The Clinical Significance of Cerebrospinal Fluid Reticulon 4 (RTN4) Levels in the Differential Diagnosis of Neurodegenerative Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Proteins Measurement
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics and CSF Concentrations of RTN-4
3.2. Correlation Analysis of RTN-4 and CSF Biomarkers
3.3. Diagnostic Usefulness of RTN-4 as a Candidate Biomarker in the Differential Diagnosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomez-Rio, M.; Caballero, M.M.; Sáez, J.M.G.; Minguez-Castellanos, A. Diagnosis of Neurodegenerative Diseases: The Clinical Approach. Curr. Alzheimer Res. 2016, 13, 469–474. [Google Scholar] [CrossRef]
- Iram, S.; Vialatte, F.-B.; Qamar, M.I. Early Diagnosis of Neurodegenerative Diseases from Gait Discrimination to Neural Synchronization. In Applied Computing in Medicine and Health; Elsevier BV: Amsterdam, The Netherlands, 2016; pp. 1–26. [Google Scholar]
- Lewczuk, P.; Riederer, P.; O’Bryant, S.E.; Verbeek, M.M.; Dubois, B.; Visser, P.J.; Jellinger, K.A.; Engelborghs, S.; Ramirez, A.; Parnetti, L.; et al. Cerebrospinal fluid and blood biomarkers for neurodegenerative dementias: An update of the Consensus of the Task Force on Biological Markers in Psychiatry of the World Federation of Societies of Biological Psychiatry. World J. Biol. Psychiatry 2018, 19, 244–328. [Google Scholar] [CrossRef]
- Robey, T.T.; Panegyres, P.K. Cerebrospinal fluid biomarkers in neurodegenerative disorders. Futur. Neurol. 2019, 14, FNL6. [Google Scholar] [CrossRef]
- Molinuevo, J.L.; Ayton, S.; Batrla, R.; Bednar, M.M.; Bittner, T.; Cummings, J.; Fagan, A.M.; Hampel, H.; Mielke, M.; Mikulskis, A.; et al. Current state of Alzheimer’s fluid biomarkers. Acta Neuropathol. 2018, 136, 821–853. [Google Scholar] [CrossRef]
- Olsson, B.; Lautner, R.; Andreasson, U.; Öhrfelt, A.; Portelius, E.; Bjerke, M.; Hölttä, M.; Rosén, C.; Olsson, C.; Strobel, G.; et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: A systematic review and meta-analysis. Lancet Neurol. 2016, 15, 673–684. [Google Scholar] [CrossRef]
- Ziemann, U.; Wahl, M.; Hattingen, E.; Tumani, H. Development of biomarkers for multiple sclerosis as a neurodegenerative disorder. Prog. Neurobiol. 2011, 95, 670–685. [Google Scholar] [CrossRef] [PubMed]
- Deisenhammer, F.; Zetterberg, H.; Fitzner, B.; Zettl, U.K. The cerebrospinal fluid in multiple sclerosis. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Wiltfang, J.; Lewczuk, P.; Otto, M. Biomarker bei Demenzen und anderen neurodegenerativen Erkrankungen. Nervenarzt 2016, 87, 1305–1309. [Google Scholar] [CrossRef]
- Chaudhry, N.; Filbin, M.T. Myelin—Associated Inhibitory Signaling and Strategies to Overcome Inhibition. Br. J. Pharmacol. 2006, 27, 1096–1107. [Google Scholar] [CrossRef]
- Schwab, M.E. Functions of Nogo proteins and their receptors in the nervous system. Nat. Rev. Neurosci. 2010, 11, 799–811. [Google Scholar] [CrossRef]
