Ultrasound-Guided Continuous Bilateral Erector Spinae Plane Blocks Are Associated with Reduced Opioid Consumption and Length of Stay for Open Cardiac Surgery: A Retrospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subject Selection
2.2. Data Collection
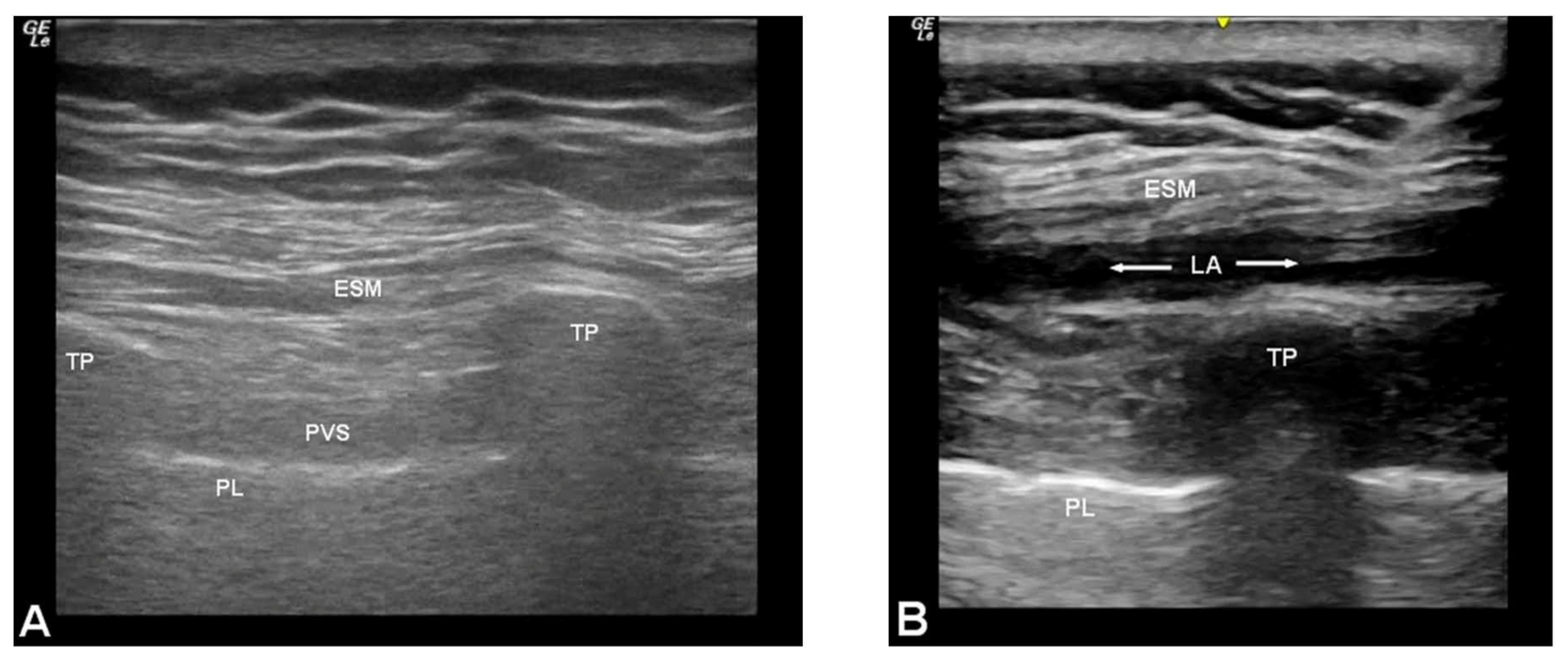
2.3. Technique for Erector Spinae Plane Blocks
2.4. Outcomes and Statistical Analysis
3. Results
4. Discussion
5. Conclusions
- Reduced opioid consumption by 238 mg oral morphine equivalents (52%);
- Reduced time to extubation by 130 min;
- Reduced intensive care unit LOS by 23.3 h;
- Reduced overall hospital LOS by 2.1 days.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kwanten, L.E.; O’Brien, B.; Anwar, S. Opioid-based anesthesia and analgesia for adult cardiac surgery: History and narrative review of the literature. J. Cardiothorac. Vasc. Anesth. 2019, 33, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Shafi, S.; Collinsworth, A.W.; Copeland, L.A.; Ogola, G.O.; Qiu, T.; Kouznetsova, M.; Liao, I.C.; Mears, N.; Pham, A.T.; Wan, G.J.; et al. Association of opioid-related adverse drug events with clinical and cost outcomes among surgical patients in a large integrated health care delivery system. JAMA Surg. 2018, 153, 757–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, H.; Soneji, N.; Ko, D.T.; Yun, L.; Wijeysundera, D. Rates and risk factors for prolonged opioid use after major surgery: Population based cohort study. BMJ 2014, 348, g1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clement, K.C.; Canner, J.K.; Lawton, J.S.; Whitman, G.J.; Grant, M.C.; Sussman, M.S. Predictors of new persistent opioid use after coronary artery bypass grafting. J. Thorac. Cardiovasc. Surg. 2020, 160, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.L.; King, A.B.; Geiger, T.M.; Grant, M.C.; Grocott, M.P.; Gupta, R.; Hah, J.M.; Miller, T.E.; Shaw, A.D.; Gan, T.J.; et al. American society for enhanced recovery and perioperative quality initiative joint consensus statement on perioperative opioid minimization in opioid-naïve patients. Anesth. Analg. 2019, 129, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Kirksey, M.A.; Duong, S.; Wu, C.L. A review of opioid-sparing modalities in perioperative pain management: Methods to decrease opioid use postoperatively. Anesth. Analg. 2017, 125, 1749–1760. [Google Scholar] [CrossRef] [PubMed]
- Perkins, F.M.; Kehlet, H. Chronic pain as an outcome of surgery. A review of predictive factors. Anesthesiology 2000, 93, 1123–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caruso, T.J.; Lawrence, K.; Tsui, B.C.H. Regional anesthesia for cardiac surgery. Curr. Opin. Anaesthesiol. 2019, 32, 674–682. [Google Scholar] [CrossRef]
