The Added Value of Subcutaneous Peripheral Nerve Field Stimulation Combined with SCS, as Salvage Therapy, for Refractory Low Back Pain Component in Persistent Spinal Pain Syndrome Implanted Patients: A Randomized Controlled Study (CUMPNS Study) Based on 3D-Mapping Composite Pain Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Selection
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Procedures and Additional PNfS Implantation
2.4. Study Outcomes
2.5. Statistical Analysis
2.5.1. Sample Size
2.5.2. Statistical Methods
3. Results
3.1. Primary Endpoint
3.2. Secondary Endpoints
3.2.1. Between-Group Analyses
3.2.2. Within-Group Analyses
3.3. Paired Comparisons of “SCS + PNfS” and “SCS Only” with a 6- and 12-Month Follow-Up
3.4. Safety
4. Discussion
4.1. Back Pain: A Real Target for Neurostimulation? Episode 2
4.2. Mechanical and Neuropathic Back Pain Component Typology Patient Characterization Suggests a Specific Role of PNfS on Mechanical Back Pain Features, as a Synergistic Approach
4.3. The Predictive Role of TENS before Considering Implanted Neurostimulation, with a Focus on PNfS
4.4. Technical Considerations to Take into Account, When Converting a Patient Already Implanted with SCS to SCS + PNfS
4.5. Study Limitations
4.5.1. PNfS and SCS Compatibility
4.5.2. Methodological Limitations
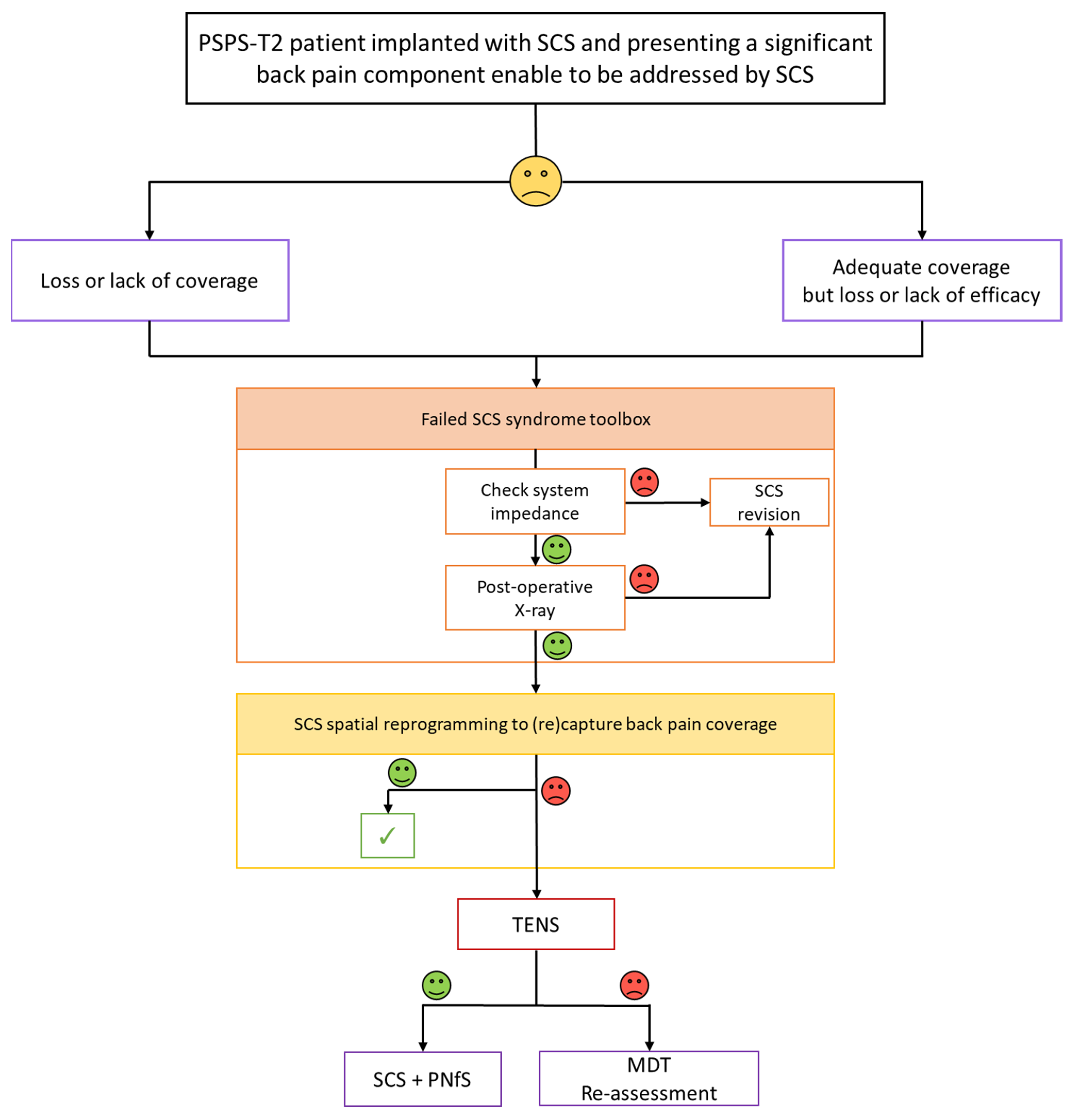
4.6. Proposal of a Salvage SCS Algorithm for Back Pain Component
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rigoard, P.; Basu, S.; Desai, M.; Taylor, R.; Annemans, L.; Tan, Y.; Johnson, M.J.; Van den Abeele, C.; North, R.; PROMISE Study Group. Multicolumn Spinal Cord Stimulation for Predominant Back Pain in Failed Back Surgery Syndrome Patients: A Multicenter Randomized Controlled Trial. Pain 2019, 160, 1410–1420. [Google Scholar] [CrossRef] [PubMed]
- Rigoard, P.; Billot, M.; Ingrand, P.; Durand-Zaleski, I.; Roulaud, M.; Peruzzi, P.; Dam Hieu, P.; Voirin, J.; Raoul, S.; Page, P.; et al. How Should We Use Multicolumn Spinal Cord Stimulation to Optimize Back Pain Spatial Neural Targeting? A Prospective, Multicenter, Randomized, Double-Blind, Controlled Trial (ESTIMET Study). Neuromodulation J. Int. Neuromodulation Soc. 2021, 24, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Taylor, R.S.; Jacques, L.; Eldabe, S.; Meglio, M.; Molet, J.; Thomson, S.; O’Callaghan, J.; Eisenberg, E.; Milbouw, G.; et al. Spinal Cord Stimulation versus Conventional Medical Management for Neuropathic Pain: A Multicentre Randomised Controlled Trial in Patients with Failed Back Surgery Syndrome. Pain 2007, 132, 179–188. [Google Scholar] [CrossRef]
- North, R.B.; Kidd, D.H.; Farrokhi, F.; Piantadosi, S.A. Spinal Cord Stimulation versus Repeated Lumbosacral Spine Surgery for Chronic Pain: A Randomized, Controlled Trial. Neurosurgery 2005, 56, 98–106, discussion 106–107. [Google Scholar] [CrossRef] [Green Version]
- Goudman, L.; De Smedt, A.; Eldabe, S.; Rigoard, P.; Linderoth, B.; De Jaeger, M.; Moens, M.; Consortium, D. High-Dose Spinal Cord Stimulation for Patients with Failed Back Surgery Syndrome: A Multicenter Effectiveness and Prediction Study. Pain 2021, 162, 582–590. [Google Scholar] [CrossRef]