- Kurowska, Z.; Brundin, P.; Schwab, M.; Li, J.-Y. Intracellular Nogo-A facilitates initiation of neurite formation in mouse midbrain neurons in vitro. Neuroscience 2014, 256, 456–466. [Google Scholar] [CrossRef]
- Zemmar, A.; Weinmann, O.; Kellner, Y.; Yu, X.; Vicente, R.; Gullo, M.; Kasper, H.; Lussi, K.; Ristic, Z.; Luft, A.R.; et al. Neutralization of Nogo-A Enhances Synaptic Plasticity in the Rodent Motor Cortex and Improves Motor Learning in Vivo. J. Neurosci. 2014, 34, 8685–8698. [Google Scholar] [CrossRef]
- Ullah, H.; Elfadl, A.; Park, S.; Kim, Y.; Chung, M.-J.; Son, J.-Y.; Yun, H.-H.; Park, J.-M.; Yim, J.-H.; Jung, S.-J.; et al. Nogo-A Is Critical for Pro-Inflammatory Gene Regulation in Myocytes and Macrophages. Cells 2021, 10, 282. [Google Scholar] [CrossRef]
- Karnezis, T.; Mandemakers, W.; McQualter, J.L.; Zheng, B.; Ho, P.P.; Jordan, K.A.; Murray, B.M.; Barres, B.; Tessier-Lavigne, M.; Bernard, C.C.A. The neurite outgrowth inhibitor Nogo A is involved in autoimmune-mediated demyelination. Nat. Neurosci. 2004, 7, 736–744. [Google Scholar] [CrossRef]
- Yan, R.; Shi, Q.; Hu, X.; Zhou, X. Reticulon proteins: Emerging players in neurodegenerative diseases. Cell. Mol. Life Sci. 2006, 63, 877–889. [Google Scholar] [CrossRef]
- Zagrebelsky, M.; Lonnemann, N.; Fricke, S.; Kellner, Y.; Preuß, E.; Michaelsen-Preusse, K.; Korte, M. Nogo-A regulates spatial learning as well as memory formation and modulates structural plasticity in the adult mouse hippocampus. Neurobiol. Learn. Mem. 2017, 138, 154–163. [Google Scholar] [CrossRef]
- He, W.; Lu, Y.; Qahwash, I.; Hu, X.-Y.; Chang, A.; Yan, R. Reticulon family members modulate BACE1 activity and amyloid-β peptide generation. Nat. Med. 2004, 10, 959–965. [Google Scholar] [CrossRef]
- Luo, H.-M.; Xiao, F.; Lin, L.-F.; Cheng, X.; Gao, Q. Nogo-66 receptor activation inhibits neurite outgrowth and increases β-amyloid protein secretion of cortical neurons. Mol. Med. Rep. 2011, 5, 619–624. [Google Scholar] [CrossRef]
- Xie, Q.-Q.; Feng, X.; Huang, Y.-Y.; Fang, N.; Yi, H.; Wang, Z.-J.; Cao, Q.-Y.; Lou, G.-F.; Pan, J.-P.; Hu, Y.; et al. Nogo-66 promotes β-amyloid protein secretion via NgR/ROCK-dependent BACE1 activation. Mol. Med. Rep. 2021, 23, 1. [Google Scholar] [CrossRef]
- Schawkat, K.; Di Santo, S.; Seiler, S.; Ducray, A.; Widmer, H.R. Loss of Nogo-A-expressing neurons in a rat model of Parkinson’s disease. Neuroscience 2015, 288, 59–72. [Google Scholar] [CrossRef]
- Seiler, S.; Di Santo, S.; Widmer, H.R. Nogo-A Neutralization Improves Graft Function in a Rat Model of Parkinson’s Disease. Front. Cell. Neurosci. 2016, 10, 87. [Google Scholar] [CrossRef]
- Inoue, H.; Lin, L.; Lee, X.; Shao, Z.; Mendes, S.; Snodgrass-Belt, P.; Sweigard, H.; Engber, T.; Pepinsky, B.; Yang, L.; et al. Inhibition of the leucine-rich repeat protein LINGO-1 enhances survival, structure, and function of dopaminergic neurons in Parkinson’s disease models. Proc. Natl. Acad. Sci. USA 2007, 104, 14430–14435. [Google Scholar] [CrossRef]
