One-Year Hemodynamic Performance of Three Cardiac Aortic Bioprostheses: A Randomized Comparative Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Inclusion and Exclusion Criteria
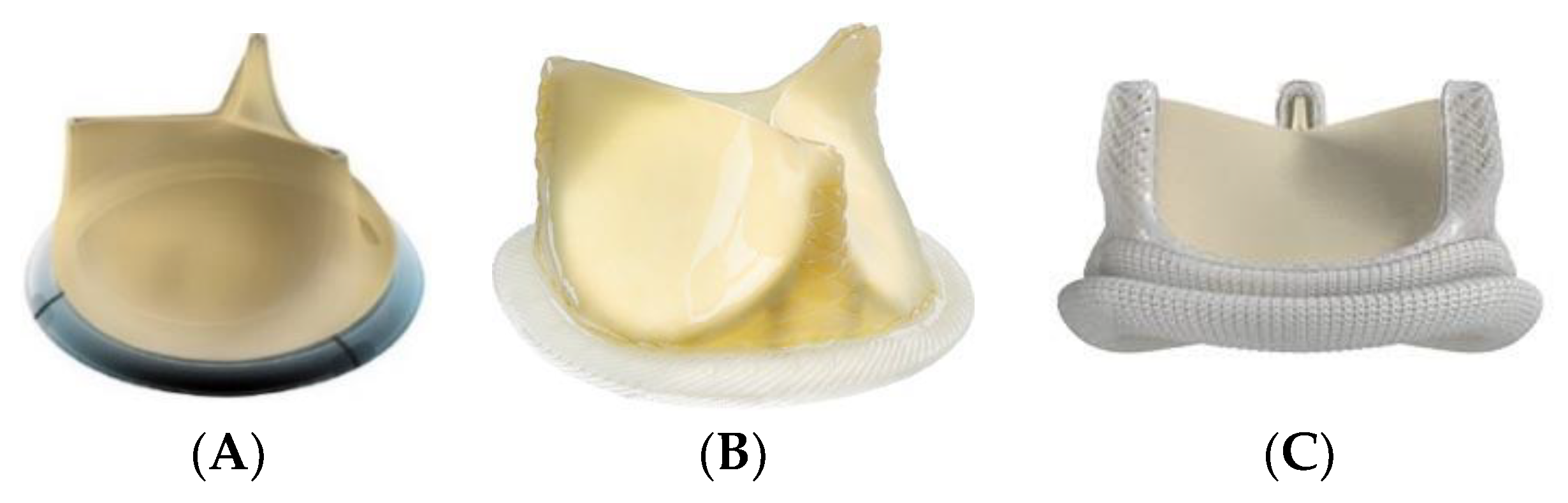
2.3. Surgical Aortic Valve Replacement
2.4. Follow-Up
2.5. Echocardiography
2.6. Statistical Analysis
3. Results
3.1. Intraoperative Data
3.2. Perioperative Outcomes
3.3. Echocardiographic Data 12 Months after Surgery
3.4. Subgroup Analyses
3.5. Postoperative Events at 12 Months
3.6. Survival at 12 Months
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Johnston, D.; Soltesz, E.; Vakil, N.; Rajeswaran, J.; Roseli, E.; Joseph, S.; Sabik, J.F.; Smedira, N.G.; Svensson, L.G.; Lytle, B.W.; et al. Long-term durability of bioprosthetic aortic valves: Implications from 12,569 implants. Ann. Thorac. Surg. 2015, 99, 1239–1247. [Google Scholar] [CrossRef] [Green Version]
- Baumgartner, H.; Falk, V.; Bax, J.; De Bonis, M.; Hamm, C.; Holm, P.; Iung, B.; Lancellotti, P.; Lansac, E.; Munoz, D.R.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. J. Cardiothorac. Surg. 2017, 52, 616–664. [Google Scholar] [CrossRef]
- Nishimura, R.; Otto, C.; Bonow, R.; Carabello, B.; Erwin, J.; Guyon, R.; O’Gara, P.T.; Ruiz, C.E.; Skubas, N.J.; Sorajja, P.; et al. 2014 AHA/ACC Guideline for the management of patients with valvular heart disease: Executive summary. Circulation 2014, 129, 2440–2492. [Google Scholar] [CrossRef]
- Wilkins, E.; Wilson, L.; Wickramasinghe, K.; Bhatnagar, P.; Leal, J.; Luengo, R.-F.; Burns, R.; Rayner, M.; Townsend, N. European Cardiovascular Disease Statistics 2017. Eur. Heart Netw. 2017. Available online: http://www.ehnheart.org/images/CVD-statistics-report-August-2017.pdf (accessed on 31 August 2021).