- Deer, T.R.; Grider, J.S.; Lamer, T.J.; Pope, J.E.; Falowski, S.; Hunter, C.W.; Provenzano, D.A.; Slavin, K.V.; Russo, M.; Carayannopoulos, A.; et al. A Systematic Literature Review of Spine Neurostimulation Therapies for the Treatment of Pain. Pain Med. Malden Mass 2020, 21, 1421–1432. [Google Scholar] [CrossRef]
- Rigoard, P.; Gatzinsky, K.; Deneuville, J.-P.; Duyvendak, W.; Naiditch, N.; Van Buyten, J.-P.; Eldabe, S. Optimizing the Management and Outcomes of Failed Back Surgery Syndrome: A Consensus Statement on Definition and Outlines for Patient Assessment. Pain Res. Manag. 2019, 2019, 3126464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Kaisy, A.; Pang, D.; Desai, M.J.; Pries, P.; North, R.; Taylor, R.S.; Mc Cracken, L.; Rigoard, P. Failed Back Surgery Syndrome: Who Has Failed? Neurochirurgie 2015, 61 (Suppl. S1), S6–S14. [Google Scholar] [CrossRef]
- Blond, S.; Mertens, P.; David, R.; Roulaud, M.; Rigoard, P. From “Mechanical” to “Neuropathic” Back Pain Concept in FBSS Patients. A Systematic Review Based on Factors Leading to the Chronification of Pain (Part C). Neurochirurgie 2015, 61 (Suppl. S1), S45–S56. [Google Scholar] [CrossRef]
- Christelis, N.; Simpson, B.; Russo, M.; Stanton-Hicks, M.; Barolat, G.; Thomson, S.; Schug, S.; Baron, R.; Buchser, E.; Carr, D.B.; et al. Persistent Spinal Pain Syndrome: A Proposal for Failed Back Surgery Syndrome and ICD-11. Pain Med. Off. J. Am. Acad. Pain Med. 2021, 22, 807–818. [Google Scholar] [CrossRef]
- Taylor, R.S.; Desai, M.J.; Rigoard, P.; Taylor, R.J. Predictors of Pain Relief Following Spinal Cord Stimulation in Chronic Back and Leg Pain and Failed Back Surgery Syndrome: A Systematic Review and Meta-Regression Analysis. Pain Pract. 2014, 14, 489–505. [Google Scholar] [CrossRef] [Green Version]
- Taylor, R.S.; Van Buyten, J.-P.; Buchser, E. Spinal Cord Stimulation for Chronic Back and Leg Pain and Failed Back Surgery Syndrome: A Systematic Review and Analysis of Prognostic Factors. Spine 2005, 30, 152–160. [Google Scholar] [CrossRef]
- Waszak, P.M.; Modrić, M.; Paturej, A.; Malyshev, S.M.; Przygocka, A.; Garnier, H.; Szmuda, T. Spinal Cord Stimulation in Failed Back Surgery Syndrome: Review of Clinical Use, Quality of Life and Cost-Effectiveness. Asian Spine J. 2016, 10, 1195–1204. [Google Scholar] [CrossRef]
- Deer, T.R.; Esposito, M.F.; McRoberts, W.P.; Grider, J.S.; Sayed, D.; Verrills, P.; Lamer, T.J.; Hunter, C.W.; Slavin, K.V.; Shah, J.M.; et al. A Systematic Literature Review of Peripheral Nerve Stimulation Therapies for the Treatment of Pain. Pain Med. 2020, 21, 1590–1603. [Google Scholar] [CrossRef]
- McRoberts, W.P.; Wolkowitz, R.; Meyer, D.J.; Lipov, E.; Joshi, J.; Davis, B.; Cairns, K.D.; Barolat, G. Peripheral Nerve Field Stimulation for the Management of Localized Chronic Intractable Back Pain: Results from a Randomized Controlled Study. Neuromodulation J. Int. Neuromodulation Soc. 2013, 16, 565–574, discussion 574–575. [Google Scholar] [CrossRef] [PubMed]