- Zhang, N.; Cui, Y.; Li, Y.; Mi, Y. A Novel Role of Nogo Proteins: Regulating Macrophages in Inflammatory Disease. Cell. Mol. Neurobiol. 2021, 1–10. [Google Scholar] [CrossRef]
- Jurewicz, A.; Matysiak, M.; Raine, C.S.; Selmaj, K. Soluble Nogo-A, an inhibitor of axonal regeneration, as a biomarker for multiple sclerosis. Neurology 2007, 68, 283–287. [Google Scholar] [CrossRef]
- Satoh, J.-I.; Onoue, H.; Arima, K.; Yamamura, T. Nogo-A and Nogo Receptor Expression in Demyelinating Lesions of Multiple Sclerosis. J. Neuropathol. Exp. Neurol. 2005, 64, 129–138. [Google Scholar] [CrossRef]
- Bedri, S.K.; Nilsson, O.B.; Fink, K.; Månberg, A.; Hamsten, C.; Ayoglu, B.; Manouchehrinia, A.; Nilsson, P.; Olsson, T.; Hillert, J.; et al. Plasma protein profiling reveals candidate biomarkers for multiple sclerosis treatment. PLoS ONE 2019, 14, e0217208. [Google Scholar] [CrossRef]
- Reindl, M.; Khantane, S.; Ehling, R.; Schanda, K.; Lutterotti, A.; Brinkhoff, C.; Oertle, T.; Schwab, M.E.; Deisenhammer, F.; Berger, T.; et al. Serum and cerebrospinal fluid antibodies to Nogo-A in patients with multiple sclerosis and acute neurological disorders. J. Neuroimmunol. 2003, 145, 139–147. [Google Scholar] [CrossRef]
- Ineichen, B.V.; Plattner, P.S.; Good, N.; Martin, R.; Linnebank, M.; Schwab, M.E. Nogo-A Antibodies for Progressive Multiple Sclerosis. CNS Drugs 2017, 31, 187–198. [Google Scholar] [CrossRef]
- Heath, J.E.; Siedlak, S.L.; Zhu, X.; Lee, H.-G.; Thakur, A.; Yan, R.; Perry, G.; Smith, M.A.; Castellani, R.J. Widespread distribution of reticulon-3 in various neurodegenerative diseases. Neuropathology 2010, 30, 574–579. [Google Scholar] [CrossRef]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef]
- Lewczuk, P.; Zimmermann, R.; Wiltfang, J.; Kornhuber, J. Neurochemical dementia diagnostics: A simple algorithm for interpretation of the CSF biomarkers. J. Neural Transm. 2009, 116, 1163–1167. [Google Scholar] [CrossRef]
- Berardelli, A.; Wenning, G.; Antonini, A.; Berg, D.; Bloem, B.R.; Bonifati, V.; Brooks, D.; Burn, D.; Colosimo, C.; Fanciulli, A.; et al. EFNS/MDS-ES recommendations for the diagnosis of Parkinson’s disease. Eur. J. Neurol. 2012, 20, 16–34. [Google Scholar] [CrossRef]
- Thompson, A.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Mroczko, B.; Groblewska, M.; Zboch, M.; Muszyński, P.; Zajkowska, A.; Borawska, R.; Szmitkowski, M.; Kornhuber, J.; Lewczuk, P. Evaluation of Visinin-Like Protein 1 Concentrations in the Cerebrospinal Fluid of Patients with Mild Cognitive Impairment as a Dynamic Biomarker of Alzheimer’s Disease. J. Alzheimer’s Dis. 2014, 43, 1031–1037. [Google Scholar] [CrossRef]
- R Core Team. 2021. Available online: https://www.r-project.org/ (accessed on 3 September 2021).
