Clinical Outcome and 8-Year Follow-Up of Alveolar Bone Tissue Engineering for Severely Atrophic Alveolar Bone Using Autologous Bone Marrow Stromal Cells with Platelet-Rich Plasma and β-Tricalcium Phosphate Granules
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
- Patients who expected to have dental implant treatment.
- Patients who had more than two continuous tooth defects in which fixed prostheses were not applicable.
- Patients who showed a severely atrophic maxilla or mandible, which required bone transplantation.
- (1)
- The width of the alveolar bone ridge at the installation sites was less than 5 mm.
- (2)
- In the maxilla, the distance between the alveolar ridge and the sinus floors was less than 5 mm.
- (3)
- In the mandible, the distance between the ridge and mandibular canal was less than 5 mm.
- Good oral hygiene was maintained.
- Aged 20 years or older, but younger than 70 years.
- An understanding of the informed consent form and provided consent for the study.
2.2. Exclusion Criteria
- Diabetes and/or autoimmune diseases.
- Hemorrhagic diathesis in which partial thromboplastin time (PT) was lower than 50% and activated partial thromboplastin time (APTT) was less than 23.5 or longer than 42.5 s.
- Uncontrollable infectious diseases.
- Osteoporosis.
- Liver dysfunction with aspartate aminotransferase (AST) values less than 10 or more than 40 IU/L, or with alanine aminotransferase (ALT) values less than 5 or more than 45 IU/L.
- Pregnant or possible pregnancy.
- Allergy to any medications used in this study and/or the presence of an allergy that requires continuous systemic medication.
- Other special conditions that the responsible physician considered inappropriate.
2.3. Number of Subjects and Duration of Study
2.4. Donor Screening
2.5. Autologous Serum Preparation
2.6. Harvest and Expansion of Bone Marrow Stromal Cells
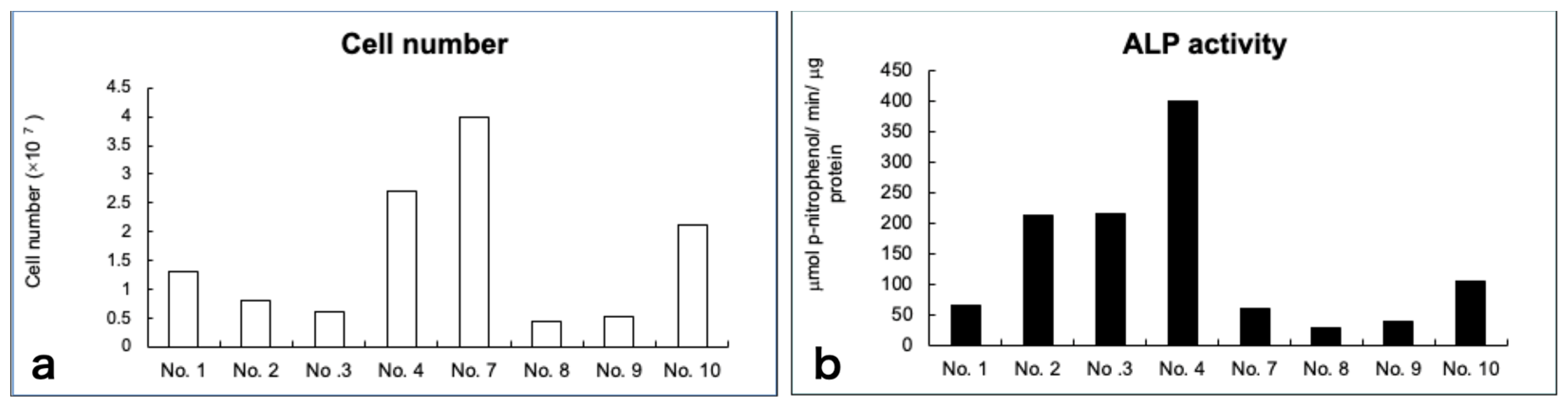
2.6.1. Alkaline Phosphatase Activity Analysis
2.6.2. Safety Tests