- Eldabe, S.S.; Taylor, R.S.; Goossens, S.; Bouche, B.; Gültuna, I.; Green, C.; Tinsley, J.; Luyet, P.-P.; Buchser, E. A Randomized Controlled Trial of Subcutaneous Nerve Stimulation for Back Pain due to Failed Back Surgery Syndrome: The SubQStim Study. Neuromodulation J. Int. Neuromodulation Soc. 2019, 22, 519–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kloimstein, H.; Likar, R.; Kern, M.; Neuhold, J.; Cada, M.; Loinig, N.; Ilias, W.; Freundl, B.; Binder, H.; Wolf, A.; et al. Peripheral Nerve Field Stimulation (PNFS) in Chronic Low Back Pain: A Prospective Multicenter Study. Neuromodulation J. Int. Neuromodulation Soc. 2014, 17, 180–187. [Google Scholar] [CrossRef]
- van Gorp, E.-J.J.A.A.; Teernstra, O.P.M.; Gültuna, I.; Hamm-Faber, T.; Bürger, K.; Schapendonk, R.; Willem Kallewaard, J.; Spincemaille, G.; Vonhögen, L.H.; Hendriks, J.C.M.; et al. Subcutaneous Stimulation as ADD-ON Therapy to Spinal Cord Stimulation Is Effective in Treating Low Back Pain in Patients With Failed Back Surgery Syndrome: A Multicenter Randomized Controlled Trial. Neuromodulation J. Int. Neuromodulation Soc. 2016, 19, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Schug, S.A.; Lavandʼhomme, P.; Barke, A.; Korwisi, B.; Rief, W.; Treede, R.-D. IASP Taskforce for the Classification of Chronic Pain The IASP Classification of Chronic Pain for ICD-11: Chronic Postsurgical or Posttraumatic Pain. Pain 2019, 160, 45–52. [Google Scholar] [CrossRef]
- Bouhassira, D.; Attal, N.; Fermanian, J.; Alchaar, H.; Gautron, M.; Masquelier, E.; Rostaing, S.; Lanteri-Minet, M.; Collin, E.; Grisart, J.; et al. Development and Validation of the Neuropathic Pain Symptom Inventory. Pain 2004, 108, 248–257. [Google Scholar] [CrossRef]
- Rigoard, P.; Nivole, K.; Blouin, P.; Monlezun, O.; Roulaud, M.; Lorgeoux, B.; Bataille, B.; Guetarni, F. A Novel, Objective, Quantitative Method of Evaluation of the Back Pain Component Using Comparative Computerized Multi-Parametric Tactile Mapping before/after Spinal Cord Stimulation and Database Analysis: The “Neuro-Pain’t” Software. Neurochirurgie 2015, 61 (Suppl. S1), S99–S108. [Google Scholar] [CrossRef]
- Philippe, R.; Farid, G. Mapping method and system, method and system for evaluating the efficacy of medullary simulation. International Patent Application No. PCT/EP2014/067231, 19 March 2015. [Google Scholar]
- Philippe, R.; Farid, G. Device and method for evaluating analgesic neurostimulation devices. International Patent Application No. PCT/FR2014/000 186, 19 March 2015. [Google Scholar]
- Philippe, R.; Farid, G. Device and method for evaluating and monitoring physical pain. International Patent Application No. PCT/FR2014/000/187, 19 March 2015. [Google Scholar]
- Fairbank, J.C.; Couper, J.; Davies, J.B.; O’Brien, J.P. The Oswestry Low Back Pain Disability Questionnaire. Physiotherapy 1980, 66, 271–273. [Google Scholar] [PubMed]
