COVID-19 and Diabetes
Abstract
:1. Diabetes and COVID-19
2. COVID-19 in Persons with Diabetes
2.1. Impact of Diabetes on COVID-19
2.2. Impact of COVID-19 on Diabetes
2.2.1. Effects of COVID-19 on Glycemic Control
2.2.2. Effects on Diabetes of the Restrictions Arising from the COVID-19 Pandemic
3. Treatment of Diabetes during the COVID-19 Pandemic: Disease Management and Drug Therapy in Various Scenarios
3.1. Diabetes Patients without COVID-19: Lockdown and Lack of Physical Exercise
3.2. Patients with Diabetes and COVID-19 Who Have Not Been Admitted to Hospital
3.2.1. Objectives of Glycemic Control
3.2.2. Glucose Monitoring
3.2.3. Pharmacologic Treatment
3.2.4. Control of Other Cardiovascular Risk Factors
3.2.5. Special Considerations in T1D
3.3. Hospitalized Patients with Diabetes and COVID-19
3.3.1. Management of Hyperglycemia in Critically Ill Patients with COVID-19
3.3.2. Management of Hyperglycemia in Non-Critically Ill Patients with COVID-19
3.3.3. Glucose Monitoring of Patients with COVID-19 in Hospital
4. Care of Patients with Diabetes during the COVID-19 Pandemic and Afterwards
- Development and broad implementation of remote diagnostic and treatment technologies that make it possible to obtain variables of interest for management of DM from the patient in his/her usual environment;
- General electronic clinical histories in health services that include all the information generated during the patient’s lifetime. System standardization and interoperability are essential if we are to ensure real integration and coordination between various care levels;
- Establishment of universal mechanisms and protocols for transferring data on variables of interest independently of where they are generated to the clinical history in standard format and in such a way that they are easily interpretable. The complexity and poor user-friendliness of the approach use up health professionals’ time and are among the main reasons this modality is discontinued. All of these actions are essential so that health professionals spend more time making decisions and less time recording data, thus leading to greater clinical efficacy and reduced resistance to change by professionals;
- In parallel, all those involved should be trained to avoid the digital gap between patients and professionals, and collaboration between professionals and care levels should be encouraged by providing reimbursement for the use of digital technology and models for evaluation and assignment of resources that discourage silo working;
- As for any other medical intervention, it is necessary to generate evidence on use—in terms of cost-effectiveness and implementation challenges—of new telemedicine systems and strategies by comparing them with previously used approaches.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Fadini, G.P.; Morieri, M.L.; Longato, E.; Avogaro, A. Prevalence and impact of diabetes among people infected with SARS- CoV-2. J. Endocrinol. Investig. 2020, 43, 867–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roncon, L.; Zuin, M.; Rigatelli, G.; Zuliani, G. Diabetic patients with COVID-19 infection are at higher risk of ICU admission and poor short-term outcome. J. Clin. Virol. 2020, 127, 104354. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Gupta, R.; Ghosh, A.; Misra, A. Diabetes in COVID-19: Prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab. Syndr. 2020, 14, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yang, J.; Zhao, F.; Zhi, L.; Wang, X.; Liu, L.; Bi, Z.; Zhao, Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin. Res. Cardiol. 2020, 109, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Barron, E.; Bakhai, C.; Kar, P.; Weaver, A.; Bradley, D.; Ismail, H.; Knighton, P.; Holman, N.; Khunti, K.; Sattar, N.; et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: A whole-population study. Lancet Diabetes Endocrinol. 2020, 8, 813–822. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Fang, L.; Karakiulakis, G.; Roth, M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020, 8, e21. [Google Scholar] [CrossRef]
- Moazzami, B.; Chaichian, S.; Kasaeian, A.; Djalalinia, S.; Akhlaghdoust, M.; Eslami, M.; Broumand, B. Metabolic risk factors and risk of COVID-19: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0243600. [Google Scholar] [CrossRef]
- Huang, I.; Lim, M.A.; Pranata, R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia. A systematic review, meta-analysis, and meta-regression. Diabetes Metab. Syndr. 2020, 14, 395–403. [Google Scholar] [CrossRef]