- Forero, M.; Adhikary, S.D.; Lopez, H.; Tsui, C.; Chin, K.J. The erector spinae plane block: A novel analgesic technique in thoracic neuropathic pain. Reg. Anesth. Pain Med. 2016, 41, 621–627. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, D.L.; Manickam, B. Erector spinae plane block for pain relief in rib fractures. Br. J. Anaesth. 2017, 118, 474–475. [Google Scholar] [CrossRef] [Green Version]
- Chin, K.J.; Adhikary, S.; Sarwani, N.; Forero, M. The analgesic efficacy of pre-operative bilateral erector spinae plane (ESP) blocks in patients having ventral hernia repair. Anaesthesia 2017, 72, 452–460. [Google Scholar] [CrossRef] [Green Version]
- Ohgoshi, Y.; Ikeda, T.; Kurahashi, K. Continuous erector spinae plane block provides effective perioperative analgesia for breast reconstruction using tissue expanders: A report of two cases. J. Clin. Anesth. 2018, 44, 1–2. [Google Scholar] [CrossRef]
- Kot, P.; Rodriguez, P.; Granell, M.; Cano, B.; Rovira, L.; Morales, J.; Broseta, A.; De Andrés, J. The erector spinae plane block: A narrative review. Korean J. Anesthesiol. 2019, 72, 209–220. [Google Scholar] [CrossRef] [Green Version]
- Krishna, S.N.; Chauhan, S.; Bhoi, D.; Kaushal, B.; Hasija, S.; Sangdup, T.; Bisoi, A.K. Bilateral erector spinae plane block for acute post-surgical pain in adult cardiac surgical patients: A randomized controlled trial. J. Cardiothorac. Vasc. Anesth. 2019, 33, 368–375. [Google Scholar] [CrossRef]
- Singh, N.G.; Nagaraja, P.S.; Ragavendran, S.; Asai, O.; Bhavya, G.; Manjunath, N.; Rajesh, K. Comparison of continuous thoracic epidural analgesia with bilateral erector spinae plane block for perioperative pain management in cardiac surgery. Ann. Card. Anaesth. 2018, 21, 323–327. [Google Scholar] [CrossRef]
- Macaire, P.; Ho, N.; Nguyen, T.; Nguyen, B.; Vu, V.; Quach, C.; Roques, V.; Capdevila, X. Ultrasound-guided continuous thoracic erector spinae plane block within an enhanced recovery program is associated with decreased opioid consumption and improved patient postoperative rehabilitation after open cardiac surgery- a patient-matched, controlled before-and-after Study. J. Cardiothorac. Vasc. Anesth. 2019, 33, 1659–1667. [Google Scholar]
- Nielsen, S.; Degenhardt, L.; Hoban, B.; Gisev, N. Comparing Opioids: A Guide to Estimating Oral Morphine Equivalents (OME) in Research; Technical Report No. 329; National Drug and Alcohol Research Centre, University of NSW: Sydney, Australia, 2014; Available online: https://ndarc.med.unsw.edu.au/sites/default/files/ndarc/resources/TR.329.pdf (accessed on 15 April 2020).