- Lee, J.Y.; Petratos, S. Multiple Sclerosis. Neuroscientist 2013, 19, 394–408. [Google Scholar] [CrossRef]
- Demirel, O.F.; Çetin, I.; Turan, Ş.; Sağlam, T.; Yıldız, N.; Duran, A. Decreased Expression of α-Synuclein, Nogo-A and UCH-L1 in Patients with Schizophrenia: A Preliminary Serum Study. Psychiatry Investig. 2017, 14, 344–349. [Google Scholar] [CrossRef]
- Gil, V.; Nicolas, O.; Mingorance, A.; Ureña, J.M.; Tang, B.L.; Hirata, T.; Sáez-Valero, J.; Ferrer, I.; Soriano, E.; Del Río, J.A. Nogo-A Expression in the Human Hippocampus in Normal Aging and in Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2006, 65, 433–444. [Google Scholar] [CrossRef]
- Shi, Q.; Ge, Y.; He, W.; Hu, X.; Yan, R. RTN1 and RTN3 protein are differentially associated with senile plaques in Alzheimer’s brains. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Murayama, K.S.; Kametani, F.; Saito, S.; Kume, H.; Akiyama, H.; Araki, W. Reticulons RTN3 and RTN4-B/C interact with BACE1 and inhibit its ability to produce amyloid β-protein. Eur. J. Neurosci. 2006, 24, 1237–1244. [Google Scholar] [CrossRef]
- Delekate, A.; Zagrebelsky, M.; Kramer, S.; Schwab, M.E.; Korte, M. NogoA restricts synaptic plasticity in the adult hippocampus on a fast time scale. Proc. Natl. Acad. Sci. USA 2011, 108, 2569–2574. [Google Scholar] [CrossRef]
- Widmer, H.R.; Seiler, S. Nogo-A and its functions beyond axonal inhibition: The controversial role of Nogo-A in Parkinson′s disease. Neural Regen. Res. 2015, 10, 1223–1224. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Ohno, K.; Sano, M.; Omura, T.; Omura, K.; Nagano, A.; Sato, K. The differential expression patterns of messenger RNAs encoding Nogo-A and Nogo-receptor in the rat central nervous system. Mol. Brain Res. 2005, 133, 119–130. [Google Scholar] [CrossRef]