- Nathaniel, S.; Saligram, S.; Leslie, A. Aortic stenosis: An update. World J. Cardiol. 2010, 2, 135–139. [Google Scholar] [CrossRef] [PubMed]
- D’Arcy, J.; Coffey, S.; Loudon, M.; Kennedy, A.; Pearson, J.; Birks, J.; Frangou, E.; Farmer, A.J.; Mant, D.; Wilson, J.; et al. Large-scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: The OxVALVE Population Cohort Study. Eur. Heart J. 2016, 37, 3515–3522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akahori, H.; Tsujino, T.; Masuyama, T.; Ishihara, M. Mechanisms of aortic stenosis. J. Cardiol. 2018, 71, 215–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, K.; Tzolos, E.; Dweck, M. Pathophysiology of aortic stenosis and future perspectives for medical therapy. Cardiol. Clin. 2020, 38, 1–12. [Google Scholar] [CrossRef]
- Kleinauskiené, R.; Jonkaitiené, R. Degenerative aortic stenosis, dyslipidemia and possibilities of medical treatment. Medicina 2018, 54, 24. [Google Scholar] [CrossRef] [Green Version]
- Kang, D.; Park, S.; Lee, S.; Lee, S.; Kim, D.; Kim, H.; Yun, S.-C.; Hong, G.-R.; Song, J.-M.; Chung, C.-H.; et al. Early surgery or conservative care for asymptomatic aortic stenosis. N. Engl. J. Med. 2020, 382, 111–119. [Google Scholar] [CrossRef]
- Head, S.; Çelik, M.; Kappetein, A. Mechanical versus bioprosthetic aortic valve replacement. Eur. Heart J. 2017, 38, 2183–2191. [Google Scholar] [CrossRef]
- Russo, M.; Tramasso, M.; Guidotti, A.; Pozzoli, A.; Nietilspach, F.; von Segesser, L.; Maisano, F. The Evolution of Surgical Valves. Cardiovasc. Med. 2017, 20, 285–292. [Google Scholar]
- Chiappini, B.; Camurri, N.; Loforte, A.; Di Marco, L.; Di Bartolomeo, R.; Marinelli, G. Outcome after aortic valve replacement in octogenarians. Ann. Thorac. Surg. 2004, 78, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.; Heller, J.; Garcia, F.; Sarnari, R.; Gordon, D.; McCarthy, P.; Barker, A.J.; Etemadi, M.; Markl, M. Detecting aortic valve-induced abnormal flow with seismocardiography and cardiac MRI. Ann. Biomed. Eng. 2020, 48, 1779–1792. [Google Scholar] [CrossRef]
- Woldendorp, K.; Bannon, P.G.; Grieve, S.M. Evaluation of aortic stenosis using cardiovascular magnetic resonance: A systematic review & meta-analysis. J. Cardiovasc. Magn. Reson. 2020, 15, 45. [Google Scholar]
- Mangieri, A.; Laricchia, A.; Montalto, C.; Palena, M.; Fiscaro, A.; Cereda, A.; Sticchi, A.; Giannini, F.; Khokhar, A.A.; Latib, A.; et al. Patient selection, procedural planning and interventional guidance for transcatheter aortic valve intervention. Minerva Cardiol. Angiol. 2021. [Google Scholar] [CrossRef]