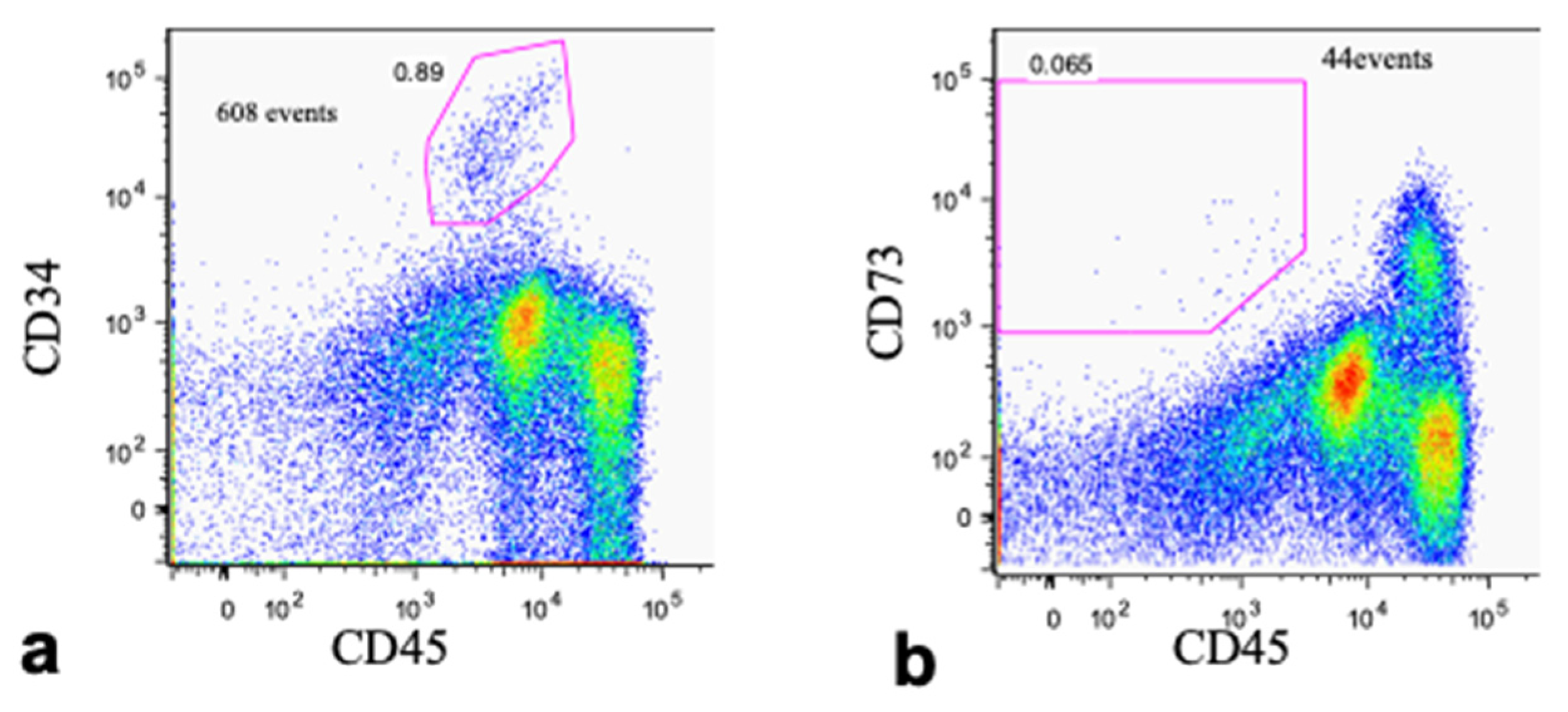
2.6.3. Flow Cytometry
2.7. Preparation of Transplants
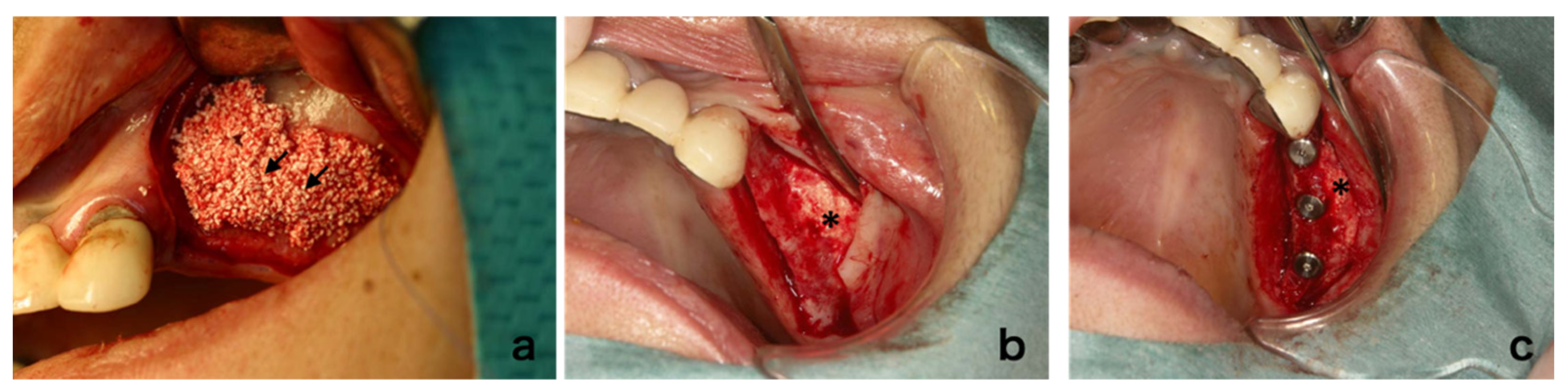
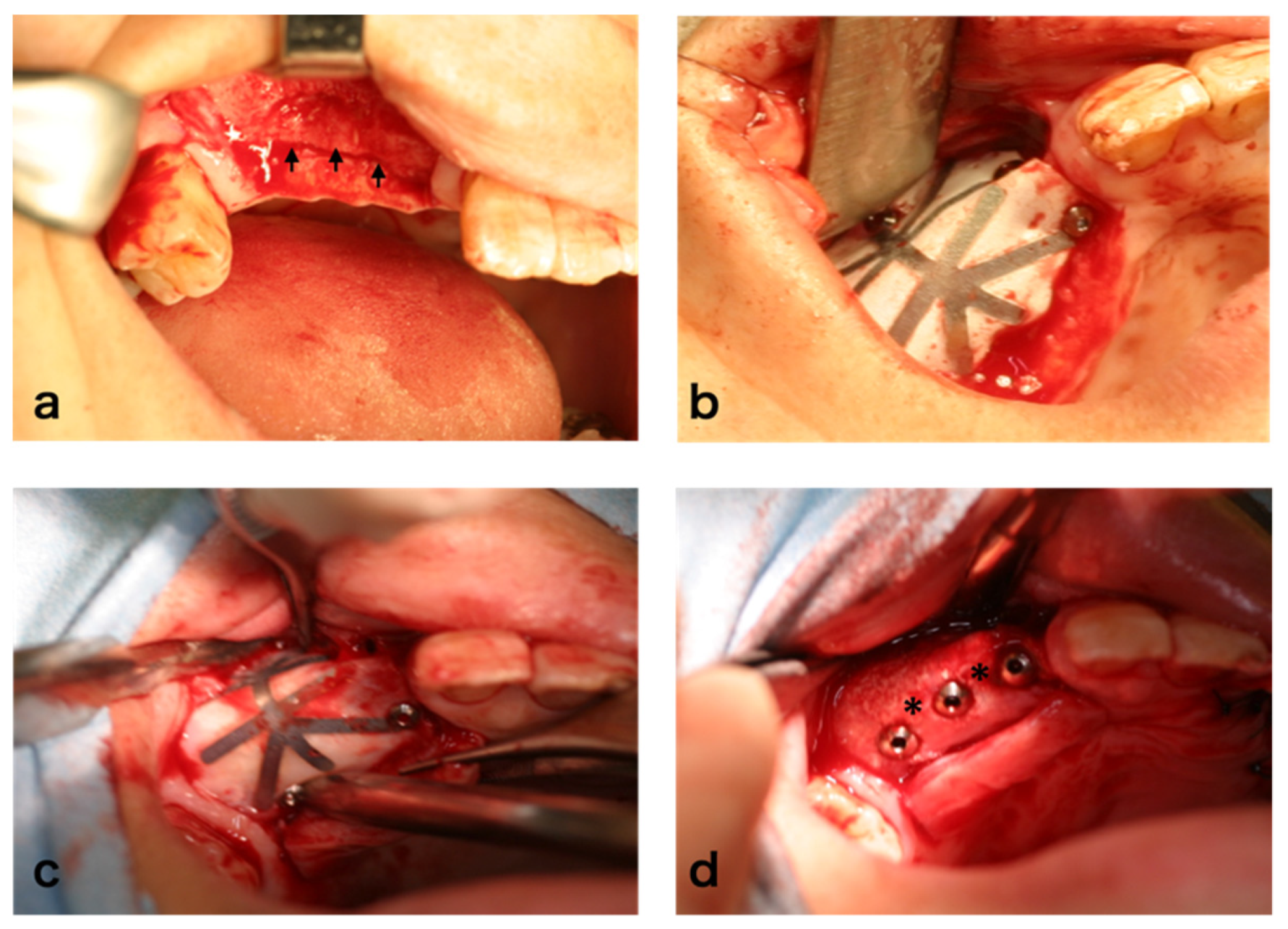
2.8. Surgical Procedure
2.9. Evaluation
Histomorphometric Analysis
2.10. Long-Term Follow-Up
3. Results
3.1. Cases
3.2. Cell Fraction Analyses of Bone Marrow Aspirate
3.3. Cell Growth and Diferentiation
3.4. Clinical Findings
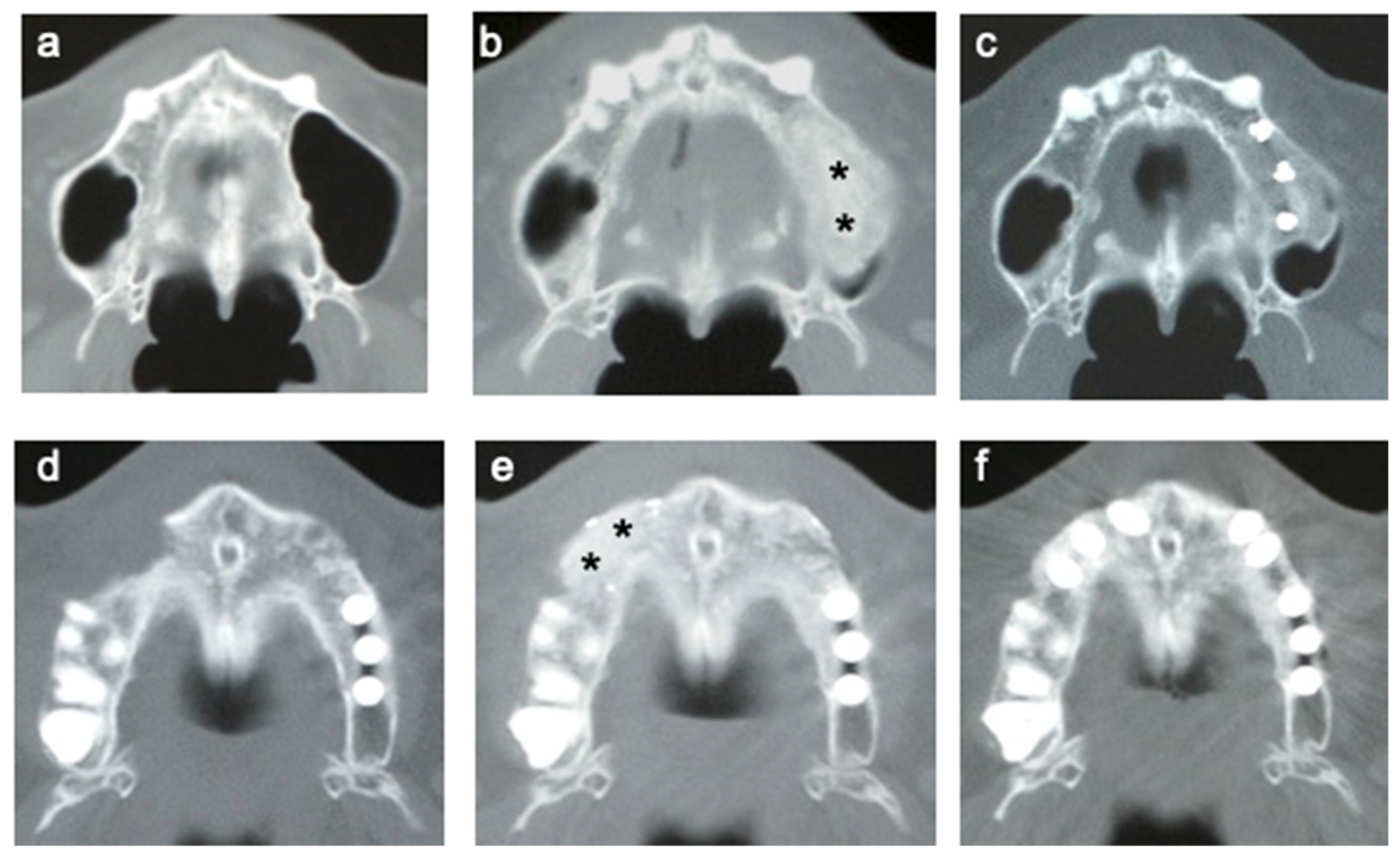
3.5. CT of Alveolar Bone
3.6. Time Course of the Volume of Regenerated Bone
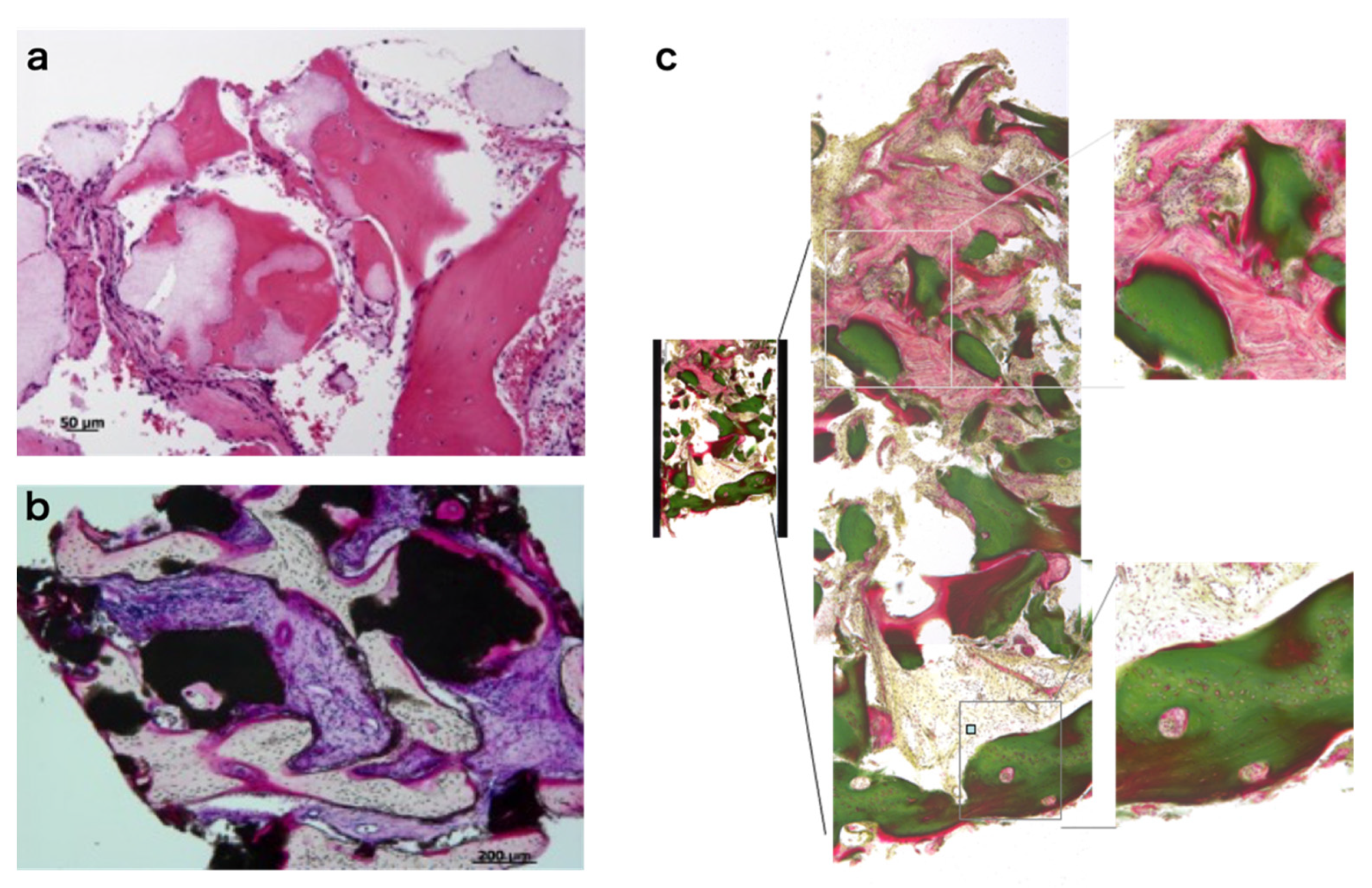
3.7. Histology of Regenerating Bone
3.8. Histomorphometric Analyses
3.9. Dental Implants
3.10. Safety Aspect of the Therapy
3.11. Long-Term Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yamada, M.; Egusa, H. Current bone substitutes for implant dentistry. J. Prosthodont. Res. 2018, 62, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials 2018, 180, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Howell, T.H.; Fiorellini, J.; Jones, A.; Alder, M.; Nummikoski, P.; Lazaro, M.; Lilly, L.; Cochran, D. A feasibility study evaluating rhBMP-2/absorbable collagen sponge device for local alveolar ridge preservation or augmentation. Int. J. Periodontics Restor. Dent. 1997, 17, 124–139. [Google Scholar]
- Simion, M.; Rocchietta, I.; Dellavia, C. Three-dimensional ridge augmentation with xenograft and recombinant human platelet-derived growth factor-BB in humans: Report of two cases. Int. J. Periodontics Restor. Dent. 2007, 27, 109–115. [Google Scholar]
- Smith, D.M.; Cooper, G.M.; Mooney, M.P.; Marra, K.G.; Losee, J.E. Bone morphogenetic protein 2 therapy for craniofacial surgery. J. Craniofac. Surg. 2008, 19, 1244–1259. [Google Scholar] [CrossRef]
- Bell, R.B.; Gregoire, C. Reconstruction of mandibular continuity defects using recombinant human bone morphogenetic protein 2: A note of caution in an atmosphere of exuberance. J. Oral Maxillofac. Surg. 2009, 67, 2673–2678. [Google Scholar] [CrossRef] [PubMed]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef]
