Effect of Aprotinin on Liver Injury after Transplantation of Extended Criteria Donor Grafts in Humans: A Retrospective Propensity Score Matched Cohort Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Donor Data
2.3. Donor Organ Assessment
2.4. Liver Transplantation Management
2.5. Aprotinin Application
2.6. Recipient Data
2.7. Postreperfusion Syndrome
2.8. Statistics
3. Results
3.1. Patients
3.2. Liver Graft Recipient Characteristics
3.3. Liver Graft Donor Characteristics
3.4. Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Manns, M.P. Liver Cirrhosis, Transplantation and Organ Shortage. Dtsch. Aerzteblatt Online 2013, 110, 83–84. [Google Scholar] [CrossRef] [PubMed]
- Weismüller, T.J.; Negm, A.; Becker, T.; Barg-Hock, H.; Klempnauer, J.; Manns, M.P.; Strassburg, C.P. The introduction of MELD-based organ allocation impacts 3-month survival after liver transplantation by influencing pretransplant patient characteristics. Transpl. Int. 2009, 22, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Kamath, P.S.; Wiesner, R.H.; Malinchoc, M.; Kremers, W.K.; Therneau, T.M.; Kosberg, C.L.; D’Amico, G.; Dickson, E.R.; Kim, W.R. A model to predict survival in patients with end-stage liver disease. Hepatology 2001, 33, 464–470. [Google Scholar] [CrossRef]
- Tacke, F.; Kroy, D.C.; Barreiros, A.P.; Neumann, U.P. Liver transplantation in Germany. Liver Transplant. 2016, 22, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Schlitt, H.J.; Loss, M.; Scherer, M.N.; Becker, T.; Jauch, K.W.; Nashan, B.; Schmidt, H.; Settmacher, U.; Rogiers, X.; Neuhaus, P.; et al. Current developments in liver transplantation in Germany: MELD-based organ allocation and incentives for transplant centres. Z. Gastroenterol. 2011, 49, 30–38. [Google Scholar] [CrossRef]
- Czigany, Z.; Lurje, I.; Schmelzle, M.; Schöning, W.; Öllinger, R.; Raschzok, N.; Sauer, I.M.; Tacke, F.; Strnad, P.; Trautwein, C.; et al. Ischemia-Reperfusion Injury in Marginal Liver Grafts and the Role of Hypothermic Machine Perfusion: Molecular Mechanisms and Clinical Implications. J. Clin. Med. 2020, 9, 846. [Google Scholar] [CrossRef]
- Kork, F.; Rimek, A.; Andert, A.; Becker, N.J.; Heidenhain, C.; Neumann, U.P.; Kroy, D.; Roehl, A.B.; Rossaint, R.; Hein, M. Visual quality assessment of the liver graft by the transplanting surgeon predicts postreperfusion syndrome after liver transplantation: A retrospective cohort study. BMC Anesthesiol. 2018, 18, 1–11. [Google Scholar] [CrossRef]
- Lentschener, C.; Roche, K.; Ozier, Y. A Review of Aprotinin in Orthotopic Liver Transplantation: Can Its Harmful Effects Offset Its Beneficial Effects? Anesth. Analg. 2005, 100, 1248–1255. [Google Scholar] [CrossRef]
- Molenaar, I.; Veldman, M.; Begliomini, B.; Groenland, H.; Januszkiewicz, A.; Lindgren, L.; Metselaar, H.; Terpstra, O.; Porte, R. Improved early graft survival in patients receiving aprotinin during orthotopic liver transplantation. Transplant. Proc. 2001, 33, 1345–1346. [Google Scholar] [CrossRef]
- Port, R.J.; Molenaar, I.Q.; Begliomini, B.; Groenland, T.H.; Januszkiewicz, A.; Lindgren, L.; Palareti, G.; Hermans, J.; Terpstra, O.T. Aprotinin and transfusion requirements in orthotopic liver transplantation: A multicentre randomised double-blind study. Lancet 2000, 355, 1303–1309. [Google Scholar] [CrossRef]