- Nyatia, E.; Lang, D. Localisation and expression of a myelin associated neurite inhibitor, Nogo-A and its receptor Nogo-receptor by mammalian CNS cells. Res. Vet. Sci. 2007, 83, 287–301. [Google Scholar] [CrossRef]
- Mi, Y.; Gao, X.; Ma, Y.; Gao, J.; Wang, Z.; Jin, W. A novel centrosome and microtubules associated subcellular localization of Nogo-A: Implications for neuronal development. Int. J. Biochem. Cell Biol. 2014, 57, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, J.W.; Crawford, M.P.; Hatfield, L.M. Soluble Nogo-A in CSF is not a useful biomarker for multiple sclerosis. Neurology 2008, 71, 35–37. [Google Scholar] [CrossRef]
- Jitoku, D.; Hattori, E.; Iwayama, Y.; Yamada, K.; Toyota, T.; Kikuchi, M.; Maekawa, M.; Nishikawa, T.; Yoshikawa, T. Association study of Nogo-related genes with schizophrenia in a Japanese case-control sample. Am. J. Med Genet. Part B Neuropsychiatr. Genet. 2011, 156, 581–592. [Google Scholar] [CrossRef]
- Beharry, C.; Cohen, L.S.; Di, J.; Ibrahim, K.; Briffa-Mirabella, S.; Alonso, A.D.C. Tau-induced neurodegeneration: Mechanisms and targets. Neurosci. Bull. 2014, 30, 346–358. [Google Scholar] [CrossRef]
- Min, S.-W.; Chen, X.; Tracy, T.E.; Li, Y.; Zhou, Y.; Wang, C.; Shirakawa, K.; Minami, S.S.; Defensor, E.; Mok, S.-A.; et al. Critical role of acetylation in tau-mediated neurodegeneration and cognitive deficits. Nat. Med. 2015, 21, 1154–1162. [Google Scholar] [CrossRef]
- Fang, Y.; Yao, L.; Li, C.; Wang, J.; Wang, J.; Chen, S.; Zhou, X.-F.; Liao, H. The blockage of the Nogo/NgR signal pathway in microglia alleviates the formation of Aβ plaques and tau phosphorylation in APP/PS1 transgenic mice. J. Neuroinflamm. 2016, 13, 56. [Google Scholar] [CrossRef]
