BCL-2 Expression in AML Patients over 65 Years: Impact on Outcomes across Different Therapeutic Strategies
Abstract
:1. Introduction
2. Methods
2.1. Patients
2.2. BCL2 Expression
2.3. Therapy
2.4. Response Definition
2.5. Statistical Analyses
3. Results
3.1. BCL2 Expression and Correlation with Other Features
3.2. Complete Remission
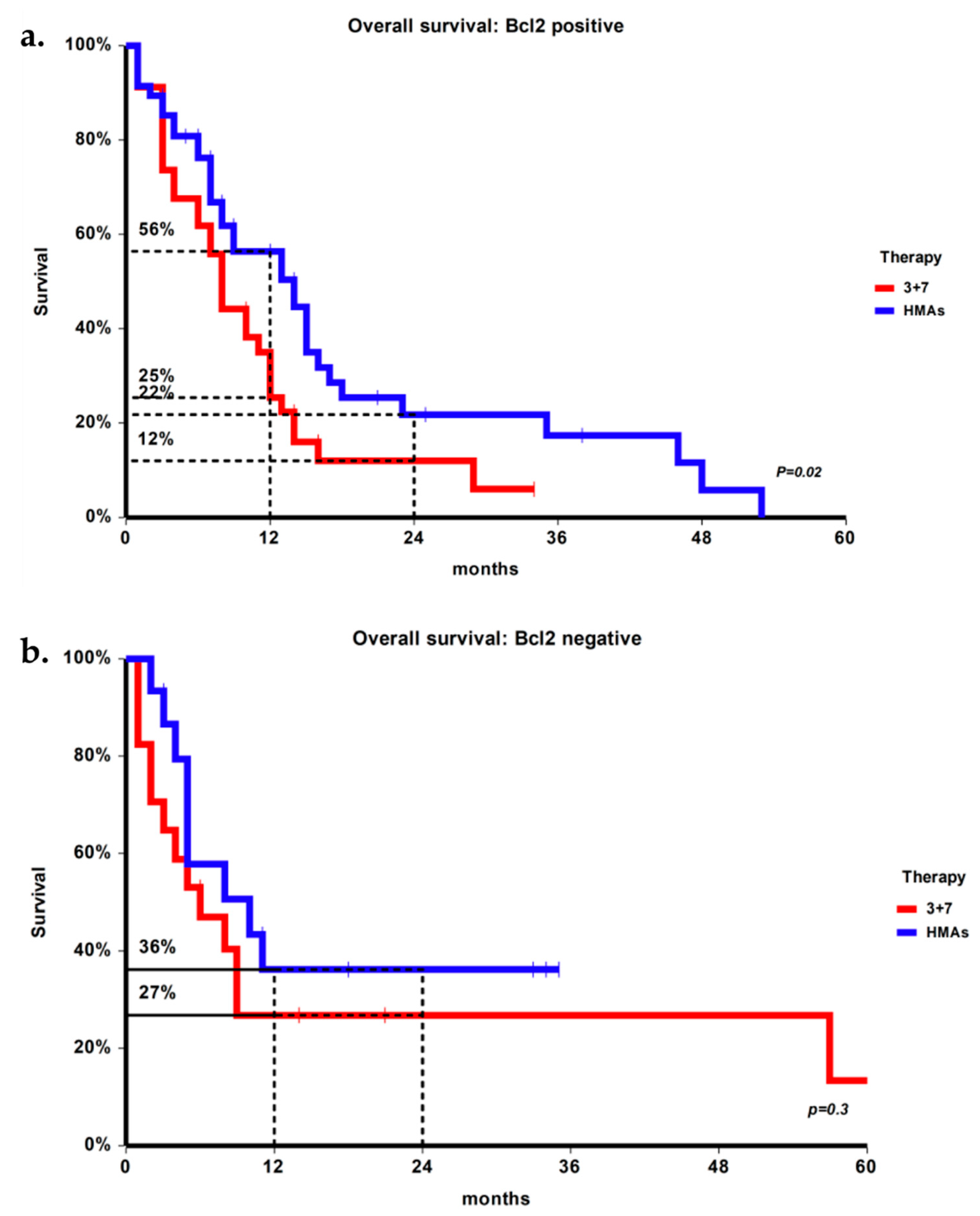
3.3. Overall Survival
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shallis, R.M.; Wang, R.; Davidoff, A.; Ma, X.; Zeidan, A.M. Epidemiology of acute myeloid leukemia: Recent progress and enduring challenges. Blood Rev. 2019, 36, 70–87. [Google Scholar] [CrossRef]
- Campos, L.; Rouault, J.P.; Sabido, O.; Oriol, P.; Roubi, N.; Vasselon, C.; Archimbaud, E.; Magaud, J.P.; Guyotat, D. High expression of bcl-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy. Blood 1993, 81, 3091–3096. [Google Scholar] [CrossRef] [Green Version]
- Karakas, T.; Maurer, U.; Weidmann, E.; Miething, C.C.; Hoelzer, D.; Bergmann, L. High expression of bcl-2 mRNA as a determinant of poor prognosis in acute myeloid leukemia. Ann. Oncol. 1998, 9, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Dendooven, A.; Mercier, M.L.; Puylaert, P.; Vermeulen, K.; Kockx, M.; Deiteren, K.; Maes, M.B.; Berneman, Z.; Anguille, S. Absence of BCL-2 expression identifies a subgroup of AML with distinct phenotypic, molecular, and clinical characteristics. J. Clin. Med. 2020, 9, 3090. [Google Scholar]
- Pan, R.; Hogdal, L.J.; Benito, J.M.; Bucci, D.; Han, L.; Borthakur, G.; Cortes, J.; DeAngelo, D.J.; DeBose, L.; Mu, H.; et al. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer Discov. 2014, 4, 362–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinardo, C.D.; Pratz, K.; Pullarkat, V.; Jonas, B.; Arellano, M.; Becker, P.S.; Frankfurt, O.; Konopleva, M.; Wei, A.H.; Kantarjian, H.M.; et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 2019, 133, 7–17. [Google Scholar] [CrossRef] [Green Version]
- Döhner, H.; Estey, E.H.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Burnett, A.K.; Dombret, H.; Fenaux, P.; Grimwade, D.; Larson, R.; et al. Diagnosis and management of acute myeloid leukemia in adults: Recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 2010, 115, 453–474. [Google Scholar] [CrossRef] [PubMed]
- Lancet, J.E. Is the overall survival for older adults with AML finally improving? Best Pract. Res. Clin. Haematol. 2018, 31, 387–390. [Google Scholar] [CrossRef]