- Warnaar, N.; Mallett, S.V.; De Boer, M.T.; Rolando, N.; Burroughs, A.K.; Nijsten, M.W.N.; Slooff, M.J.H.; Rolles, K.; Porte, R.J. The Impact of Aprotinin on Renal Function After Liver Transplantation: An Analysis of 1043 Patients. Arab. Archaeol. Epigr. 2007, 7, 2378–2387. [Google Scholar] [CrossRef] [PubMed]
- Warnaar, N.; Mallett, S.V.; Klinck, J.R.; De Boer, M.T.; Rolando, N.; Burroughs, A.K.; Jamieson, N.V.; Rolles, K.; Porte, R.J. Aprotinin and the risk of thrombotic complications after liver transplantation: A retrospective analysis of 1492 patients. Liver Transplant. 2009, 15, 747–753. [Google Scholar] [CrossRef]
- Royston, D.; De Hert, S.; van der Linden, J.; Ouattara, A.; Zacharowski, K. A special article following the relicence of aprotinin injection in Europe. Anaesth. Crit. Care Pain Med. 2017, 36, 97–102. [Google Scholar] [CrossRef]
- Neuhaus, P.; Bechstein, W.; Lefèbre, B.; Blumhardt, G.; Slama, K. Effect of Aprotinin on Intraoperative Bleeding and Fibrinolysis in Liver Transplantation. Lancet 1989, 334, 924–925. [Google Scholar] [CrossRef]
- Kuyvenhoven, J.P.; Molenaar, Q.; Veldman, M.G.; Palareti, G.; Legnani, C.; Moolenburgh, S.E.; Terpstra, O.T.; Lamers, C.B.; Van Hoek, B.; Porte, R.J.; et al. Plasma MMP–2 and MMP–9 and their inhibitors TIMP-1 and TIMP-2 during human orthotopic liver transplantation. Thromb. Haemost. 2004, 91, 506–513. [Google Scholar] [CrossRef][Green Version]
- Bittner, H.B.; Richter, M.; Kuntze, T.; Rahmel, A.; Dahlberg, P.; Hertz, M.; Mohr, F.W. Aprotinin decreases reperfusion injury and allograft dysfunction in clinical lung transplantation☆. Eur. J. Cardio-Thorac. Surg. 2006, 29, 210–215. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lie, T.S.; Seger, R.; Hong, G.S.; Preissinger, H.; Ogawa, K. Protective Effect of Aprotinin on Ischemic Hepatocellular Damage. Transplantation 1989, 48, 396–399. [Google Scholar] [CrossRef]
- Maksan, S.M.; Maksan, M.O.; Gebhard, M.M.; Herfarth, C.; Klar, E. Reduction of Hepatic Reperfusion Injury by Antithrombin Iii and Aprotinin. Transpl. Int. 2000, 13, S562–S564. [Google Scholar] [CrossRef]
- Tuffs, A. Bayer withdraws heart surgery drug. BMJ 2007, 335, 1015. [Google Scholar] [CrossRef]
- Mossdorf, A.; Ulmer, F.; Junge, K.; Heidenhain, C.; Hein, M.; Temizel, I.; Neumann, U.P.; Schöning, W.; Schmeding, M. Bypass during Liver Transplantation: Anachronism or Revival? Liver Transplantation Using a Combined Venovenous/Portal Venous Bypass—Experiences with 163 Liver Transplants in a Newly Established Liver Transplantation Program. Gastroenterol. Res. Pract. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Yersiz, H.; Lee, C.; Kaldas, F.M.; Hong, J.C.; Rana, A.; Schnickel, G.; Wertheim, J.; Zarrinpar, A.; Agopian, V.G.; Gornbein, J.; et al. Assessment of hepatic steatosis by transplant surgeon and expert pathologist: A prospective, double-blind evaluation of 201 donor livers. Liver Transplant. 2013, 19, 437–449. [Google Scholar] [CrossRef] [PubMed]
- McCormack, L.; Dutkowski, P.; El-Badry, A.M.; Clavien, P.-A. Liver transplantation using fatty livers: Always feasible? J. Hepatol. 2011, 54, 1055–1062. [Google Scholar] [CrossRef]
- Görlinger, K. Coagulation management during liver transplantation. Hämostaseologie 2006, 26, 64–76. [Google Scholar] [CrossRef]
- Eurotransplant. Annual Report. 2013. Available online: http://www.eurotransplant.org/wp-content/uploads/2019/12/AR2015.pdf (accessed on 22 September 2021).