- Herdman, M.; Gudex, C.; Lloyd, A.; Janssen, M.; Kind, P.; Parkin, D.; Bonsel, G.; Badia, X. Development and Preliminary Testing of the New Five-Level Version of EQ-5D (EQ-5D-5L). Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil. 2011, 20, 1727–1736. [Google Scholar] [CrossRef] [Green Version]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Harden, R.N.; Weinland, S.R.; Remble, T.A.; Houle, T.T.; Colio, S.; Steedman, S.; Kee, W.G. Medication Quantification Scale Version III: Update in Medication Classes and Revised Detriment Weights by Survey of American Pain Society Physicians. J. Pain 2005, 6, 364–371. [Google Scholar] [CrossRef]
- Rigoard, P.; Delmotte, A.; D’Houtaud, S.; Misbert, L.; Diallo, B.; Roy-Moreau, A.; Durand, S.; Royoux, S.; Giot, J.-P.; Bataille, B. Back Pain: A Real Target for Spinal Cord Stimulation? Neurosurgery 2012, 70, 574–584, discussion 584–585. [Google Scholar] [CrossRef]
- Duarte, R.V.; McNicol, E.; Colloca, L.; Taylor, R.S.; North, R.B.; Eldabe, S. Randomized Placebo-/Sham-Controlled Trials of Spinal Cord Stimulation: A Systematic Review and Methodological Appraisal. Neuromodulation J. Int. Neuromodulation Soc. 2019, 23, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Sator-Katzenschlager, S.; Fiala, K.; Kress, H.G.; Kofler, A.; Neuhold, J.; Kloimstein, H.; Ilias, W.; Mozes-Balla, E.-M.; Pinter, M.; Loining, N.; et al. Subcutaneous Target Stimulation (STS) in Chronic Noncancer Pain: A Nationwide Retrospective Study. Pain Pract. Off. J. World Inst. Pain 2010, 10, 279–286. [Google Scholar] [CrossRef] [PubMed]
- van Gorp, E.-J.; Eldabe, S.; Slavin, K.V.; Rigoard, P.; Goossens, S.; Mielke, D.; Barolat, G.; Declerck, C.; Gilmore, C.; Gültuna, I.; et al. Peripheral Nerve Field Stimulation for Chronic Back Pain: Therapy Outcome Predictive Factors. Pain Pract. Off. J. World Inst. Pain 2020, 20, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Assaker, R.; Zairi, F. Failed Back Surgery Syndrome: To Re-Operate or Not to Re-Operate? A Retrospective Review of Patient Selection and Failures. Neurochirurgie 2015, 61 (Suppl. S1), S77–S82. [Google Scholar] [CrossRef] [PubMed]
- Mertens, P.; Blond, S.; David, R.; Rigoard, P. Anatomy, Physiology and Neurobiology of the Nociception: A Focus on Low Back Pain (Part A). Neurochirurgie 2015, 61 (Suppl. S1), S22–S34. [Google Scholar] [CrossRef]
- Rigoard, P.; Blond, S.; David, R.; Mertens, P. Pathophysiological Characterisation of Back Pain Generators in Failed Back Surgery Syndrome (Part B). Neurochirurgie 2015, 61 (Suppl. S1), S35–S44. [Google Scholar] [CrossRef]
- Rigoard, P.; Roulaud, M.; Goudman, L.; Ounajim, A.; Adjali, N.; Voirin, J.; Perruchoud, C.; Bouche, B.; Page, P.; Guillevin, R.; et al. Comparison of Spinal Cord Stimulation vs. Dorsal Root Ganglion Stimulation vs. Association of Both in Patients with Refractory Chronic Back and/or Lower Limb Neuropathic Pain: An International, Prospective, Randomized, Double Blinded, Crossover Protocol Trial (BOOST-DRG Study). 2021; Submitted. Available online: https://clinicaltrials.gov/ct2/show/NCT04852107 (accessed on 31 August 2021).