- Colli, A.; Marchetto, G.; Salizzoni, S.; Rinaldi, M.; Di Marco, L.; Pacini, D.; Di Bartolomeo, R.; Nicolini, F.; Gherli, T.; Agrifoglio, M.; et al. The TRIBECA study: (TRI)fecta (B)ioprosthesis (E)valuation versus (C)arpentier Magna-Ease in (A)ortic position. Eur. J. Cardio Thoraic Surg. 2016, 49, 478–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suri, R.; Michelena, H.; Burkhart, H.; Greason, K.; Daly, R.; Dearani, J.; Park, S.J.; Jouce, L.D.; Stulak, J.M.; Snudt, T.M.; et al. A prospective, randomized comparison of 3 contemporary bioprosthetic aortic valves: Should hemodynamic performance influence device selection? J. Thorac. Cardiovasc. Surg. 2012, 144, 1387–1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bach, D.; Patel, H.; Kolias, T.; Deeb, M. Randomized comparison of exercise haemodynamics of Freestyle, Magna Ease and Trifecta bioprostheses after aortic valve replacement for severe aortic stenosis. Eur. J. Cardio Thoraic Surg. 2016, 50, 361–367. [Google Scholar] [CrossRef] [Green Version]
- Van Linden, A.; Arsalan, M.; Körschgen, T.; Bleumenstein, J.; Kempfert, J.; Hecker, F.; Walther, T. Randomized (CO)mparison of (TRI)fecta and (P)erimount Magna Ease Supraannular Aortic. Thorac. Cardiovasc. Surg. 2019, 67, 266–273. [Google Scholar]
- Fiegl, K.; Deutsch, M.; Rondak, I.; Lange, R.; Guenzinger, R. Matched comparison of two different biological prostheses for complete supra-annular aortic valve replacement. Thorac. Cardiovasc. Surg. 2015, 63, 459–466. [Google Scholar] [PubMed] [Green Version]
- Kilic, A.; Sultan, I.; Navid, F.; Aranda, E.; Chu, D.; Thoma, F.; Gleason, T.G. Trifecta Aortic Bioprosthesis: Midterm results in 1953 patients from a single center. Ann. Thorac. Surg. 2019, 107, 1356–1362. [Google Scholar] [CrossRef] [PubMed]




| Magna EaseTM (n = 48) | Crown PRTTM (n = 51) | TrifectaTM (n = 55) | Global (n = 154) | p | |
|---|---|---|---|---|---|
| BASELINE CLINICAL CHARACTERISTICS | |||||
| Male gender | 34 (70.83%) | 24 (47.06%) | 34 (61.82%) | 92 (59.74%) | 0.051 |
| Age (years) | 76.5 (IQR 72.5–79) | 76.5 (IQR 68.5–81.5) | 77.5 (IQR 72.5 79.5) | 76.5 (IQR 71.5–79.5) | 0.916 Ψ |
| Body mass index | 28.4 (26.1–31.1) | 28.1 (24.9–30.8) | 28.6 (27.0–31.4) | 28.4 (25.5–31.2) | 0.428 Ψ |
| Arterial hypertension | 35 (72.92%) | 40 (78.43%) | 42 (76.36%) | 117 (75.97%) | 0.817 Ψ |
| Diabetes | 20 (41.67%) | 17 (33.33%) | 17 (30.91%) | 54 (35.06%) | 0.497 Ψ |
| Smoking | 1 (2.0%) | 4 (7.84%) | 5 (9.09%) | 10 (6.49%) | 0.670 Ψ |
| Dyslipemia | 34 (70.83%) | 34 (66.67%) | 36 (65.45%) | 104 (67.53%) | 0.833 |
| COPD | 8 (16.67%) | 5 (9.8%) | 2 (3.64%) | 15 (9.74%) | 0.083 Ψ |
| Renal failure | 7 (14.58%) | 6 (11.76%) | 6 (10.91%) | 19 (12.34%) | 0.866 Ψ |
| NYHA IV | 14 (29.16%) | 19 (37.35%) | 14 (25.4%) | 47 (30.52%) | 0.932 Ψ |
| Elective surgery | 41 (85.42%) | 34 (66.67%) | 34 (61.82%) | 109 (70.78%) | 0.020 |