- Vorweg, M.; Hanke, A.; Gorlinger, K.; Lier, H. Thromboelastometry guided therapy of severe bleeding. Hämostaseologie 2013, 33, 51–61. [Google Scholar] [CrossRef]
- Olthoff, K.M.; Kulik, L.; Samstein, B.; Kaminski, M.; Abecassis, M.; Emond, J.; Shaked, A.; Christie, J.D. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transplant. 2010, 16, 943–949. [Google Scholar] [CrossRef]
- Hoyer, D.P.; Paul, A.; Gallinat, A.; Molmenti, E.P.; Reinhardt, R.; Minor, T.; Saner, F.H.; Canbay, A.; Treckmann, J.W.; Sotiropoulos, G.C.; et al. Donor information based prediction of early allograft dysfunction and outcome in liver transplantation. Liver Int. 2014, 35, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Pezzati, D.; Ghinolfi, D.; De Simone, P.; Balzano, E.; Filipponi, F. Strategies to optimize the use of marginal donors in liver transplantation. World J. Hepatol. 2015, 7, 2636–2647. [Google Scholar] [CrossRef] [PubMed]
- Andert, A.; Becker, N.; Ulmer, F.; Schöning, W.; Hein, M.; Neumann, U.P.; Schmeding, M. Liver Transplantation and Donor Body Mass Index >30—Use or Refuse? Ann. Transpl. 2016, 21, 185–193. [Google Scholar] [CrossRef]
- Patrassi, G.; Viero, M.; Sartori, M.; Silvestro, G.; Rossaro, L.; Burra, P.; Nolli, M.; Piccinni, P.; Bassi, N. Aprotinin efficacy on intraoperative bleeding and transfusion requirements in orthotopic liver transplantation. Transfusion 1994, 34, 507–511. [Google Scholar] [CrossRef]
- Marcel, R.J.; Stegall, W.C.; Suit, C.T.; Arnold, J.C.; Vera, R.L.; Ramsay, M.A.; O’Donnell, M.B.; Swygert, T.H.; Hein, H.A.; Whitten, C.W. Continuous Small-Dose Aprotinin Controls Fibrinolysis During Orthotopic Liver Transplantation. Anesth. Analg. 1996, 82, 1122–1125. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, I.Q.; Begliomini, B.; Martinelli, G.; Putter, H.; Terpstra, O.T.; Porte, R.J. Reduced Need for Vasopressors in Patients Receiving Aprotinin during Orthotopic Liver Transplantation. J. Am. Soc. Anesthesiol. 2001, 94, 433–438. [Google Scholar] [CrossRef]