- Centers for Disease Control and Prevention. Calculating Total Daily Dose of Opioids for Safer Dosage. Updated August 28; 2019. Available online: https://www.cdc.gov/drugoverdose/pdf/calculating_total_daily_dose-a.pdf (accessed on 15 April 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org/ (accessed on 4 August 2020).
- Baxter, R.; Squiers, J.; Conner, W.; Kent, M.; Fann, J.; Lobdell, K.; DiMaio, J.M. Enhanced recovery after surgery: A narrative review of its application in cardiac surgery. Ann. Thorac. Surg. 2020, 109, 1937–1945. [Google Scholar] [CrossRef]
- Williams, J.B.; McConnell, G.; Allender, J.E.; Woltz, P.; Kane, K.; Smith, P.K.; Engelman, D.; Bradford, W.T. One-year results from the first US-based enhanced recovery after cardiac surgery (ERAS Cardiac) program. J. Thorac. Cardiovasc. Surg. 2019, 157, 1881–1888. [Google Scholar] [CrossRef]
- Noss, C.; Anderson, K.J.; Gregory, A.J. Erector spinae plane block for open-heart surgery: A potential tool for improved analgesia. J. Cardiothorac. Vasc. Anesth. 2019, 33, 376–377. [Google Scholar] [CrossRef] [Green Version]
- Ilfeld, B.M.; Vandenborne, K.; Duncan, P.W.; Sessler, D.I.; Enneking, F.K.; Shuster, J.J.; Theriaque, D.W.; Chmielewski, T.L.; Spadoni, E.H.; Wright, T.W. Ambulatory continuous interscalene nerve blocks decrease the time to discharge readiness after total shoulder arthroplasty: A randomized, triple-masked, placebo-controlled study. Anesthesiology 2006, 105, 999–1007. [Google Scholar] [CrossRef]
- Ilfeld, B.M.; Mariano, E.R.; Girard, P.J.; Loland, V.J.; Meyer, R.S.; Donovan, J.F.; Pugh, G.A.; Le, L.T.; Sessler, D.I.; Shuster, J.J.; et al. A multicenter, randomized, triple-masked, placebo-controlled trial of the effect of ambulatory continuous femoral nerve blocks on discharge-readiness following total knee arthroplasty in patients on general orthopaedic wards. Pain 2010, 150, 477–484. [Google Scholar] [CrossRef] [Green Version]
- Ilfeld, B.M.; Madison, S.J.; Suresh, P.J.; Sandhu, N.S.; Kormylo, N.J.; Malhotra, N.; Loland, V.J.; Wallace, M.S.; Mascha, E.J.; Xu, Z.; et al. Persistent postmastectomy pain and pain-related physical and emotional functioning with and without a continuous paravertebral nerve block: A prospective 1-year follow-up assessment of a randomized, triple-masked, placebo-controlled study. Ann. Surg. Oncol. 2015, 22, 2017–2025. [Google Scholar] [CrossRef] [Green Version]
- Verret, M.; Lauzier, F.; Zarychanski, R.; Savard, X.; Cossi, M.-J.; Pinard, A.M.; Leblanc, G.; Turgeon, A.F. Perioperative use of gabapentinoids for the management of postoperative acute pain: A systematic review and meta-analysis. Anesthesiology 2020, 133, 265–279. [Google Scholar] [CrossRef]


| Control (n = 50) | Case (n = 28) | Mean Difference | p-Value | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | (95% CI) | ||
| Age | 65.8 ± 7.7 | 65.6 ± 8.5 | 0.2 (−3.7 to 4.1) | 0.921 |
| Male (%) | 38 (76%) | 21 (75%) | - | 1.000 |
| BMI | 31.1 ± 5.3 | 29.9 ± 4.7 | 1.2 (−1.2 to 3.5) | 0.326 |