- Deckers, K.; De Smedt, K.; Mitchell, B.; Vivian, D.; Russo, M.; Georgius, P.; Green, M.; Vieceli, J.; Eldabe, S.; Gulve, A.; et al. New Therapy for Refractory Chronic Mechanical Low Back Pain-Restorative Neurostimulation to Activate the Lumbar Multifidus: One Year Results of a Prospective Multicenter Clinical Trial. Neuromodulation J. Int. Neuromodulation Soc. 2018, 21, 48–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, B.; Deckers, K.; De Smedt, K.; Russo, M.; Georgius, P.; Green, M.; Gulve, A.; van Buyten, J.-P.; Smet, I.; Mehta, V.; et al. Durability of the Therapeutic Effect of Restorative Neurostimulation for Refractory Chronic Low Back Pain. Neuromodulation J. Int. Neuromodulation Soc. 2021, 24, 1024–1032. [Google Scholar] [CrossRef]
- West, S.J.; Bannister, K.; Dickenson, A.H.; Bennett, D.L. Circuitry and Plasticity of the Dorsal Horn—Toward a Better Understanding of Neuropathic Pain. Neuroscience 2015, 300, 254–275. [Google Scholar] [CrossRef] [PubMed]
- Deyo, R.A.; Walsh, N.E.; Martin, D.C.; Schoenfeld, L.S.; Ramamurthy, S. A Controlled Trial of Transcutaneous Electrical Nerve Stimulation (TENS) and Exercise for Chronic Low Back Pain. N. Engl. J. Med. 1990, 322, 1627–1634. [Google Scholar] [CrossRef]
- Rushton, D.N. Electrical Stimulation in the Treatment of Pain. Disabil. Rehabil. 2002, 24, 407–415. [Google Scholar] [CrossRef]
- Gibson, W.; Wand, B.M.; Meads, C.; Catley, M.J.; O’Connell, N.E. Transcutaneous Electrical Nerve Stimulation (TENS) for Chronic Pain—An Overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2019, 4, CD011890. [Google Scholar] [CrossRef] [PubMed]
- Gibson, W.; Wand, B.M.; O’Connell, N.E. Transcutaneous Electrical Nerve Stimulation (TENS) for Neuropathic Pain in Adults. Cochrane Database Syst. Rev. 2017, 9, CD011976. [Google Scholar] [CrossRef] [Green Version]
- Almeida, C.C.; de Silva, V.Z.M.; da Júnior, G.C.; Liebano, R.E.; Durigan, J.L.Q. Transcutaneous Electrical Nerve Stimulation and Interferential Current Demonstrate Similar Effects in Relieving Acute and Chronic Pain: A Systematic Review with Meta-Analysis. Braz. J. Phys. Ther. 2018, 22, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-C.; Weng, P.-W.; Chen, C.-H.; Huang, Y.-Y.; Tsuang, Y.-H.; Chiang, C.-J. Literature Review and Meta-Analysis of Transcutaneous Electrical Nerve Stimulation in Treating Chronic Back Pain. Reg. Anesth. Pain Med. 2018, 43, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Khadilkar, A.; Milne, S.; Brosseau, L.; Wells, G.; Tugwell, P.; Robinson, V.; Shea, B.; Saginur, M. Transcutaneous Electrical Nerve Stimulation for the Treatment of Chronic Low Back Pain: A Systematic Review. Spine 2005, 30, 2657–2666. [Google Scholar] [CrossRef] [PubMed]
- Mathew, L.; Winfree, C.; Miller-Saultz, D.; Sonty, N. Transcutaneous Electrical Nerve Stimulator Trial May Be Used as a Screening Tool Prior to Spinal Cord Stimulator Implantation. Pain 2010, 150, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Melzack, R.; Wall, P.D. Pain Mechanisms: A New Theory. Science 1965, 150, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Eldabe, S.; Buchser, E.; Duarte, R.V. Complications of Spinal Cord Stimulation and Peripheral Nerve Stimulation Techniques: A Review of the Literature. Pain Med. Malden Mass 2016, 17, 325–336. [Google Scholar] [CrossRef] [Green Version]
- North, R.; Desai, M.J.; Vangeneugden, J.; Raftopoulos, C.; Van Havenbergh, T.; Deruytter, M.; Remacle, J.-M.; Shipley, J.; Tan, Y.; Johnson, M.J.; et al. Postoperative Infections Associated with Prolonged Spinal Cord Stimulation Trial Duration (PROMISE RCT). Neuromodulation J. Int. Neuromodulation Soc. 2020, 23, 620–625. [Google Scholar] [CrossRef] [Green Version]
- Law, J.D. Targeting a Spinal Stimulator to Treat the “Failed Back Surgery Syndrome”. Appl. Neurophysiol. 1987, 50, 437–438. [Google Scholar] [CrossRef]
- Holsheimer, J.; Barolat, G. Spinal Geometry and Paresthesia Coverage in Spinal Cord Stimulation. Neuromodulation J. Int. Neuromodulation Soc. 1998, 1, 129–136. [Google Scholar] [CrossRef]
- North, R.B.; Kidd, D.H.; Olin, J.C.; Sieracki, J.M. Spinal Cord Stimulation Electrode Design: Prospective, Randomized, Controlled Trial Comparing Percutaneous and Laminectomy Electrodes-Part I: Technical Outcomes. Neurosurgery 2002, 51, 381–389, discussion 389–390. [Google Scholar]
- Rigoard, P.; Jacques, L.; Delmotte, A.; Poon, K.; Munson, R.; Monlezun, O.; Roulaud, M.; Prevost, A.; Guetarni, F.; Bataille, B.; et al. An Algorithmic Programming Approach for Back Pain Symptoms in Failed Back Surgery Syndrome Using Spinal Cord Stimulation with a Multicolumn Surgically Implanted Epidural Lead: A Multicenter International Prospective Study. Pain Pract. Off. J. World Inst. Pain 2015, 15, 195–207. [Google Scholar] [CrossRef]
- Veizi, E.; Hayek, S.M.; North, J.; Brent Chafin, T.; Yearwood, T.L.; Raso, L.; Frey, R.; Cairns, K.; Berg, A.; Brendel, J.; et al. Spinal Cord Stimulation (SCS) with Anatomically Guided (3D) Neural Targeting Shows Superior Chronic Axial Low Back Pain Relief Compared to Traditional SCS-LUMINA Study. Pain Med. Malden Mass 2017, 18, 1534–1548. [Google Scholar] [CrossRef] [Green Version]
- Le Tutor, T.; Collin, S.; Elhouari, K.; Ye, W.; Germaneau, A.; Caillé, L.; Roulaud, M.; Ounajim, A.; Billot, M.; North, R.; et al. The Challenge of Spinal Cord Stimulation Computerized Modeling. Past and Future Directions. J. Clin. Med. submitted.