- Mangano, D.T.; Tudor, I.C.; Dietzel, C.; Group Multicenter Study of Perioperative Ischemia Research, Research Ischemia, and Foundation Education. The Risk Associated with Aprotinin in Cardiac Surgery. N. Engl. J. Med. 2006, 354, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Fergusson, D.; Hébert, P.C.; Mazer, C.D.; Fremes, S.; MacAdams, C.; Murkin, J.M.; Teoh, K.; Duke, P.C.; Arellano, R.; Blajchman, M.A.; et al. A Comparison of Aprotinin and Lysine Analogues in High-Risk Cardiac Surgery. N. Engl. J. Med. 2008, 358, 2319–2331. [Google Scholar] [CrossRef] [PubMed]
- Schofield, N.; Sugavanam, A.; Thompson, K.; Mallett, S.V. No increase in blood transfusions during liver transplantation since the withdrawal of aprotinin. Liver Transplant. 2014, 20, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Walkey, C.J.; Maretti-Mira, A.C.; Wang, L.; Johnson, D.L.; DeLeve, L.D. Susceptibility of Rat Steatotic Liver to Ischemia–Reperfusion Is Treatable with Liver-Selective Matrix Metalloproteinase Inhibition. Hepatology 2020, 72, 1771–1785. [Google Scholar] [CrossRef]
- Roberts, R.F.; Nishanian, G.P.; Carey, J.N.; Darbinian, S.H.; Kim, J.D.; Sakamaki, Y.; Chang, J.Y.; Starnes, V.A.; Barr, M.L. Addition of aprotinin to organ preservation solutions decreases lung reperfusion injury. Ann. Thorac. Surg. 1998, 66, 225–230. [Google Scholar] [CrossRef]
- Baran, D.; Tenstad, O.; Aukland, K. Aprotinin uptake in the proximal tubules in the rat kidney. II. Uptake site relative to glomerulus. J. Struct. Biol. 2003, 142, 409–415. [Google Scholar] [CrossRef]
- El-Badry, A.M.; Breitenstein, S.; Jochum, W.; Washington, K.; Paradis, V.; Rubbia-Brandt, L.; Puhan, M.A.; Slankamenac, K.; Graf, R.; Clavien, P.A. Assessment of Hepatic Steatosis by Expert Pathologists: The End of a Gold Standard. Ann. Surg. 2009, 250, 691–697. [Google Scholar] [CrossRef]
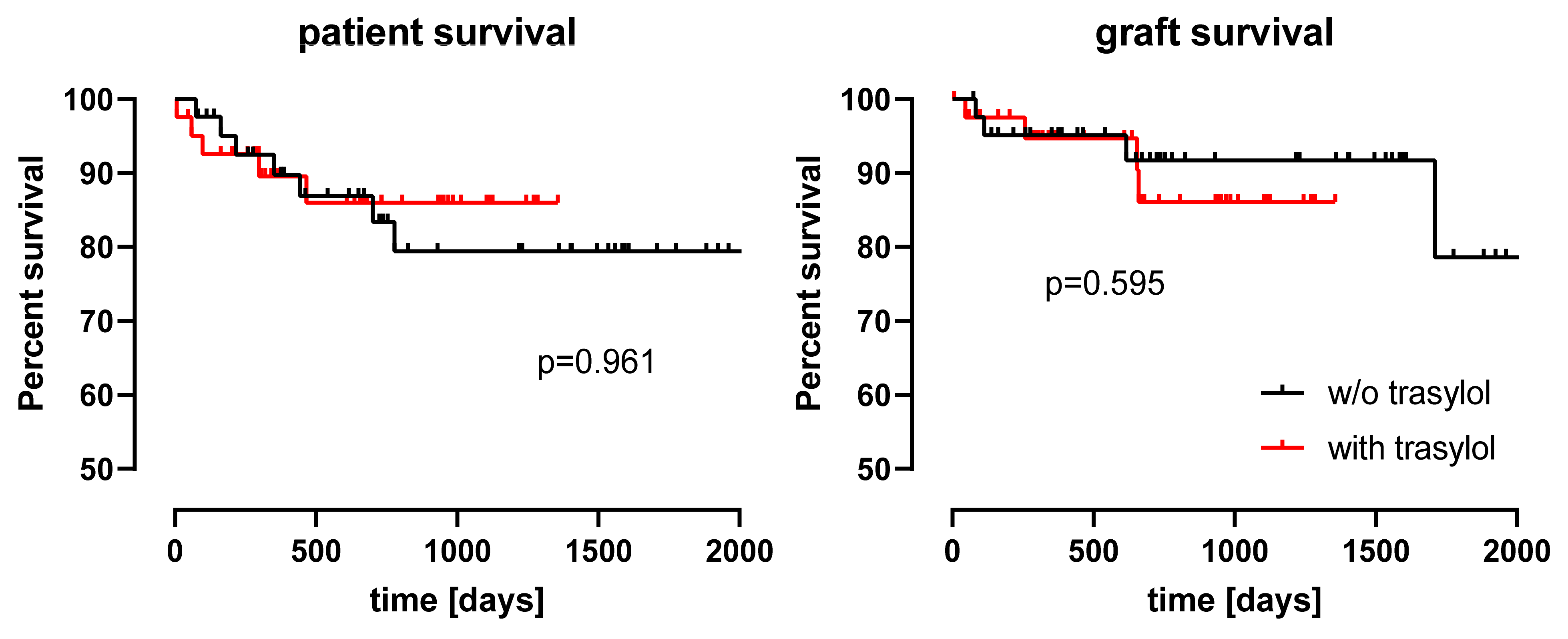
| Whole Cohort | Matched Cohort | ||||
|---|---|---|---|---|---|
| Controls (n = 232) | p | Aprotinin (n = 42) | p | Controls (n = 42) | |
| Age (years) | 53.5 ± 10.8 | 0.048 | 57.0 ± 6.6 | 0.937 | 57.1 ± 7.2 |
| Sex (female. n) | 79 (34.1%) | 0.214 | 10 (23.8%) | 0.601 | 10 (23.8%) |
