urol. 2019, 27, 67–71. [Google Scholar] [CrossRef]
- Rapisarda, S.; Bada, M.; Crocetto, F.; Barone, B.; Arcaniolo, D.; Polara, A.; Imbimbo, C.; Grosso, G. The role of multiparametric resonance and biopsy in prostate cancer detection: Comparison with definitive histological report after laparoscopic/robotic radical prostatectomy. Abdom. Radiol. 2020, 45, 4178–4184. [Google Scholar] [CrossRef]
- Gross, M.D.; Awamlh, B.A.H.A.; Shoag, J.E.; Mauer, E.; Banerjee, S.; Margolis, D.J.; Mosquera, J.M.; Hamilton, A.S.; Schumura, M.J.; Hu, J.C. Race and prostate imaging: Implications for targeted biopsy and image-based prostate cancer interventions. BMJ Surg. Interv. Health. Technol. 2019, 1, e000010. [Google Scholar] [CrossRef]


| PI-RADs Score | 3 (n = 199) | 4 (n = 403) | 5 (n = 109) | p |
|---|---|---|---|---|
| Age | 63.0 (58.0–69.0) | 64.0 (59.0–70.0) | 67.0 (63.0–72.0) | <0.001 |
| PSA (ng/mL) | 4.9 (3.8–6.8) | 4.8 (3.7–6.7) | 6.1 (4.6–8.6) | <0.001 |
| Biopsies (%) | 0.058 | |||
| Initial | 102 (51.3%) | 239 (59.3%) | 70 (64.2%) | |
| Repeat | 97 (48.7%) | 164 (40.7%) | 39 (35.8%) | |
| Prostate volume (mL) | 42.9 (33.8–55.3) | 37.3 (27.8–50.4) | 31.6 (24.7–44.0) | <0.001 |
| PSA density (ng/mL2) | 0.14 (0.03–0.52) | 0.16 (0.03–1.04) | 0.23 (0.05–0.80) | <0.001 |
| Days between biopsy and MRI (day) | 51.1 (23.4–79.9) | 39.5 (22.9–66.6) | 31.7 (18.7–47.2) | <0.001 |
| Total biopsy core number | 12.0 (12.0–14.0) | 12.0 (12.0–14.0) | 12.0 (12.0–13.0) | 0.069 |
| Targeted biopsy core | 3.0 (2.0–6.0) | 4.0 (2.0–6.0) | 3.0 (3.0–6.0) | 0.017 |
| Systematic biopsy core | 10.0 (6.0–12.0) | 9.0 (6.0–10.0) | 8.0 (6.0–10.0) | 0.032 |
| PI-RADs Group | 3 (n = 199) | 4 (n = 403) | 5 (n = 109) | p |
|---|---|---|---|---|
| Cancer detection core, n (%) | 33 (16.6%) | 190 (47.1%) | 88 (80.7%) | <0.001 |
| Combination core G/S, n (%) | <0.001 | |||
| 6 | 23 (69.7%) | 74 (39.2%) | 12 (13.6%) | |
| 7 | 8 (24.2%) | 85 (45.0%) | 52 (59.1%) | |
| ≥8 | 2 (6.1%) | 30 (15.9%) | 24 (27.3%) | |
| Targeted core G/S, n (%) | 0.003 | |||
| 6 | 14 (63.6%) | 58 (36.7%) | 18 (21.2%) | |
| 7 | 6 (27.3%) | 76 (48.1%) | 47 (55.3%) | |
| ≥8 | 2 (9.1%) | 24 (15.2%) | 20 (23.5%) | |
| Systematic core G/S, n (%) | <0.001 | |||
| 6 | 19 (79.2%) | 76 (54.3%) | 18 (24.7%) | |
| 7 | 5 (20.8%) | 47 (33.6%) | 40 (54.8%) | |
| ≥8 | 0 (0.0%) | 17 (12.1%) | 15 (20.5%) | |
| Targeted core upgrading (Targeted—systematic G/S) | 0.651 | |||
| Increased | 4 (30.8%) | 26 (24.1%) | 18 (25.7%) | |
| None | 9 (69.2%) | 71 (65.7%) | 42 (60.0%) | |
| Decreased | 0 (0.0%) | 11 (10.2%) | 10 (14.3%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.U.; Choi, J.; Sung, S.H.; Chung, J.H.; Song, W.; Kang, M.; Sung, H.H.; Jeong, B.C.; Seo, S.I.; Jeon, S.S.; et al. The Role of Prostate Combination Biopsy Consisting of Targeted and Additional Systematic Biopsy. J. Clin. Med. 2021, 10, 4804. https://doi.org/10.3390/jcm10214804
Lee CU, Choi J, Sung SH, Chung JH, Song W, Kang M, Sung HH, Jeong BC, Seo SI, Jeon SS, et al. The Role of Prostate Combination Biopsy Consisting of Targeted and Additional Systematic Biopsy. Journal of Clinical Medicine. 2021; 10(21):4804. https://doi.org/10.3390/jcm10214804
Chicago/Turabian StyleLee, Chung Un, Joongwon Choi, Si Hyun Sung, Jae Hoon Chung, Wan Song, Minyong Kang, Hyun Hwan Sung, Byong Chang Jeong, Seong Il Seo, Seong Soo Jeon, and et al. 2021. "The Role of Prostate Combination Biopsy Consisting of Targeted and Additional Systematic Biopsy" Journal of Clinical Medicine 10, no. 21: 4804. https://doi.org/10.3390/jcm10214804
APA StyleLee, C. U., Choi, J., Sung, S. H., Chung, J. H., Song, W., Kang, M., Sung, H. H., Jeong, B. C., Seo, S. I., Jeon, S. S., Lee, H. M., & Jeon, H. G. (2021). The Role of Prostate Combination Biopsy Consisting of Targeted and Additional Systematic Biopsy. Journal of Clinical Medicine, 10(21), 4804. https://doi.org/10.3390/jcm10214804