- Rigoard, P.; Le Tutor, T.; Collin, S.; Elhouari, K.; Ye, W.; Germaneau, A.; Caillé, L.; Roulaud, M.; Hervochon, R.; Ounajim, A.; et al. The “Neuro-Fiber-Mapping”: An Original Concept Using Live Electrostimulation Mapping to (Re)Explore Spinal Cord Neural Networks with a Focus on the Conus Medullaris. J. Clin. Med. submitted.
- De Ridder, D.; Plazier, M.; Kamerling, N.; Menovsky, T.; Vanneste, S. Burst Spinal Cord Stimulation for Limb and Back Pain. World Neurosurg. 2013, 80, 642-649.e1. [Google Scholar] [CrossRef]
- De Andres, J.; Monsalve-Dolz, V.; Fabregat-Cid, G.; Villanueva-Perez, V.; Harutyunyan, A.; Asensio-Samper, J.M.; Sanchis-Lopez, N. Prospective, Randomized Blind Effect-on-Outcome Study of Conventional vs. High-Frequency Spinal Cord Stimulation in Patients with Pain and Disability due to Failed Back Surgery Syndrome. Pain Med. Malden Mass 2017, 18, 2401–2421. [Google Scholar] [CrossRef] [Green Version]
- Bolash, R.; Creamer, M.; Rauck, R.; Vahedifar, P.; Calodney, A.; Fox, I.; Özaktay, C.; Panchal, S.; Vanquathem, N.; Yasin, M. Wireless High-Frequency Spinal Cord Stimulation (10 KHz) Compared with Multiwaveform Low-Frequency Spinal Cord Stimulation in the Management of Chronic Pain in Failed Back Surgery Syndrome Subjects: Preliminary Results of a Multicenter, Prospective Randomized Controlled Study. Pain Med. Malden Mass 2019, 20, 1971–1979. [Google Scholar] [CrossRef] [Green Version]
- Karri, J.; Orhurhu, V.; Wahezi, S.; Tang, T.; Deer, T.; Abd-Elsayed, A. Comparison of Spinal Cord Stimulation Waveforms for Treating Chronic Low Back Pain: Systematic Review and Meta-Analysis. Pain Physician 2020, 23, 451–460. [Google Scholar]
- Hagedorn, J.M.; Romero, J.; Ha, C.T.; Bendel, M.A.; D’Souza, R.S. Paresthesia-Based Versus High-Frequency Spinal Cord Stimulation: A Retrospective, Real-World, Single-Center Comparison. Neuromodulation Technol. Neural Interface. 2021. [Google Scholar] [CrossRef]
- Billot, M.; Naiditch, N.; Brandet, C.; Lorgeoux, B.; Baron, S.; Ounajim, A.; Roulaud, M.; Roy-Moreau, A.; de Montgazon, G.; Charrier, E.; et al. Comparison of Conventional, Burst and High-Frequency Spinal Cord Stimulation on Pain Relief in Refractory Failed Back Surgery Syndrome Patients: Study Protocol for a Prospective Randomized Double-Blinded Cross-over Trial (MULTIWAVE Study). Trials 2020, 21, 696. [Google Scholar] [CrossRef]
- Hunter, C.W.; Carlson, J.; Yang, A.; Patterson, D.; Lowry, B.; Mehta, P.; Rowe, J.; Deer, T. BURST(Able): A Retrospective, Multicenter Study Examining the Impact of Spinal Cord Stimulation with Burst on Pain and Opioid Consumption in the Setting of Salvage Treatment and “Upgrade”. Pain Physician 2020, 23, E643–E658. [Google Scholar]
- Andrade, P.; Heiden, P.; Visser-Vandewalle, V.; Matis, G. 1.2 KHz High-Frequency Stimulation as a Rescue Therapy in Patients With Chronic Pain Refractory to Conventional Spinal Cord Stimulation. Neuromodulation J. Int. Neuromodulation Soc. 2021, 24, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Rigoard, P.; Ounajim, A.; Goudman, L.; Banor, T.; Héroux, F.; Roulaud, M.; Babin, E.; Bouche, B.; Page, P.; Lorgeoux, B.; et al. The Challenge of Converting “Failed Spinal Cord Stimulation Syndrome” Back to Clinical Success, Using SCS Reprogramming as Salvage Therapy, Through Neurostimulation Adapters Combined with 3D-Computerized Pain Mapping Assessment. A Real-Life Retrospective Cohort Analysis. Preprints 2021. [Google Scholar] [CrossRef]