| BM (kg/cm2) | 26.6 ± 5.2 | 0.702 | 27.0 ± 5.3 | 0.361 | 28.1 ± 5.7 |
| labMELD score | 19.9 ± 10.9 | 0.993 | 19.9 ± 8.1 | 0.061 | 16.4 ± 8.6 |
| Reason for transplantation | |||||
| Alcoholic Cirrhosis (n) | 57 (24.7%) | 0.784 | 15 (35.7%) | 0.596 | 17 (40.5%) |
| Hepatocellular Carcinoma (n) | 56 (24.2%) | 9 (21.4%) | 14 (33.3%) | ||
| Acute Liver Failure (n) | 28 (12.1%) | 3 (7.1%) | 1 (2.4%) | ||
| Primary Biliary Cirrhosis (n) | 22 (9.5%) | 2 (4.8%) | 2 (4.8%) | ||
| HBV or HCV Cirrhosis (n) | 15 (6.5%) | 3 (7.1%) | 2 (4.8%) | ||
| Graft Failure (n) | 14 (6.1%) | 3 (7.1%) | 0 (0.0%) | ||
| Nonalcoholic Steatohepatitis (n) | 7 (3.0%) | 2 (4.8%) | 2 (4.8%) | ||
| Other (n) | 33 (14.2%) | 5 (11.9%) | 4 (9.5%) | ||
| Preoperative Clinical Chemistry | |||||
| Creatinine (mg/dl) | 1.6 ± 1.5 | 0.342 | 1.8 ± 1.5 | 0.189 | 1.4 ± 1.4 |
| AST (U/L) | 374.2 ± 1297.0 | 0.568 | 518.5 ± 1998.9 | 0.188 | 80.6 ± 100.7 |
| ALT (U/L) | 303.3 ± 1081.7 | 0.859 | 269.5 ± 1007.9 | 0.205 | 54.2 ± 55.3 |
| Bilirubin (mg/dL) | 7.7 ± 10.0 | 0.672 | 7.0 ± 8.5 | 0.113 | 4.3 ± 6.3 |
| GGT (U/L) | 177.8 ± 239.1 | 0.694 | 160.8 ± 278.4 | 0.817 | 173.4 ± 184.2 |
| GLDH (U/L) | 191.9 ± 936.0 | 0.682 | 275.2 ± 1274.6 | 0.249 | 8.8 ± 10.6 |
| Whole Cohort | Matched Cohort | ||||
|---|---|---|---|---|---|
| Controls (n = 232) | p | Aprotinin (n = 42) | p | Controls (n = 42) | |
| Age (years) | 54.3 ± 16.7 | 0.021 | 59.0 ± 10.8 | 0.354 | 61.6 ± 14.5 |
| Sex (female) | 107 (46.1%) | 0.243 | 24 (57.1%) | 0.127 | 17 (40.5%) |
| BMI (kg/cm2) | 28.8 ± 7.4 | 0.001 | 34.7 ± 8.9 | 0.018 | 30.3 ± 7.5 |
| Clinical chemistry | |||||
| AST (U/L) | 108.1 ± 182.6 | 0.564 | 127.9 ± 256.8 | 0.869 | 72.2 ± 75.9 |
| ALT (U/L) | 93.3 ± 223.2 | 0.837 | 85.7 ± 114.2 | 0.032 | 43.6 ± 38.6 |
| Bilirubin (mmol/L) | 0.8 ± 1.1 | 0.755 | 0.7 ± 0.9 | 0.193 | 1.1 ± 1.5 |
| Sodium (mmol/L) | 149.4 ± 10.3 | 0.984 | 149.4 ± 7.9 | 0.182 | 148.4 ± 7.2 |
| ICU length of stay (days) | 4.9 ± 5.1 | 0.132 | 6.1 ± 9.7 | 0.587 | 6.4 ± 8.0 |
| Organ Quality a | |||||
| good | 188 (81.0%) | 0.001 | 12 (28.6%) | 0.960 | 12 (28.6%) |