- Yamada, Y.; Ueda, M.; Hibi, H.; Nagasaka, T. Translational research for injectable tissue-engineered bone regeneration using mesenchymal stem cells and platelet-rich plasma: From basic research to clinical case study. Cell Transplant. 2004, 13, 343–355. [Google Scholar] [CrossRef] [Green Version]
- Meijer, G.J.; de Bruijn, J.D.; Koole, R.; van Blitterswijk, C.A. Cell based bone tissue engineering in jaw defects. Biomaterials 2008, 29, 3053–3061. [Google Scholar] [CrossRef]
- Schimming, R.; Schmelzeisen, R. Tissue-engineered bone for maxillary sinus augmentation. J. Oral Maxillofac. Surg. 2004, 67, 724–729. [Google Scholar] [CrossRef]
- Springer, I.N.; Nocini, P.F.; Schlegel, K.A.; De Santis, D.; Park, J.; Warnke, P.H.; Terheyden, H.; Zimmermann, R.; Chiarini, L.; Gardner, K.; et al. Two techniques for the preparation of cell-scaffold constructs suitable for sinus augmentation: Steps into clinical application. Tissue Eng. 2006, 12, 2649–2659. [Google Scholar] [CrossRef]
- Mesimäki, K.; Lindroos, B.; Törnwall, J.; Mauno, J.; Lindqvist, C.; Kontio, R.; Miettinen, S.; Suuronen, R. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int. J. Oral Maxillofac. Surg. 2009, 38, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Sándor, G.K.; Numminen, J.; Wolff, J.; Thesleff, T.; Miettinen, A.; Tuovinen, V.J.; Mannerström, B.; Patrikoski, M.; Seppänen, R.; Miettinen, S.; et al. Adipose stem cells used to reconstruct 13 cases with cranio-maxillofacial hard-tissue defects. Stem. Cells Transl. Med. 2014, 3, 530–540. [Google Scholar] [CrossRef]
- Amable, P.R.; Teixeira, M.V.; Carias, R.B.; Granjeiro, J.M.; Borojevic, R. Mesenchymal stromal cell proliferation, gene expression and protein production in human platelet-rich plasma-supplemented media. PLoS ONE 2014, 9, e104662. [Google Scholar] [CrossRef]