- Bloomgarden, Z.T. Diabetes and COVID-19. J. Diabetes 2020, 12, 347–348. [Google Scholar] [CrossRef] [Green Version]
- Bartsch, S.M.; Ferguson, M.C.; McKinnell, J.A.; O’Shea, K.J.; Wedlock, P.T.; Siegmund, S.S.; Lee, B.Y. The potential health care costs and resource use associated with COVID-19 in The United States. Health Aff. 2020, 39, 927–935. [Google Scholar] [CrossRef] [Green Version]
- Muniyappa, R.; Gubbi, S. Covid-19 pandemic, coronaviruses, and diabetes mellitus. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E736–E741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klonoff, D.C.; Messler, J.C.; Umpiérrez, G.E.; Peng, L.; Booth, R.; Crowe, J.; Garrett, V.; McFarland, R.; Pasquel, F.J. Association between achieving inpatient glycemic control and clinical outcomes in hospitalized patients with COVID-19: A multicenter, retrospective hospital-based analysis. Diabetes Care 2021, 44, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Sánchez, F.J.; López-Carmona, M.D.; Martínez-Marcos, F.J.; Pérez-Belmonte, L.M.; Hidalgo-Jiménez, A.; Buonaiuto, V.; Fernández Suárez, C.; Freire Castro, S.J.; Luordo, D.; Pesqueira Fontan, P.M. Admission hyperglycaemia as a predictor of mortality in patients hospitalized with COVID-19 regardless of diabetes status: Data from the Spanish SEMI-COVID-19 Registry. Ann. Med. 2021, 53, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Sardu, C.; D’Onofrio, N.; Balestrieri, M.L.; Barbieri, M.; Rizzo, M.R.; Messina, V.; Maggi, P.; Coppola, N.; Paolisso, G.; Marfella, R. Outcomes in patients with hyperglycemia affected by COVID-19: Can we do more on glycemic control? Diabetes Care 2020, 43, 1408–1415. [Google Scholar] [CrossRef]
- Iacobellis, G. COVID-19 and diabetes: Can DPP4 inhibition play a role? Diabetes Res. Clin. Pract. 2020, 162, 108125. [Google Scholar] [CrossRef]
- Rao, S.; Lau, A.; Hon-Cheong, S. Exploring diseases/traits and blood proteins causally related to expression of ACE2, the putative receptor of SARS-CoV-2: A Mendelian randomization analysis highlights tentative relevance of diabetes-related traits. Diabetes Care 2020, 43, 1416–14126. [Google Scholar] [CrossRef]
- Bindom, S.M.; Lazartigues, E. The sweeter side of ACE2: Physiological evidence for a role in diabetics. Mol. Cell Endocrinol. 2009, 301, 193–202. [Google Scholar] [CrossRef]
- Bode, B.; Garrett, V.; Messler, J.; McFarland, R.; Crowe, J.; Booth, R.; Klonoff, D.C. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J. Diabetes Sci. Technol. 2020, 14, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tan, J. Diabetes patients with COVID-19 need better blood glucose management in Wuhan, China. Metabolism 2020, 107, 154216. [Google Scholar] [CrossRef]
- Wu, L.; Girgis, C.M.; Cheung, N.W. COVID-19 and diabetes: Insulin requirements parallel illness severity in critically unwell patients. Clin. Endocrinol. 2020, 93, 390–393. [Google Scholar] [CrossRef]
- Pal, R.; Banerjee, M.; Yadav, U.; Bhattacharjee, S. Clinical profile and outcomes in COVID-19 patients with diabetic ketoacidosis: A systematic review of literature. Diabetes Metab. Syndr. 2020, 14, 1563–1569. [Google Scholar] [CrossRef]
- Yang, J.K.; Lin, S.S.; Ji, X.J.; Guo, L.M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010, 47, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wang, X.; Chen, J.; Zuo, X.; Zhang, H.; Deng, A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes. Metab. 2020, 22, 1935–1941. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Bae, J.H.; Kwon, H.S.; Nauck, M.A. COVID-19 and diabetes mellitus: From pathophysiology to clinical management. Nat. Rev. Endocrinol. 2021, 17, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Perez, A.; Jansen-Chaparro, S.; Saigi, I.; Bernal-López, M.R.; Miñambres, I.; Gómez-Huelgas, R. Glucocorticoid-induced hyperglycemia. J. Diabetes 2014, 6, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J.; Marre, M.; Thivolet, C. Prognostic factors in patients with diabetes hospitalized for COVID-19: Findings from the CORONADO study and other recent reports. Diabetes Metab. 2020, 46, 265–271. [Google Scholar] [CrossRef]
- Fernández, E.; Cortázar, A.; Bellido, V. Impact of COVID-19 lockdown on glycemic control in patients with type 1 diabetes. Diabetes Res. Clin. Pract. 2020, 166, 108348. [Google Scholar] [CrossRef]
- Capaldo, B.; Annuzzi, G.; Creanza, A.; Giglio, C.; De Angelis, R.; Lupoli, R.; Masulli, M.; Riccardi, G.; Albarosa Rivellese, A.; Bozzetto, L. Blood glucose control during lockdown for COVID-19: CGM metrics in Italian adults with type 1 diabetes. Diabetes Care 2020, 43, e88–e89. [Google Scholar] [CrossRef]