| acceptable | 30 (12.9%) | 22 (52.4%) | 21 (50%) | ||
| poor | 14 (6.0%) | 8 (19%) | 9 (21.4%) | ||
| Graft fat content | |||||
| Macrovesicular (%) | 21.3 ± 22.8 | 0.057 | 32.1 ± 23.0 | 0.319 | 23.5 ± 25.1 |
| Microvesicular (%) | 42.3 ± 28.5 | 0.198 | 50.6 ± 22.9 | 0.246 | 42.7 ± 29.9 |
| Time to transplantation | |||||
| Cold Ischemia Time (min) | 496.8 ± 125.3 | 0.195 | 524.6 ± 137.5 | 0.281 | 509.5 ± 119.0 |
| Warm Ischemia Time (min) | 45.0 ± 8.1 | 0.363 | 46.2 ± 8.0 | 0.593 | 44.2 ± 9.1 |
| Extended donor criteria | |||||
| Age > 65 years (n) | 62 (26.7%) | 0.851 | 12 (28.6%) | 0.340 | 17 (40.5%) |
| BMI > 30 (n) | 61 (26.3%) | 0.001 | 24 (57.1%) | 0.008 | 12 (28.6%) |
| ICU stay > 7 days (n) | 50 (21.6%) | 0.312 | 7 (16.7%) | 0.287 | 11 (26.2%) |
| Elevated Transaminases b (n) | 49 (21.1%) | 0.416 | 10 (23.8%) | 0.154 | 5 (11.9%) |
| CIT > 10 h | 41 (17.7%) | 0.079 | 12 (28.6%) | 0.450 | 9 (21.4%) |
| Bilirubin > 3 mmol/L | 9 (3.9%) | 0.530 | 1 (2.4%) | 0.645 | 5 (11.9%) |
| Steatosis > 40% | 45 (19.4%) | 0.001 | 20 (47.6%) | 0.268 | 15 (35.7%) |
| Sodium > 165 mmol/L | 19 (8.2%) | 0.345 | 2 (4.8%) | 0.645 | 3 (7.1%) |
| Number of Extented donor criteria | |||||
| 0 (n) | 64 (27.6%) | 0.111 | 4 (9.5%) | 0.682 | 4 (9.5%) |
| 1 or 2 (n) | 138 (59.5%) | 28 (66.6%) | 32 (76.2%) | ||
| ≥ 3 (n) | 29 (12.5%) | 10 (23.8%) | 6 (14.3%) | ||
| Whole Cohort | Matched Cohort | ||||
|---|---|---|---|---|---|
| Controls (n = 232) | p | Aprotinin (n = 42) | p | Controls (n = 42) | |
| Intraoperative Complications | |||||
| Postreperfusion Syndrome (n) | 72 (31.0%) | 0.013 | 22 (52.4%) | 0.414 | 20 (47.6%) |
| Hyperfibrinolysis (n) | 10 (4.3%) | 0.319 | 3 (7.1%) | 1.000 | 4 (9.5%) |
| Intraoperative Transfusions | |||||
| Packed Red Blood Cells (U) | 8.1 ± 7.5 | 0.296 | 13.7 ± 32.0 | 0.374 | 8.8 ± 6.9 |
| Fresh Frozen Plasma (U) | 15.7 ± 8.3 | 0.744 | 15.2 ± 9.0 | 0.380 | 17.1 ± 9.5 |
| Platelets (U) | 0.9 ± 1.3 | 0.267 | 1.2 ± 1.2 | 0.191 | 0.8 ± 1.1 |
| Fibrinogen (g) | 2.3 ± 2.9 | 0.013 | 3.6 ± 3.2 | 0.094 | 2.4 ± 3.0 |