- Allen, M.R.; Hock, J.M.; Burr, D.B. Periosteum: Biology, regulation, and response to osteoporosis therapies. Bone 2004, 35, 1003–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kagami, H.; Agata, H.; Tojo, A. Bone marrow stromal cells (bone marrow-derived multipotent mesenchymal stromal cells) for alveolar bone tissue engineering: Basic science to clinical translation. Int. J. Biochem. Cell Biol. 2011, 43, 286–289. [Google Scholar] [CrossRef]
- Arthur, A.; Gronthos, S. Clinical Application of Bone Marrow Mesenchymal Stem/Stromal Cells to Repair Skeletal Tissue. Int. J. Mol. Sci. 2020, 21, 9759. [Google Scholar] [CrossRef]
- Sheehy, E.J.; Kelly, D.J.; O’Brien, F.J. Biomaterial-based endochondral bone regeneration: A shift from traditional tissue engineering paradigms to developmentally inspired strategies. Mater. Today Biol. 2019, 3, 100009. [Google Scholar] [CrossRef]
- Simonpieri, A.; Del Corso, M.; Vervelle, A.; Jimbo, R.; Inchingolo, F.; Sammartino, G.; Dohan Ehrenfest, D.M. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 2: Bone graft, implant and reconstructive surgery. Curr. Pharm. Biotechnol. 2012, 13, 1231–1256. [Google Scholar] [CrossRef]
- Varshney, S.; Dwivedi, A.; Pandey, V. Efficacy of autologous stem cells for bone regeneration during endosseous dental implants insertion-A systematic review of human studies. J. Oral Biol. Craniofac. Res. 2020, 10, 347–355. [Google Scholar] [CrossRef]
- Egido-Moreno, S.; Valls-Roca-Umbert, J.; Céspedes-Sánchez, J.M.; López-López, J.; Velasco-Ortega, E. Clinical Efficacy of Mesenchymal Stem Cells in Bone Regeneration in Oral Implantology. Systematic Review and Meta-Analysis. Int. J. Env. Res. Public Health 2021, 18, 894. [Google Scholar] [CrossRef]
- Kagami, H.; Agata, H.; Inoue, M.; Asahina, I.; Tojo, A.; Yamashita, N.; Imai, K. The use of bone marrow stromal cells (bone marrow-derived multipotent mesenchymal stromal cells) for alveolar bone tissue engineering: Basic science to clinical translation. Tissue Eng. Part B Rev. 2014, 20, 229–232. [Google Scholar] [CrossRef] [Green Version]