| Previous mayor CVA | 2 (4.17%) | 1 (1.96%) | 2 (3.64%) | 5 (3.25%) | 0.866 Ψ |
| Previous MI | 3 (6.25%) | 4 (7.84%) | 10 (18.18%) | 17 (11.04%) | 0.176 Ψ |
| EUROSCORE II (%) | 2.0 (IQR 1.2–2.8) | 2.3 (IQR 1.4–4.6) | 2.4 (IQR 1.5–4.8) | 2.3 (IQR 1.4–4) | 0.125 Ψ |
| EUROSCORE I (%) | 5.5 (IQR 4.3–7.5) | 6.0 (IQR 4.1–8.4) | 6.6 (IQR 5.1–8.4) | 6.2 (IQR 4.4–8.5) | 0.312 Ψ |
| PREOPERATIVE ECHOCARDIOGRAPHIC FINDINGS | |||||
| Severe aortic stenosis | 44 (91.67%) | 43 (84.31%) | 48 (87.27%) | 135 (87.66%) | 0.225 Ψ |
| Severe aortic regurgitation | 3 (6.25%) | 7 (13.73%) | 5 (9.09%) | 15 (9.74%) | 0.877 Ψ |
| PASP > 55 mmhg | 1 (2.08%) | 1 (1.96%) | 0 (0.0%) | 2 (1.3%) | 0.604 Ψ |
| LVEF (%) | 65 (IQR 60.3–72.2) | 62.7 (IQR 59–70.7) | 65 (IQR 57.7–65) | 65 (IQR 59.2–71.8) | 0.65 Ψ |
| Peak aortic gradient (mmHg) | 74.2 (±20.4) | 69.7 (±29.2) | 71.4 (±22.5) | 71.7 (±24.3) | 0.658 |
| Mean aortic gradient (mmHg) | 42.4 (±12.6) | 41.5 (±18.2) | 42.5 (±14.9) | 42.1(±15.34) | 0.931 |
| Peak aortic velocity (cm/s) | 429.4 (IQR 395.3–458.9) | 424 (IQR 363.8–480.4) | 429 (IQR 388.6–478.1) | 428 (IQR 388.6–472.4) | 0.829 Ψ |
| Aortic valve area (cm2) | 0.7 (IQR 0.6–0.9) | 0.7 (IQR 0.6 – 0.9) | 0.71 (IQR 0.6–0.9) | 0.7 (IQR 0.6–0.9) | 0.466 Ψ |
| Left ventricular mass (g) | 220.8 (IQR 167.9–277) | 232.8 (IQR 185.6–294) | 236 (IQR 187.8–298.6) | 226.6 (IQR 179.3–293.5) | 0.377 Ψ |
| LVTSV (mL) | 38.8 (IQR 25.4–48.7) | 36.6 (IQR 26.6–50.1) | 35.9 (IQR 22.6–49.3) | 36.1 (IQR 24–49) | 0.892 Ψ |
| LVTDV (mL) | 93.4 (IQR 64.1–124.7) | 97.3 (IQR 73.3–128.7) | 96.4 (IQR 74.5–117.8) | 96.8 (IQR 70.8 124.7) | 0.866 Ψ |
| TAPSE (cm) | 2.2 (IQR 2–2.4) | 2.2 (IQR 1.9–2.3) | 2.1 (IQR 2–2.4) | 2.2 (IQR 2–2.4) | 0.545 Ψ |
| PROCEDURAL CHARACTERISTICS | |||||
| Mini-sternotomy | 11 (22.92%) | 8 (15.69%) | 4 (7.27%) | 23 (14.94%) | 0.083 |
| CBP time (min) | 78.5 (IQR 61.5–92.5) | 73 (IQR 56–94) | 76 (IQR 65–98) | 75 (IQR 61–94) | 0.624 Ψ |
| Aortic cross-clamp time (min) | 61 (IQR 51–78) | 57 (IQR 47–67) | 61 (IQR 51–81) | 60 (IQR 50–77) | 0.352 Ψ |
| Hegar sizer (mm) | 0.892 | ||||
| ≤22 | 20 (41.67%) | 28 (54.90%) | 28 (50.90%) | 76 (49.39%) | |
| >22 | 28 (58.33%) | 23 (45.14%) | 27 (49.09%) | 78 (50.64%) | |
| Prostheses sizer (mm) | 0.894 | ||||
| ≤21 | 24 (50.00%) | 28 (54.90%) | 29 (52.72%) | 81 (52.60%) | |
| >21 | 24 (50.00%) | 23 (45.09%) | 26 (47.27%) | 73 (47.40%) | |
| CABG | 9 (18.75%) | 12 (23.53%) | 15 (27.27%) | 36 (23.38%) | 0.604 Ψ |
| Magna EaseTM (n = 47) | Crown PRTTM (n = 44) | TrifectaTM (n = 51) | Global (n = 142) | p | p-Value | |||
|---|---|---|---|---|---|---|---|---|
| Crown vs. Magna Ease | Trifecta vs. Magna Ease | Trifecta vs. Crown | ||||||
| Peak aortic gradient (mmHg) | 17.5 (IQR 11.3–26) | 21.4 (IQR 14.5–26.7) | 13 (IQR 9.6–17.8) | 16.9 (IQR 11.1–25) | 0.003 | 0.90 | 0.51 | 0.03 |