- Del Poeta, G.; Venditti, A.; Del Principe, M.I.; Maurillo, L.; Buccisano, F.; Tamburini, A.; Cox, M.C.; Franchi, A.; Bruno, A.; Mazzone, C.; et al. Amount of spontaneous apoptosis detected by Bax/Bcl-2 ratio predicts outcome in acute myeloid leukemia (AML). Blood 2003, 101, 2125–2131. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, D.A.; Russell, N.H. Comparative quantitative expression of bcl-2 by normal and leukaemic myeloid cells. Br. J. Haematol. 1995, 91, 374–379. [Google Scholar] [CrossRef]
- Lauria, F.; Raspadori, D.; Rondelli, D.; Ventura, M.; Fiacchini, M.; Visani, G.; Forconi, F.; Tura, S. High bcl-2 expression in acute myeloid leukemia cells correlates with CD34 positivity and complete remission rate. Leuk. 1997, 11, 2075–2078. [Google Scholar] [CrossRef] [Green Version]
- DiNardo, C.D.; Pratz, K.W.; Letai, A.; Jonas, B.; Wei, A.H.; Thirman, M.; Arellano, M.; Frattini, M.G.; Kantarjian, H.; Popovic, R.; et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: A non-randomised, open-label, phase 1b study. Lancet Oncol. 2018, 19, 216–228. [Google Scholar] [CrossRef]
- Wei, A.H.; Strickland , S.A., Jr.; Hou, J.-Z.; Fiedler, W.; Lin, T.; Walter, R.B.; Enjeti, A.; Tiong, I.S.; Savona, M.; Lee, S.; et al. Venetoclax combined with low-dose cytarabine for previously untreated patients with acute myeloid leukemia: Results from a phase ib/ii study. J. Clin. Oncol. 2019, 37, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Maiti, A.; DiNardo, C.D.; Cortes, J.E.; Borthakur, G.; Pemmaraju, N.; Benton, C.B.; Kadia, T.M.; Takahashi, K.; Naqvi, K.; Ravandi, F.; et al. Interim analysis of phase II study of venetoclax with 10-day decitabine (DEC10-VEN) in acute myeloid leukemia and myelodysplastic syndrome. Blood 2018, 132, 286. [Google Scholar] [CrossRef]
- Dombret, H.; Seymour, J.F.; Butrym, A.; Wierzbowska, A.; Selleslag, D.; Jang, J.H.; Kumar, R.; Cavenagh, J.; Schuh, A.C.; Candoni, A.; et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 2015, 126, 291–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kantarjian, H.M.; Thomas, X.G.; Dmoszynska, A.; Wierzbowska, A.; Mazur, G.; Mayer, J.; Gau, J.-P.; Chou, W.-C.; Buckstein, R.; Cermak, J.; et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J. Clin. Oncol. 2012, 30, 2670–2677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.D.; Zhang, T.J.; Xu, Z.J.; Gu, Y.; Ma, J.C.; Li, X.X.; Guo, H.; Wen, X.M.; Zhang, W.; Yang, L.; et al. BCL2 overexpression: Clinical implication and biological insights in acute myeloid leukemia. Diagn. Pathol. 2019, 14, 68. [Google Scholar] [CrossRef]
- Kulsoom, B.; Shamsi, T.S.; Afsar, N.A.; Memon, Z.; Ahmed, N.; Hasnain, S.N. Bax, Bcl-2, and Bax/Bcl-2 as prognostic markers in acute myeloid leukemia: Are we ready for Bcl-2-directed therapy? Cancer Manag. Res. 2018, 10, 403–416. [Google Scholar] [CrossRef] [Green Version]