- Agata, H.; Asahina, I.; Yamazaki, Y.; Uchida, M.; Shinohara, Y.; Honda, M.; Kagami, H.; Ueda, M. Effective bone engineering using periosteum-derived cells. J. Dent. Res. 2007, 86, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Harasawa, R.; Mizuswa, H.; Nozawa, K.; Asada, K.; Kato, I. Detection and tentative identification of dominant mycoplasma species in cell cultured by restriction analysis of the 16S–23S, rNFA intergenic spacer regions. Res. Microbiol. 1993, 144, 489–493. [Google Scholar] [CrossRef]
- Cricchio, G.; Lundgren, S. Donor site morbidity in two different approaches to anterior iliac crest bone harvesting. Clin. Implant. Dent. Relat. Res. 2003, 5, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Quatro, R.; Mastrogiacomo, M.; Cancedda, R. Repair of large bone defects with the use of autologous bone marrow stromal cells. N. Eng. J. Med. 2001, 344, 385–386. [Google Scholar] [CrossRef]
- Marcacci, M.; Kon, E.; Moukhachev, V.; Lavroukov, A.; Kutepov, S.; Quarto, R.; Mastrogiacomo, M.; Cancedda, R. Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng. 2007, 13, 947–955. [Google Scholar] [CrossRef] [Green Version]
- Hallman, M.; Sennerby, L.; Lundgren, S.A. Clinical and histologic evaluation of implant integration in the posterior maxilla after sinus floor augmentation with autogenous bone, bovine hydroxyapatite, or a 20:80 mixture. Int. J. Oral Maxillofac. Implant. 2002, 17, 635–643. [Google Scholar]
- Proussaefs, P.; Lozada, J. The use of intraorally harvested autogenous block grafts for vertical alveolar ridge augmentation: A human study. Int. J. Periodontics Restor. Dent. 2005, 25, 351–363. [Google Scholar]
- Szabó, G.; Huys, L.; Coulthard, P.; Maiorana, C.; Garagiola, U.; Barabás, J.; Németh, Z.; Hrabák, K.; Suba, Z.A. Prospective multicenter randomized clinical trial of autogenous bone versus beta-tricalcium phosphate graft alone for bilateral sinus elevation: Histologic and histomorphometric evaluation. Int. J. Oral Maxillofac. Implant. 2005, 20, 371–381. [Google Scholar]