- Zuo, Y.-C.; Li, H.-L.; Xiong, N.-X.; Shen, J.-Y.; Huang, Y.-Z.; Fu, P.; Zhao, H.-Y. Overexpression of Tau Rescues Nogo-66-Induced Neurite Outgrowth Inhibition In Vitro. Neurosci. Bull. 2016, 32, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Gil, V.; Bichler, Z.; Lee, J.K.; Seira, O.; Llorens, F.; Bribian, A.; Morales-Carbajal, R.; Claverol-Tinture, E.; Soriano, E.; Sumoy, L.; et al. Developmental Expression of the Oligodendrocyte Myelin Glycoprotein in the Mouse Telencephalon. Cereb. Cortex 2009, 20, 1769–1779. [Google Scholar] [CrossRef] [PubMed]
- Mingorance, A.; Soriano-García, E.; Del Rio, J.A. [Nogo-A functions during the development of the central nervous system and in the adult]. Rev. De Neurol. 2004, 39, 39. [Google Scholar]
- Kumari, A.; Thakur, M.K. Age-Dependent Decline of Nogo-A Protein in the Mouse Cerebrum. Cell. Mol. Neurobiol. 2014, 34, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- VanGuilder, H.D.; Bixler, G.V.; Sonntag, W.; Freeman, W.M. Hippocampal expression of myelin-associated inhibitors is induced with age-related cognitive decline and correlates with deficits of spatial learning and memory. J. Neurochem. 2012, 121, 77–98. [Google Scholar] [CrossRef] [PubMed]
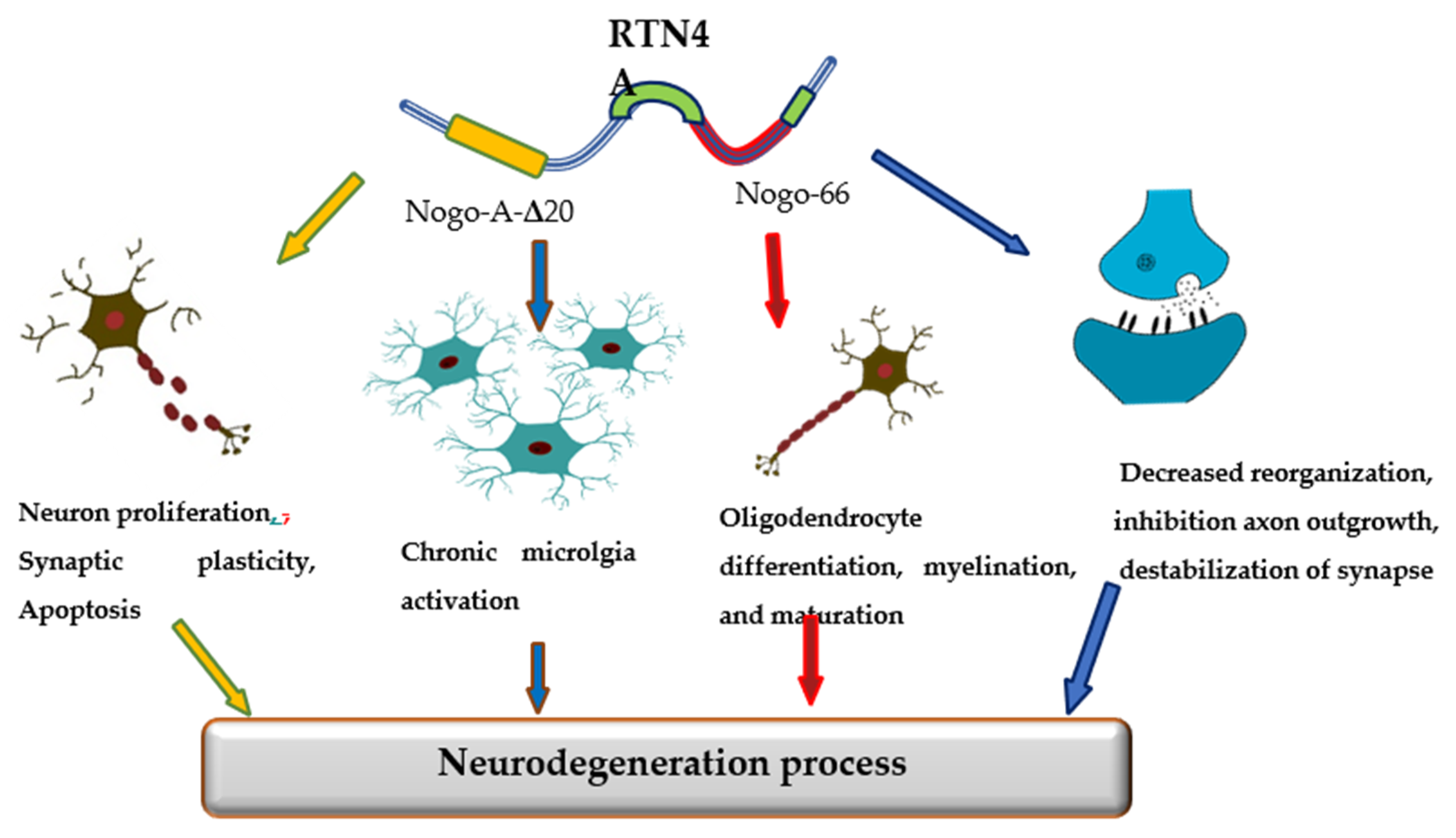
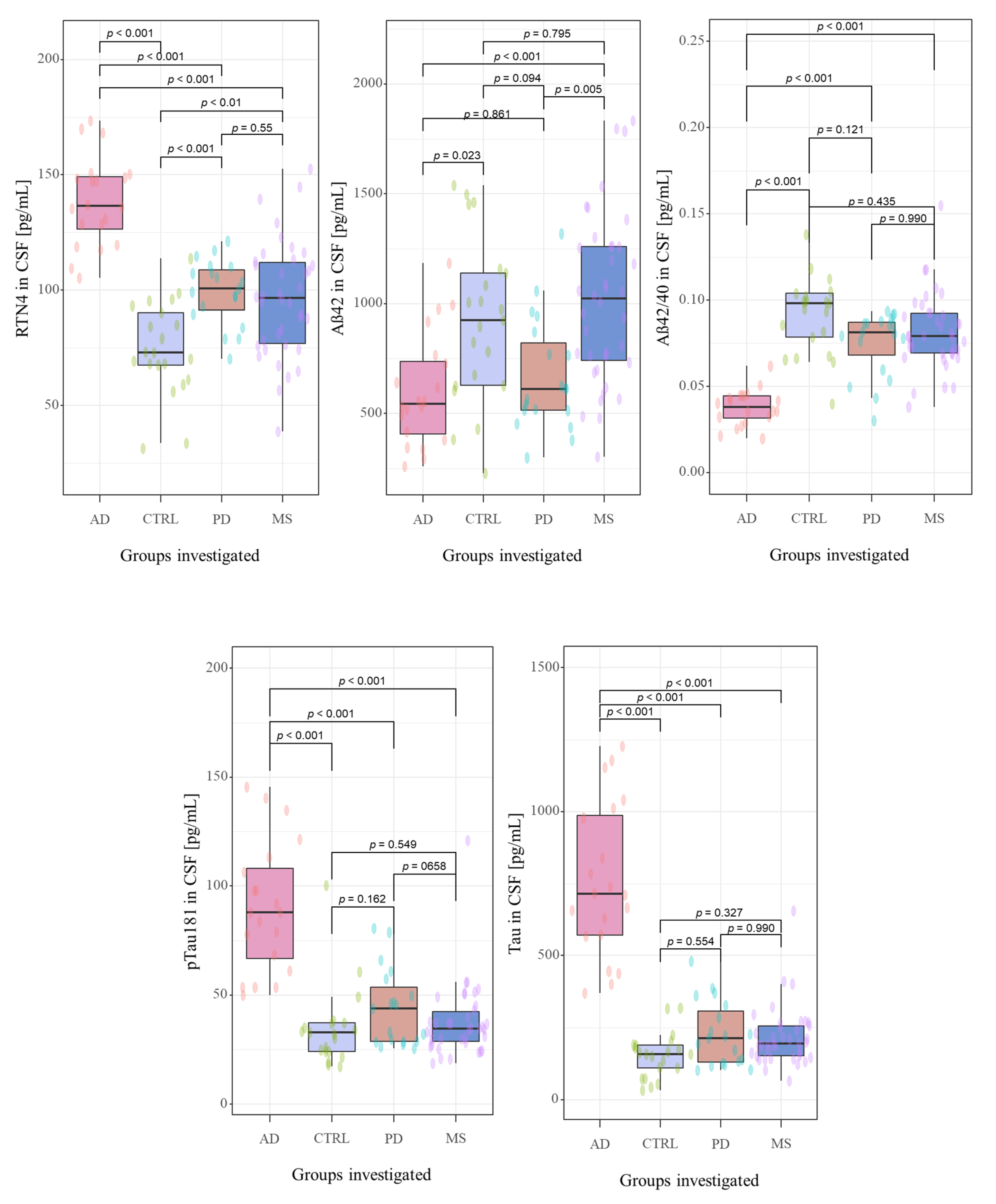

| Biomarkers | AD | PD | MS | CTRL | Kruskal-Wallis Test | Significance (Dwass Steel Critchlow-Fligner Test) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Median (Interquartile Range) | Median (Interquartile Range) | Median (Interquartile Range) | Median (Interquartile Range) | AD vs. CTRL | PD vs. CTRL | MS vs. CTRL | AD vs. PD | PD vs. MS | AD vs. MS | ||
| RTN-4 (pg/mL) | 137 | 101 | 97 | 73 | <0.001 | <0.001 | 0.001 | 0.009 | <0.001 | 0.929 | <0.001 |
| (126–149) | (91–109) | (77–112) | (68–90) | ||||||||
| Aβ-42 (pg/mL) | 544 | 612 | 1023 | 925 | <0.001 | 0.023 | 0.094 | 0.795 | 0.861 | 0.005 | <0.001 |
| (407–736) | (515–821) | (741–1260) | (627–1084) | ||||||||