| 4F-PCC (IU) | 1010.9 ± 1534.3 | 0.492 | 1200.0 ± 1450.1 | 0.566 | 986.8 ± 1710.4 |
| Postoperative Complications | |||||
| Early Allograft Dysfunction (n) | 72 (31.0%) | 0.001 | 27 (64.3%) | 0.029 | 17 (40.5%) |
| Rejection Episodes (n) | 51 (22.0%) | 0.406 | 12 (14.3%) | 0.241 | 6 (7.1%) |
| Acute Kidney Injury (n) | 51 (22.0%) | 0.001 | 20 (48.8%) | 0.033 | 11 (26.2%) |
| Renal Replacement Therapy (n) | 31 (13.4%) | 0.001 | 10 (24.4%) | 0.015 | 3 (7.1%) |
| Retransplantation | |||||
| 30 Day Retransplantation (n) | 7 (3.0%) | 0.070 | 4 (9.5%) | 0.167 | 1 (2.4%) |
| 1 Year Retransplantation (n) | 10 (4.3%) | 0.242 | 4 (9.5%) | 0.693 | 3 (7.1%) |
| Reasons for Retransplantation | |||||
| Arterial Thrombosis (n) | 0 (0%) | 0.036 | 1 (2.4%) | 0.306 | 0 |
| Primary Non-Function (n) | 6 (2.6%) | 3 (7.1%) | 1 (2.4%) | ||
| Ischemic Type Biliary Lesions (n) | 2 (0.9%) | 0 (0.0%) | 2 (4.8%) | ||
| Tumor (n) | 1 (0.4%) | 0 (0.0%) | 1 (2.4%) | ||
| Postoperative Clinical Chemistry | |||||
| Creatinine. peak (mg/dL) | 2.3 ± 1.7 | 0.150 | 2.7 ± 1.6 | 0.812 | 2.6 ± 2.2 |
| AST. peak (U/L) | 1743.9 ± 2317.2 | 0.001 | 3193.6 ± 2273.5 | 0.010 | 1984.2 ± 1883.9 |
| ALT. peak (U/L) | 1024 ± 1212.6 | 0.004 | 1611.3 ± 1152.4 | 0.007 | 975.2 ± 937.4 |
| Bilirubine. peak (mg/L) | 6.3 ± 5.5 | 0.155 | 7.6 ± 5.1 | 0.167 | 6.2 ± 4.2 |
| GGT. peak (U/L) | 436.4 ± 349.7 | 0.413 | 502.9 ± 499.7 | 0.405 | 424.1 ± 349.1 |
| GLDH. peak (U/L) | 731.6 ± 1285.4 | 0.025 | 1199.4 ± 894.8 | 0.459 | 1002.3 ± 1464.7 |
| Mortality | |||||
| 30 Day Mortality (n) | 8 (3.4%) | 0.654 | 2 (4.8%) | 0.152 | 0 (0.0%) |
| 1 Year Mortality (n) | 22 (9.5%) | 0.580 | 5 (11.9%) | 0.724 | 4 (9.5%) |
| Overall Mortality (n) | 31 (13.4%) | 0.810 | 6 (14.3%) | 0.763 | 7 (16.7%) |
| Early Allograft Dysfunction | Peak AST after Transplantation | |||||
|---|---|---|---|---|---|---|
| OR | (95%CI) | p | beta | (95%CI) | p | |
| Recipient data | ||||||
| Age (years) | 0.95 | (0.86–1.04) | 0.242 | −33.4 | (−107.8–41.0) | 0.373 |
| Sex (female) | 0.64 | (0.15–2.66) | 0.534 | −437. 6 | (−1632.9–757.7) | 0.467 |