| Mean aortic gradient (mmHg) | 7.8 (IQR 6.1–12) | 10.4 (IQR 7–13.3) | 6.6 (IQR 4.8–8.6) | 7.75 (IQR 5.3–11.9) | 0.003 | 0.77 | 0.51 | 0.03 |
| Peak aortic velocity (cm/s) | 227.1 (IQR 202.0 –268.8) | 237.8 (IQR 195.9 –261.9) | 209.7 (IQR 176.5 –241.4) | 222.6 (IQR 194.1–259.2) | 0.025 | 1.00 | 0.33 | 0.38 |
| Effective orifice area (cm2) | 1.4 (IQR 1.3–1.7) | 1.4 (IQR 1.2–1.7) | 1.65 (IQR 1.4–2) | 1.55 (IQR 1.2–1.8) | 0.242 | - | - | - |
| LVEF % | 62.9 (IQR 60–67.9) | 61 (IQR 58.6–65.7) | 60.7 (IQR 56.1–66.9) | 61.5 (IQR 57.2–67.5) | 0.738 | - | - | - |
| LV mass (g) | 168.7 (IQR 134.7–214) | 184.7 (IQR 147.8 –229.9) | 191 (IQR 143.9–230.2) | 182.3 (IQR 144.5–230.1) | 0.533 | - | - | - |
| LVTSV (mL) | 26.8 (IQR 20.1–38.5) | 34 (IQR 24–39.4) | 30.6 (IQR 20–43) | 31.5 (IQR 20.4–48) | 0.839 | - | - | - |
| LVTDV (mL) | 80.9 (IQR 61.7–121.1) | 79.6 (IQR 59.5–97.8) | 79.4 (IQR 60.8–102.5) | 80.1 (IQR 60.8–105.8) | 0.599 | - | - | - |
| TAPSE (cm) | 1.8 (IQR 1.7–1.9) | 1.7 (IQR 1.6–2.0) | 1.8 (IQR 1.6–2.0) | 1.7 (IQR 1.6–1.9) | 0.467 | - | - | - |
| Size | Magna EaseTM | Crown PRTTM | TrifectaTM | p | p-Value | |||
|---|---|---|---|---|---|---|---|---|
| Crown vs. Magna Ease | Trifecta vs. Magna Ease | Trifecta vs. Crown | ||||||
| Nº ≤ 21 | n = 23 | n = 25 | n = 29 | |||||
| Peak aortic gradient (mmHg) | 17.8 (IQR 13.6–25.8) | 26 (IQR 19.4–34.3) | 12.3 (IQR 9.3–19.9) | 0.001 Ψ | 0.216 | 0.551 | 0.004 | |
| Mean aortic gradient (mmHg) | 8 (IQR 6.9–12.0) | 12 (IQR 9.4–18.0) | 6.7 (IQR 4.3–10.5) | 0.004 Ψ | 0.204 | 1.000 | 0.014 | |
| Peak aortic velocity (cm/s) | 236.8 (IQR 209–267.8) | 251.4 (IQR 228.5–271.5) | 209.7 (IQR 176.5–241.9) | 0.033 Ψ | 1.000 | 0.576 | 0.079 | |
| Effective orifice area (cm2) | 1.4 (IQR 1.2–1.6) | 1.2 (IQR 1.2–1.3) | 1.55 (IQR 1.3–1.8) | 0.287 Ψ | - | - | - | |
| Nº > 21 | n = 24 | n = 19 | n = 22 | |||||
| Peak aortic gradient (mmHg) | 17.5 (IQR 11–27) | 16 (IQR 9.2–21.9) | 13.3 (IQR 10.2–17.8) | 0.455 Ψ | - | - | - | |
| Mean aortic gradient (mmHg) | 7.5 (IQR 5.4–13.0) | 8.6 (IQR 5.1–11.4) | 6.6 (IQR 4.9–7.3) | 0.254 Ψ | - | - | - | |
| Peak aortic velocity (cm/s) | 223.4 (IQR 194.4–270.7) | 217.3 (IQR 182.5–252.5) | 209.7 (IQR 175.1–229.9) | 0.274 Ψ | - | - | - | |
| Effective orifice area (cm2) | 1.7 (IQR 1.5–2.4) | 1.65 (IQR 1.5–1.8) | 1.8 (IQR 1.6–2.1) | 0.527 Ψ | - | - | - | |
| Size | Magna EaseTM | Crown PRTTM | TrifectaTM | p | p-Value | |||
|---|---|---|---|---|---|---|---|---|
| Crown vs. Magna Ease | Trifecta vs. Magna Ease | Trifecta vs. Crown | ||||||
| Nº ≤ 22 | n = 19 | n = 24 | n = 26 | |||||
| Peak aortic gradient (mmHg) | 17.8 (IQR 13.6–26.5) | 26 (IQR 19.4–28.7) | 13.6 (IQR 10.2–17.0) | 0.001 | 0.467 | 0.479 | 0.006 | |