| Aβ-42/Aβ-40 ratio | 0.038 | 0.081 | 0.079 | 0.095 | |||||||
| (0.032–0.045) | (0.068–0.087) | (0.069–0.092) | (0.070–0.102) | <0.001 | <0.001 | 0.121 | 0.435 | <0.001 | 0.990 | <0.001 | |
| Tau (pg/mL) | 714 | 213 | 194 | 167 | <0.001 | < 0.001 | 0.554 | 0.327 | <0.001 | 0.990 | <0.001 |
| (571–987) | (130–306) | (152–255) | (110–190) | ||||||||
| pTau181 (pg/mL) | 88 | 44 | 35 | 33 | <0.001 | < 0.001 | 0.162 | 0.549 | <0.001 | 0.658 | <0.001 |
| (67–108) | (29–54) | (29–42) | (24–37) | ||||||||
| Variable Tested | ROC Criteria in AD Compared to CTRL | |||
|---|---|---|---|---|
| AUC | SE | 95% C.I. (AUC) | p | |
| RTN4 | 0.995 | 0.006 | 0.9839–1 | <0.001 |
| Aβ42 | 0.762 | 0.077 | 0.6103–0.9135 | <0.001 |
| Aβ42/40 | 0.976 | 0.024 | 0.9283–1 | <0.001 |
| Tau | 0.895 | 0.066 | 0.7663–1 | <0.001 |
| pTau181 | 1.00 | 0.00 | 1–1 | <0.001 |
| ROC criteria inPD compared to CTRL | ||||
| RTN4 | 0.847 | 0.061 | 0.7267–0.9675 | <0.001 |
| Aβ42 | 0.719 | 0.084 | 0.5546–0.884 | 0.005 |
| Aβ42/40 | 0.739 | 0.083 | 0.5159–0.8575 | 0.002 |
| Tau | 0.687 | 0.091 | 0.5159–0.8575 | 0.063 |
| pTau181 | 0.639 | 0.087 | 0.4607–0.8175 | 0.016 |
| ROC criteria inAD compared to PD | ||||
| RTN4 | 0.958 | 0.034 | 0.9024–1 | <0.001 |
| Aβ42 | 0.574 | 0.093 | 0.3896–0.7578 | 0.216 |
| Aβ42/40 | 0.932 | 0.044 | 0.8441–1 | <0.001 |
| Tau | 0.984 | 0.021 | 0.957–1 | <0.001 |
| pTau181 | 0.926 | 0.045 | 0.85–1 | <0.001 |
| ROC criteria inMS compared to CTRL | ||||
| RTN4 | 0.749 | 0.065 | 0.6226–0.8762 | <0.001 |
| Aβ42 | 0.554 | 0.081 | 0.3953–0.7125 | 0.253 |
| Aβ42/40 | 0.653 | 0.078 | 0.4936–0.8122 | 0.030 |
| Tau | 0.698 | 0.073 | 0.5547–0.8413 | 0.003 |
| pTau181 | 0.566 | 0.083 | 0.4023–0.7293 | 0.215 |
| ROC criteria inAD compared to MS | ||||
| RTN4 | 0.909 | 0.038 | 0.8346–0.9838 | <0.001 |
| Aβ42 | 0.824 | 0.054 | 0.7144–0.9343 | <0.001 |