| BMI (kg/cm2) | 1.02 | (0.9–1.15) | 0.768 | −19.0 | (−120.3–82.3) | 0.709 |
| MELD score | 0.98 | (0.92–1.06) | 0.671 | −47.4 | (−104.7–10.0) | 0.104 |
| Donor data | ||||||
| Age (years) | 0.98 | (0.94–1.03) | 0.478 | −64.0 | (−103.7–−24.3) | 0.002 |
| BMI (kg/cm2) | 0.96 | (0.89–1.02) | 0.181 | −22.9 | (−79.6–33.9) | 0.423 |
| AST (U/L) | 1.00 | (1.00–1.00) | 0.833 | −2.0 | (−4.6–0.5) | 0.115 |
| Cold Ischemia Time (minutes) | 1.00 | (1.00–1.01) | 0.994 | 1.4 | (−2.5–5.2) | 0.483 |
| Organ Quality a | ||||||
| acceptable | 4.95 | (1.26–19.46) | 0.022 | 1770.0 | (814.4–3031.2) | 0.001 |
| poor | 11.78 | (1.99–69.55) | 0.007 | 1324.8 | (387.6–3152.4) | 0.013 |
| Treatment with Aprotinin | 4.12 | (1.21–14.00) | 0.023 | −33.4 | (354.0–2295.6) | 0.008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roehl, A.B.; Andert, A.; Junge, K.; Neumann, U.P.; Hein, M.; Kork, F. Effect of Aprotinin on Liver Injury after Transplantation of Extended Criteria Donor Grafts in Humans: A Retrospective Propensity Score Matched Cohort Analysis. J. Clin. Med. 2021, 10, 5232. https://doi.org/10.3390/jcm10225232
Roehl AB, Andert A, Junge K, Neumann UP, Hein M, Kork F. Effect of Aprotinin on Liver Injury after Transplantation of Extended Criteria Donor Grafts in Humans: A Retrospective Propensity Score Matched Cohort Analysis. Journal of Clinical Medicine. 2021; 10(22):5232. https://doi.org/10.3390/jcm10225232
Chicago/Turabian StyleRoehl, Anna B., Anne Andert, Karsten Junge, Ulf P. Neumann, Marc Hein, and Felix Kork. 2021. "Effect of Aprotinin on Liver Injury after Transplantation of Extended Criteria Donor Grafts in Humans: A Retrospective Propensity Score Matched Cohort Analysis" Journal of Clinical Medicine 10, no. 22: 5232. https://doi.org/10.3390/jcm10225232
APA StyleRoehl, A. B., Andert, A., Junge, K., Neumann, U. P., Hein, M., & Kork, F. (2021). Effect of Aprotinin on Liver Injury after Transplantation of Extended Criteria Donor Grafts in Humans: A Retrospective Propensity Score Matched Cohort Analysis. Journal of Clinical Medicine, 10(22), 5232. https://doi.org/10.3390/jcm10225232