- Felice, P.; Marchetti, C.; Iezzi, G.; Piattelli, A.; Worthington, H.; Pellegrino, G.; and Esposito, M. Vertical ridge augmentation of the atrophic posterior mandible with interpositional bloc grafts: Bone from the iliac crest vs. bovine anorganic bone. Clinical and histological results up to one year after loading from a randomized-controlled clinical trial. Clin. Oral Implant. Res. 2009, 20, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Boëck-Neto, R.J.; Gabrielli, M.F.; Shibli, J.A.; Marcantonio, E.; Lia, R.C.; Marcantonio, E., Jr. Histomorphometric evaluation of human sinus floor augmentation healing responses to placement of calcium phosphate or Ricinus communis polymer associated with autogenous bone. Clin. Implant. Dent. Relat. Res. 2005, 7, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Zijderveld, S.A.; Schulten, E.A.; Aartman, I.H.; ten Bruggenkate, C.M. Long-term changes in graft height after maxillary sinus floor elevation with different grafting materials: Radiographic evaluation with a minimum follow-up of 4.5 years. Clin. Oral Implant. Res. 2009, 20, 691–700. [Google Scholar] [CrossRef]
- Zizelmann, C.; Schoen, R.; Metzger, M.C.; Schmelzeisen, R.; Schramm, A.; Dott, B.; Bormann, K.H.; Gellrich, N.C. Bone formation after sinus augmentation with engineered bone. Clin. Oral Implant. Res. 2007, 18, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Agata, H.; Asahina, I.; Watanabe, N.; Ishii, Y.; Kubo, N.; Ohshima, S.; Yamazaki, M.; Tojo, A.; Kagami, H. Characteristic change and loss of in vivo osteogenic abilities of human bone marrow stromal cells during passage. Tissue Eng. Part A 2010, 16, 663–673. [Google Scholar] [CrossRef] [Green Version]
- Horwitz, E.M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.J.; Krause, D.S.; Keating, A. International Society for Cellular Therapy. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005, 7, 393–395. [Google Scholar] [CrossRef]