| Aβ42/40 | 0.979 | 0.017 | 0.9488–1 | <0.001 |
| Tau | 0.968 | 0.021 | 0.9161–1 | <0.001 |
| pTau181 | 0.963 | 0.023 | 0.9163–1 | <0.001 |
| ROC criteria inPD compared to MS | ||||
| RTN4 | 0.550 | 0.080 | 0.4003–0.6994 | 0.257 |
| Aβ42 | 0.773 | 0.062 | 0.645–0.9007 | <0.001 |
| Aβ42/40 | 0.525 | 0.081 | 0.3147–0.6354 | 0.380 |
| Tau | 0.501 | 0.082 | 0.317–0.6802 | 0.494 |
| pTau181 | 0.594 | 0.078 | 0.4262–0.7621 | 0.136 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulczyńska-Przybik, A.; Dulewicz, M.; Słowik, A.; Borawska, R.; Kułakowska, A.; Kochanowicz, J.; Mroczko, B. The Clinical Significance of Cerebrospinal Fluid Reticulon 4 (RTN4) Levels in the Differential Diagnosis of Neurodegenerative Diseases. J. Clin. Med. 2021, 10, 5281. https://doi.org/10.3390/jcm10225281
Kulczyńska-Przybik A, Dulewicz M, Słowik A, Borawska R, Kułakowska A, Kochanowicz J, Mroczko B. The Clinical Significance of Cerebrospinal Fluid Reticulon 4 (RTN4) Levels in the Differential Diagnosis of Neurodegenerative Diseases. Journal of Clinical Medicine. 2021; 10(22):5281. https://doi.org/10.3390/jcm10225281
Chicago/Turabian StyleKulczyńska-Przybik, Agnieszka, Maciej Dulewicz, Agnieszka Słowik, Renata Borawska, Alina Kułakowska, Jan Kochanowicz, and Barbara Mroczko. 2021. "The Clinical Significance of Cerebrospinal Fluid Reticulon 4 (RTN4) Levels in the Differential Diagnosis of Neurodegenerative Diseases" Journal of Clinical Medicine 10, no. 22: 5281. https://doi.org/10.3390/jcm10225281
APA StyleKulczyńska-Przybik, A., Dulewicz, M., Słowik, A., Borawska, R., Kułakowska, A., Kochanowicz, J., & Mroczko, B. (2021). The Clinical Significance of Cerebrospinal Fluid Reticulon 4 (RTN4) Levels in the Differential Diagnosis of Neurodegenerative Diseases. Journal of Clinical Medicine, 10(22), 5281. https://doi.org/10.3390/jcm10225281