- Haider, N.; Ligham, D.; Quave, B.; Harum, K.E.; Garcia, E.A.; Gilmore, C.A.; Miller, N.; Moore, G.A.; Bains, A.; Lechleiter, K.; et al. Spinal Cord Stimulation (SCS) Trial Outcomes After Conversion to a Multiple Waveform SCS System. Neuromodulation J. Int. Neuromodulation Soc. 2018, 21, 504–507. [Google Scholar] [CrossRef]
- Reddy, R.D.; Moheimani, R.; Yu, G.G.; Chakravarthy, K.V. A Review of Clinical Data on Salvage Therapy in Spinal Cord Stimulation. Neuromodulation J. Int. Neuromodulation Soc. 2020, 23, 562–571. [Google Scholar] [CrossRef]
- Ghosh, P.E.; Gill, J.S.; Simopoulos, T. The Evolving Role of High-Frequency Spinal Cord Stimulation as Salvage Therapy in Neurostimulation. Pain Pract. Off. J. World Inst. Pain 2020, 20, 706–713. [Google Scholar] [CrossRef] [PubMed]





| Variable at Baseline | SCS + PNfS Group (n = 6) | SCS Only Group (n = 7) | p-Value |
|---|---|---|---|
| Gender | 0.56 | ||
| Male | 2 (33.3%) | 1 (14.3%) | |
| Female | 4 (66.7%) | 6 (85.7%) | |
| Age (years) | 51.7 ± 12.2 | 45.1 ± 9.5 | 0.39 |
| Body mass index (kg/cm²) | 28.8 ± 6.7 | 29.0 ± 2.8 | 0.95 |
| Time between pain onset and SCS implantation (years) * | 6 ± 5.3 | 4 ± 12.5 | 0.83 |
| Time between SCS and PNfS implantation (years)* | 1.5 ± 1.75 | 2 ± 2 | 0.38 |
| Back pain DN4 Positive (≥4) | 5 (83.3%) | 3 (42.9%) | 0.27 |
| Back pain DN4 Negative (<4) | 1 (16.7%) | 4 (57.1%) | |
| Back pain VAS (/100 mm) | 76.6 ± 18.0 | 78.6 ± 14.6 | 0.99 |
| Back pain surface (cm²) | 100.4 ± 57.6 | 130.6 ± 162.4 | 0.84 |
| Baseline back pain paresthesia coverage (%) | 0.0 ± 13.2 | 8.9 ± 12.4 | 0.21 |
| ODI score (%) | 50.3 ± 11.8 | 48.3 ± 16.1 | 0.83 |
| EQ-5D-3L index | 0.34 ± 0.20 | 0.46 ± 0.26 | 0.43 |
| Anxiety HADS score | 10.3 ± 3.1 | 6.3 ± 4.6 | 0.15 |
| Depression HADS score | 8.3 ± 4.1 | 5.4 ± 2.5 | 0.25 |
| MQS III score | 21.5 ± 14.8 | 15.8 ± 17.1 | 0.28 |
| SCS Lead implantation level | 0.33 | ||
| T8 | 1 (16.7%) | 0 (0%) | |
| T9 | 3 (50.0%) | 5 (71.4%) | |
| T10 | 2 (33.3%) | 0 (0%) | |
| T11 | 0 (0%) | 1 (14.3%) | |
| T12 | 0 (0%) | 1 (14.3%) |
| SCS + PNfS Group (n = 6); Mean ± SD | SCS Only Group (n = 7); Mean ± SD | p-Value | |
|---|---|---|---|
| At 1 month | |||
| Back pain surface | −89.2 ± 9.4% | −19.3 ± 84.9% | 0.003 |
| Back pain paresthesia coverage | 10.5 ± 17.0% | −8.9 ± 15.3% | 0.017 |
| Back pain VAS | −65.4 ± 20.4% | 8.6 ± 26.6% | 0.001 |
| ODI score | −33.8 ± 39.9% | 12.3 ± 41.1% | 0.034 |
| EQ-5D-3L index | 0.20 ± 0.30 | −0.04 ± 0.27 | 0.40 |
| HADS anxiety score | 0.83 ± 2.66 | 2.0 ± 1.03 | 0.190 |
| HADS depression score | 1.67 ± 3.67 | 0.0 ± 1.38 | 0.40 |
| MQS-III score | −3.57 ± 5.63 | 3.94 ± 6.73 | 0.07 |
| At 3 months | |||
| Back pain surface | −80.2 ± 21.3% | 13.2 ± 94.8% | 0.012 |
| Back pain paresthesia coverage | 16.05 ± 16.16% | −0.94 ± 2.2% | 0.016 |
| Back pain VAS | −68.8 ± 19.9% | 4.0 ± 15.0% | <0.0001 |
| ODI score | −31.5 ± 34.1% | 5.0 ± 29.7% | 0.07 |
| EQ-5D-3L index | 0.23 ± 0.33 | 0.02 ± 0.17 | 0.18 |
| HADS anxiety score | −0.33 ± 2.34 | 0.14 ± 2.79 | 0.70 |
| HADS depression score | 1.83 ± 4.07 | 0.57 ± 1.51 | 0.80 |
| MQS-III score | −6.32 ± 10.72 | 7.29 ± 12.20 | 0.07 |
| Endpoints | SCS + PNfS Group (n = 6) | SCS Only Group (n = 7) | ||||
|---|---|---|---|---|---|---|
| Baseline | 1-Month | 3-Month | Baseline | 1-Month | 3-Month | |
| Leg pain VAS (mm) | 23.5 ± 26.6 | 19.7 ± 18.7 | 25.1 ± 25.4 | 31.5 ± 13.6 | 33.5 ± 20.1 | 42.1 ± 22.0 |