- Kaigler, D.; Avila-Ortiz, G.; Travan, S.; Taut, A.D.; Padial-Molina, M.; Rudek, I.; Wang, F.; Lanis, A.; Giannobile, W.V. Bone Engineering of Maxillary Sinus Bone Deficiencies Using Enriched CD90+ Stem Cell Therapy: A Randomized Clinical Trial. J. Bone Miner. Res. 2015, 30, 1206–1216. [Google Scholar] [CrossRef] [Green Version]
- Rickert, D.; Sauerbier, S.; Nagursky, H.; Menne, D.; Vissink, A.; Raghoebar, G.M. Maxillary sinus floor elevation with bovine bone mineral combined with either autogenous bone or autogenous stem cells: A prospective randomized clinical trial. Clin. Oral Implant. Res. 2011, 22, 251–258. [Google Scholar] [CrossRef]
- Gjerde, C.; Mustafa, K.; Hellem, S.; Rojewski, M.; Gjengedal, H.; Yassin, M.A.; Feng, X.; Skaale, S.; Berge, T.; Rosen, A.; et al. Cell therapy induced regeneration of severely atrophied mandibular bone in a clinical trial. Stem Cell Res. Ther. 2018, 9, 213. [Google Scholar] [CrossRef]
- Kaigler, D.; Pagni, G.; Park, C.H.; Braun, T.M.; Holman, L.A.; Yi, E.; Tarle, S.A.; Bartel, R.L.; Giannobile, W.V. Stem cell therapy for craniofacial bone regeneration: A randomized, controlled feasibility trial. Cell Transplant. 2013, 22, 767–777. [Google Scholar] [CrossRef] [Green Version]
- Bajestan, M.N.; Rajan, A.; Edwards, S.P.; Aronovich, S.; Cevidanes, L.H.S.; Polymeri, A.; Travan, S.; Kaigler, D. Stem cell therapy for reconstruction of alveolar cleft and trauma defects in adults: A randomized controlled, clinical trial. Clin. Implant. Dent. Relat. Res. 2017, 19, 793–801. [Google Scholar] [CrossRef] [PubMed]



| No. | Age | Sex | Target Region and Procedure | Cell No. (×106) | PRP (mL) | Plt. Count (104/uL) | β-TCP (g) | No. of Implants |
|---|---|---|---|---|---|---|---|---|
| 1 | 40 | F | rt. SFA | 13.3 | 2.8 | ND | 2.0 | 2 |
| 2 | 52 | M | rt. SFA | 8.2 | 3.0 | ND | 1.5 | 2 |
| 3 | 53 | F | lt. SFA | 6.0 | 3.5 | 73 | 2.0 | 3 |
| 4 | 52 | F | lt. SFA | 26.8 | 2.7 | 49.3 | 1.4 | 2 |
| 5 | 51 | F | - | - | - | - | - | - |