- Zjablovskaja, P.; Florian, M.C. Acute Myeloid Leukemia: Aging and Epigenetics. Cancers 2020, 12, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lachowiez, C.A.; Loghavi, S.; Kadia, T.M.; Daver, N.; Borthakur, G.; Pemmaraju, N.; Naqvi, K.; Alvarado, Y.; Yilmaz, M.; Short, N.; et al. Outcomes of older patients with NPM1-mutated AML: Current treatments and the promise of venetoclax-based regimens. Blood Adv. 2020, 4, 1311–1320. [Google Scholar] [CrossRef]
- Chua, C.C.; Roberts, A.W.; Reynolds, J.; Fong, C.Y.; Ting, S.B.; Salmon, J.M.; MacRaild, S.; Ivey, A.; Tiong, I.S.; Fleming, S.; et al. Chemotherapy and venetoclax in elderly acute myeloid leukemia trial (CAVEAT): A phase ib dose-escalation study of venetoclax combined with modified intensive chemotherapy. J. Clin. Oncol. 2020, 38, 3506–3517. [Google Scholar] [CrossRef] [PubMed]
| BCL-2+ (n = 81) | BCL-2- (n = 32) | p | |
|---|---|---|---|
| Median age (range), years | 73 (65–85) | 74 (65–83) | 0.85 |
| AML type | 0.15 | ||
| De novo | 31 | 17 | |
| Secondary | 50 | 15 | |
| Mean WBC (IQR), ×109/L | 6.6 (4.3–9.5) | 8.5 (4.0–33.0) | 0.048 |
| ELN cytogenetic/molecular risk | 0.045 | ||
| Favorable | 11 | 6 | |
| Intermediate | 29 | 18 | |
| Unfavorable | 41 | 8 | |
| FLT3 | 0.96 | ||
| ITD/TKD | 13 | 5 | |
| WT | 68 | 27 | |
| NPM | 0.31 | ||
| Mutated | 13 | 4 | |
| WT | 67 | 26 | |
| NA | 1 | 2 | |
| CD34 | 0.90 | ||
| Positive | 65 | 26 | |
| Negative | 16 | 6 | |
| CD56 | 0.09 | ||
| Positive | 22 | 14 | |
| Negative | 59 | 18 |
| CR | Failure | p | |
|---|---|---|---|
| BCL-2+ | 28 | 53 | 0.98 |
| BCL-2- | 11 | 21 | |
| 3 + 7 | 15 | 36 | 0.30 |
| HMA | 24 | 38 | |
| BCL-2+ with 3 + 7 | 8 | 27 | 0.05 |
| BCL-2+ with HMA | 20 | 26 | |
| BCL-2- with 3 + 7 | 7 | 9 | 0.26 |
| BCL-2- with HMA | 4 | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiribelli, M.; Michelutti, A.; Cavallin, M.; Di Giusto, S.; Simeone, E.; Fanin, R.; Damiani, D. BCL-2 Expression in AML Patients over 65 Years: Impact on Outcomes across Different Therapeutic Strategies. J. Clin. Med. 2021, 10, 5096. https://doi.org/10.3390/jcm10215096
Tiribelli M, Michelutti A, Cavallin M, Di Giusto S, Simeone E, Fanin R, Damiani D. BCL-2 Expression in AML Patients over 65 Years: Impact on Outcomes across Different Therapeutic Strategies. Journal of Clinical Medicine. 2021; 10(21):5096. https://doi.org/10.3390/jcm10215096
Chicago/Turabian StyleTiribelli, Mario, Angela Michelutti, Margherita Cavallin, Sara Di Giusto, Erica Simeone, Renato Fanin, and Daniela Damiani. 2021. "BCL-2 Expression in AML Patients over 65 Years: Impact on Outcomes across Different Therapeutic Strategies" Journal of Clinical Medicine 10, no. 21: 5096. https://doi.org/10.3390/jcm10215096