| ODI score | 50.3 ± 11.8 | 35.0 ± 21.2 * | 37.3 ± 24.0 | 48.3 ± 16.1 | 49.1 ± 8.2 | 46.9 ± 8.6 |
| EQ-5D-3L score | 0.34 ± 0.20 | 0.54 ± 0.24 | 0.57 ± 0.29 | 0.46 ± 0.26 | 0.42 ± 0.21 | 0.48 ± 0.21 |
| HADS anxiety score | 10.3 ± 3.1 | 9.5 ± 2.9 | 10.7 ± 4.0 | 6.3 ± 4.6 | 4.3 ± 4.2 | 6.1 ± 4.0 |
| HADS depression score | 8.3 ± 4.1 | 6.7 ± 5.0 | 6.5 ± 5.5 | 5.4 ± 2.5 | 5.4 ± 2.3 | 4.9 ± 2.9 |
| Endpoints | Difference between Baseline and 6-Month Follow-Up | Difference between Baseline and 12-Month Follow-Up | ||||
|---|---|---|---|---|---|---|
| Difference | CI95% | p-Value | Difference | CI95% | p-Value | |
| Back pain surface | −79.81 cm² | (−182.92; 23.30) | 0.013 | −32.98 cm² | (−107.61; 40.66) | 0.27 |
| Back pain VAS | −41.6 mm | (−59.4; −23.8) | 0.0003 | −39.4 mm | (−57.7; −21.0) | 0.001 |
| Leg pain VAS | −5.8 mm | (−21.7; 10.1) | 0.4 | −2.2 mm | (−18.9; 14.5) | 0.8 |
| ODI score | −11.9% | (−21.6; −2.1) | 0.02 | −10.8% | (−20.6; −1.1) | 0.03 |
| EQ-5D-3L score | 0.19 | (0.04; 0.33) | 0.017 | 0.16 | (0.02; 0.34) | 0.1 |
| HADS anxiety score | −2.1 | (−3.9; −0.3) | 0.03 | −2.0 | (−3.3; −0.7) | 0.008 |
| HADS depression score | −0.9 | (−3.3; 1.4) | 0.4 | −1.0 | (−3.5; 1.6) | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rigoard, P.; Ounajim, A.; Goudman, L.; Bouche, B.; Roulaud, M.; Page, P.; Lorgeoux, B.; Baron, S.; Nivole, K.; Many, M.; et al. The Added Value of Subcutaneous Peripheral Nerve Field Stimulation Combined with SCS, as Salvage Therapy, for Refractory Low Back Pain Component in Persistent Spinal Pain Syndrome Implanted Patients: A Randomized Controlled Study (CUMPNS Study) Based on 3D-Mapping Composite Pain Assessment. J. Clin. Med. 2021, 10, 5094. https://doi.org/10.3390/jcm10215094
Rigoard P, Ounajim A, Goudman L, Bouche B, Roulaud M, Page P, Lorgeoux B, Baron S, Nivole K, Many M, et al. The Added Value of Subcutaneous Peripheral Nerve Field Stimulation Combined with SCS, as Salvage Therapy, for Refractory Low Back Pain Component in Persistent Spinal Pain Syndrome Implanted Patients: A Randomized Controlled Study (CUMPNS Study) Based on 3D-Mapping Composite Pain Assessment. Journal of Clinical Medicine. 2021; 10(21):5094. https://doi.org/10.3390/jcm10215094
Chicago/Turabian StyleRigoard, Philippe, Amine Ounajim, Lisa Goudman, Benedicte Bouche, Manuel Roulaud, Philippe Page, Bertille Lorgeoux, Sandrine Baron, Kevin Nivole, Mathilde Many, and et al. 2021. "The Added Value of Subcutaneous Peripheral Nerve Field Stimulation Combined with SCS, as Salvage Therapy, for Refractory Low Back Pain Component in Persistent Spinal Pain Syndrome Implanted Patients: A Randomized Controlled Study (CUMPNS Study) Based on 3D-Mapping Composite Pain Assessment" Journal of Clinical Medicine 10, no. 21: 5094. https://doi.org/10.3390/jcm10215094
APA StyleRigoard, P., Ounajim, A., Goudman, L., Bouche, B., Roulaud, M., Page, P., Lorgeoux, B., Baron, S., Nivole, K., Many, M., Adjali, N., Charrier, E., Rannou, D., Poupin, L., Wood, C., David, R., Héraud, D., Moens, M., & Billot, M. (2021). The Added Value of Subcutaneous Peripheral Nerve Field Stimulation Combined with SCS, as Salvage Therapy, for Refractory Low Back Pain Component in Persistent Spinal Pain Syndrome Implanted Patients: A Randomized Controlled Study (CUMPNS Study) Based on 3D-Mapping Composite Pain Assessment. Journal of Clinical Medicine, 10(21), 5094. https://doi.org/10.3390/jcm10215094