| Mean aortic gradient (mmHg) | 8.95 (IQR 7.0–12.3) | 12.6 (IQR 9.4–18) | 6.6 (IQR 4.9–9.1) | 0.003 | 0.435 | 0.840 | 0.018 | |
| Peak aortic velocity (cm/s) | 236.8 (IQR 209–269) | 256.8 (IQR 230.2–271.5) | 207.7 (IQR 184.2–241.9) | 0.014 | 0.920 | 0.592 | 0.048 | |
| Effective orifice area (cm2) | 1.4 (IQR 1.2–1.7) | 1.2 (IQR 1.2–1.3) | 1.5 (IQR 1.3–1.6) | 0.304 | - | - | - | |
| Nº > 22 | n = 28 | n = 20 | n = 25 | |||||
| Peak aortic gradient (mmHg) | 17.5 (IQR 11–25.5) | 16 (IQR 9.4–21.5) | 12.7 (IQR 9.3–19.8) | 0.376 | - | - | - | |
| Mean aortic gradient (mmHg) | 7.5 (IQR 5.4–11.9) | 8.5 (IQR 5.1–11.1) | 6.6 (IQR 4.5–8.2) | 0.313 | - | - | - | |
| Peak aortic velocity (cm/s) | 224.8 (IQR 194.4–266.7) | 210.8 (IQR 180–247.6) | 210 (IQR 176.5–230.4) | 0.197 | - | - | - | |
| Effective orifice area (cm2) | 1.6 (IQR 1.3–2.4) | 1.7 (IQR 1.5–1.8) | 1.8 (IQR 1.6–2.1) | 0.650 | - | - | - | |
| Magna EaseTM (n = 48) | Crown PRTTM (n = 51) | TrifectaTM (n = 55) | Global (n = 154) | p | |
|---|---|---|---|---|---|
| Myocardial infarction | 0 (0.00%) | 4 (7.84%) | 3 (5.45%) | 7 (4.55%) | 0.218 |
| Cerebrovascular events | 2 (4.17%) | 3 (5.88%) | 2 (3.64%) | 7 (4.55%) | 0.880 |
| Thromboembolic complications | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | - |
| Prosthetic valve deterioration | 0 (0.00%) | 0 (0.00%) | 1 (1.82%) | 1 (0.65%) | - |
| Endocarditis | 2 (4.17%) | 0 (0.00%) | 2 (3.64%) | 4 (2.60%) | 0.910 |
| Reintervention | 0 (0.00%) | 0 (0.00%) | 1 (1.82%) | 1 (0.65%) | - |
| Event-free survival | 43 (89.6%) | 42 (82.4%) | 47 (85.5%) | 132 (85.7%) | 0.356 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montero-Cruces, L.; Carnero-Alcázar, M.; Reguillo-Lacruz, F.J.; Cobiella-Carnicer, F.J.; Pérez-Camargo, D.; Campelos-Fernández, P.; Maroto-Castellanos, L.C. One-Year Hemodynamic Performance of Three Cardiac Aortic Bioprostheses: A Randomized Comparative Clinical Trial. J. Clin. Med. 2021, 10, 5340. https://doi.org/10.3390/jcm10225340
Montero-Cruces L, Carnero-Alcázar M, Reguillo-Lacruz FJ, Cobiella-Carnicer FJ, Pérez-Camargo D, Campelos-Fernández P, Maroto-Castellanos LC. One-Year Hemodynamic Performance of Three Cardiac Aortic Bioprostheses: A Randomized Comparative Clinical Trial. Journal of Clinical Medicine. 2021; 10(22):5340. https://doi.org/10.3390/jcm10225340
Chicago/Turabian StyleMontero-Cruces, Lourdes, Manuel Carnero-Alcázar, Fernando José Reguillo-Lacruz, Francisco Javier Cobiella-Carnicer, Daniel Pérez-Camargo, Paula Campelos-Fernández, and Luis Carlos Maroto-Castellanos. 2021. "One-Year Hemodynamic Performance of Three Cardiac Aortic Bioprostheses: A Randomized Comparative Clinical Trial" Journal of Clinical Medicine 10, no. 22: 5340. https://doi.org/10.3390/jcm10225340