| 6 | 38 | F | - | - | - | - | - | - |
| 7 | 63 | M | rt. SFA | 39.6 | 4.0 | 57.3 | 1.0 | 4 |
| 8 | 57 | F | bil. SFA 22~23 ARA | 4.5 | 8.0 | ND | 3.0 | 6 |
| 9 | 60 | F | 15~12, 23~24 ARA. | 5.2 | 4.0 | ND | 1.0 | 5 |
| 10 | 57 | F | bil. SFA 24~25 ARA. | 21.1 | 10.0 | 80.2 | 3.0 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asahina, I.; Kagami, H.; Agata, H.; Honda, M.J.; Sumita, Y.; Inoue, M.; Nagamura-Inoue, T.; Tojo, A. Clinical Outcome and 8-Year Follow-Up of Alveolar Bone Tissue Engineering for Severely Atrophic Alveolar Bone Using Autologous Bone Marrow Stromal Cells with Platelet-Rich Plasma and β-Tricalcium Phosphate Granules. J. Clin. Med. 2021, 10, 5231. https://doi.org/10.3390/jcm10225231
Asahina I, Kagami H, Agata H, Honda MJ, Sumita Y, Inoue M, Nagamura-Inoue T, Tojo A. Clinical Outcome and 8-Year Follow-Up of Alveolar Bone Tissue Engineering for Severely Atrophic Alveolar Bone Using Autologous Bone Marrow Stromal Cells with Platelet-Rich Plasma and β-Tricalcium Phosphate Granules. Journal of Clinical Medicine. 2021; 10(22):5231. https://doi.org/10.3390/jcm10225231
Chicago/Turabian StyleAsahina, Izumi, Hideaki Kagami, Hideki Agata, Masaki J. Honda, Yoshinori Sumita, Minoru Inoue, Tokiko Nagamura-Inoue, and Arinobu Tojo. 2021. "Clinical Outcome and 8-Year Follow-Up of Alveolar Bone Tissue Engineering for Severely Atrophic Alveolar Bone Using Autologous Bone Marrow Stromal Cells with Platelet-Rich Plasma and β-Tricalcium Phosphate Granules" Journal of Clinical Medicine 10, no. 22: 5231. https://doi.org/10.3390/jcm10225231
APA StyleAsahina, I., Kagami, H., Agata, H., Honda, M. J., Sumita, Y., Inoue, M., Nagamura-Inoue, T., & Tojo, A. (2021). Clinical Outcome and 8-Year Follow-Up of Alveolar Bone Tissue Engineering for Severely Atrophic Alveolar Bone Using Autologous Bone Marrow Stromal Cells with Platelet-Rich Plasma and β-Tricalcium Phosphate Granules. Journal of Clinical Medicine, 10(22), 5231. https://doi.org/10.3390/jcm10225231