| Smoking status | ||||
| Never smoker (%) | 23 (46%) | 13 (46%) | - | 1.000 |
| Former smoker (%) | 23 (46%) | 12 (43%) | - | 0.976 |
| Current smoker (%) | 4 (8%) | 3 (11%) | - | 1.000 |
| Left ventricular ejection fraction | 52 ± 8 | 54 ± 8 | 2 (−5.8 to 1.6) | 0.268 |
| Cross-clamp time, minutes | 78 ± 23 | 81 ± 24 | 3 (−14 to 8) | 0.598 |
| Cardiopulmonary bypass time, minutes | 98 ± 25 | 102 ± 25 | 4 (−15 to 8) | 0.540 |
| Procedure time, minutes | 235 ± 45 | 235 ± 41 | 0 (−20 to 20) | 0.997 |
| Control | Case | Mean Difference | p-Value | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | (95% CI) | ||
| Total OME, mg | 461 ± 185 | 224 ± 108 | 237 (172 to 304) | <0.001 |
| Total acetaminophen, mg | 9947 ± 3086 | 9370 ± 4861 | 577 (−1480 to 2633) | 0.574 |
| Intraoperative ketamine used | 3 (6%) | 0 (0%) | - | 0.479 |
| Postoperative OME, mg | 238 ± 154 | 190 ± 104 | 48 (−10 to 107) | 0.104 |
| Postoperative gabapentin, mg | 1708 ± 827 | 1375 ± 480 | 333 (38 to 628) | 0.027 |
| Postoperative NSAIDS used | 8 (16%) | 3 (10.7%) | - | 0.761 |
| Control | Case | Median Difference | p-Value | |
| Median (IQR) | Median (IQR) | (95% CI) | ||
| Intraoperative OME, mg | 223.6 (188–259) | 34.1 (27.5–40.7) | −165 (−170 to −155) | <0.001 |
| Highest reported pain score, POD #0 | 8 (7.0–9.0) | 8 (7.0–9.5) | 3.6 × 10−5 (−1.9 × 10−5 to 1.0) | 0.246 |
| Highest reported pain score, POD #1 | 8 (7.0–9.75) | 8 (7.0–9.25) | −6.6 × 10−5 (−1.0 to 1.0) | 0.949 |
| Highest reported pain score, POD #2 | 7 (5.25–8.0) | 6 (5.0–8.0) | −4.6 × 10−5 (−1.0 to 1.0) | 0.442 |
| Highest reported pain score, POD #3 | 6 (3.25–7.0) | 4.5 (3.0–6.0) | −1.0 (−2.0 to 1.6 × 10−5) | 0.139 |
| Highest reported pain score, POD #4 | 5 (0.0–7.0) | 4 (0.0–6.0) | −2.6 × 10−5 (−2.0 to 8.9 × 10−6) | 0.423 |
| Highest reported pain score, POD #5 | 4 (0.0–6.0) | 5 (0.0–6.25) | 7.0 × 10−5 (−1.0 to 3.0) | 0.637 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaughan, B.N.; Bartone, C.L.; McCarthy, C.M.; Answini, G.A.; Hurford, W.E. Ultrasound-Guided Continuous Bilateral Erector Spinae Plane Blocks Are Associated with Reduced Opioid Consumption and Length of Stay for Open Cardiac Surgery: A Retrospective Cohort Study. J. Clin. Med. 2021, 10, 5022. https://doi.org/10.3390/jcm10215022
Vaughan BN, Bartone CL, McCarthy CM, Answini GA, Hurford WE. Ultrasound-Guided Continuous Bilateral Erector Spinae Plane Blocks Are Associated with Reduced Opioid Consumption and Length of Stay for Open Cardiac Surgery: A Retrospective Cohort Study. Journal of Clinical Medicine. 2021; 10(21):5022. https://doi.org/10.3390/jcm10215022
Chicago/Turabian StyleVaughan, Brian N., Cheryl L. Bartone, Catherine M. McCarthy, Geoffrey A. Answini, and William E. Hurford. 2021. "Ultrasound-Guided Continuous Bilateral Erector Spinae Plane Blocks Are Associated with Reduced Opioid Consumption and Length of Stay for Open Cardiac Surgery: A Retrospective Cohort Study" Journal of Clinical Medicine 10, no. 21: 5022. https://doi.org/10.3390/jcm10215022