- Garofolo, M.; Aragona, M.; Rodia, C.; Falcetta, P.; Bertolotto, A.; Campi, F.; Del Prato, S.; Penno, G. Glycaemic control during the lockdown for COVID-19 in adults with type 1 diabetes: A meta-analysis of observational studies. Diabetes Res. Clin. Pract. 2021, 180, 109066. [Google Scholar] [CrossRef]
- Fisher, L.; Polonsky, W.; Asuni, A.; Jolly, Y.; Hessler, D. The early impact of the COVID-19 pandemic on adults with type 1 or type 2 diabetes: A national cohort study. J. Diabetes Complicat. 2020, 34, 107748. [Google Scholar] [CrossRef] [PubMed]
- Tejera-Pérez, C.; Moreno-Pérez, Ó.; Ríos, J.; Reyes-García, R. People living with type 1 diabetes point of view in COVID-19 times (covidT1 study): Disease impact, health system pitfalls and lessons for the future. Diabetes Res. Clin. Pract. 2021, 171, 108547. [Google Scholar] [CrossRef] [PubMed]
- Biancalana, E.; Parolini, F.; Mengozzi, A.; Solini, A. Short-term impact of COVID-19 lockdown on metabolic control of patients with well-controlled type 2 diabetes: A single-centre observational study. Acta Diabetol. 2021, 58, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Carr, M.J.; Wright, A.K.; Leelarathna, L.; Thabit, H.; Milne, N.; Kanumilli, N.; Ashcroft, D.M.; Rutter, M.K. Impact of COVID-19 on diagnoses, monitoring and mortality in people with type 2 diabetes: A UK-wide cohort study involving 14 million people in primary care. Lancet Diabetes Endocrinol. 2021, 9, 413–415. [Google Scholar] [CrossRef]
- Jacob, L.; Rickwood, S.; Rathmann, W.; Kostev, K. Change in glucose-lowering medication regimens in individuals with type 2 diabetes mellitus during the COVID-19 pandemic in Germany. Diabetes Obes. Metab. 2020, 23, 910–915. [Google Scholar] [CrossRef]
- Wang, A.; Zhao, W.; Xu, Z.; Gu, J. Timely blood glucose management for the outbreak of 2019 novel coronavirus disease (COVID-19) is urgently needed. Diabetes Res. Clin. Pract. 2020, 162, 108118. [Google Scholar] [CrossRef]
- Katulanda, P.; Dissanayake, H.A.; Ranathunga, I.; Ratnasamy, V.; Wijewickrama, P.S.A.; Yogendranathan, N.; Gamage, K.K.K.; de Silva, N.L.; Sumanatilleke, M.; Somasundaram, N.; et al. Prevention and management of COVID-19 among patients with diabetes: An appraisal of the literature. Diabetologia 2020, 63, 1440–1452. [Google Scholar] [CrossRef]
- Pal, R.; Bhadada, S.K.; Misra, A. COVID-19 vaccination in patients with diabetes mellitus: Current concepts, uncertainties and challenges. Diabetes Metab. Syndr. 2021, 15, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Marfella, R.; D’Onofrio, N.; Sardu, C.; Scisciola, L.; Maggi, P.; Coppola, N.; Romano, C.; Messina, V.; Turriziani, F.; Siniscalchi, M. Does poor glycaemic control affect the immunogenicity of the COVID-19 vaccination in patients with type 2 diabetes: The CAVEAT study. Diabetes Obes. Metab. 2021. [Google Scholar] [CrossRef]
- Pascual Fuster, V.; Pérez Pérez, A.; Carretero Gómez, J.; Caixàs Pedragós, A.; Gómez-Huelgas, R.; Pérez-Martínez, P. Executive summary: Updates to the dietary treatment of prediabetes and type 2 diabetes mellitus. Clin. Investig. Arteriosler. 2021, 33, 73–84. [Google Scholar]
- Reyes-García, R.; Moreno-Pérez, Ó.; Tejera-Pérez, C.; Fernández-García, D.; Bellido-Castañeda, V.; De la Torre Casares, M.L.; Rozas-Moreno, P.; Fernández-García, J.C.; Martínez Marco, A.; Escalada-San Martín, J. Documento de abordaje integral de la diabetes tipo 2. Endocrinol. Diabetes Nutr. 2019, 66, 443–458. [Google Scholar] [CrossRef]
- Marçal, I.R.; Fernandes, B.; Viana, A.A.; Ciolac, E.G. The urgent need for recommending physical activity for the management of diabetes during and beyond COVID-19 outbreak. Front. Endocrinol. 2020, 11, 584642. [Google Scholar] [CrossRef]
- Philippou, A.; Chryssanthopoulos, C.; Maridaki, M.; Dimitriadis, G.; Koutsilieris, M. Exercise metabolism in health and disease. In Cardiorespiratory Fitness in Cardiometabolic Diseases; Kokkinos, P., Narayan, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Koliaki, C.; Tentolouris, A.; Eleftheriadou, I.; Melidonis, A.; Dimitriadis, G.; Tentolouris, N. Clinical management of diabetes mellitus in the era of COVID-19: Practical issues, peculiarities and concerns. J. Clin. Med. 2020, 9, 2288. [Google Scholar] [CrossRef]
- Hussain, A.; Bhowmik, B.; Do Vale Moreira, N.C. COVID-19 and diabetes: Knowledge in progress. Diabetes Res. Clin. Pract. 2020, 162, 108142. [Google Scholar] [CrossRef]
- American Diabetes Association. 6. Glycemic targets-Standards of medical care in diabetes—2021. Diabetes Care 2021, 44 (Suppl. S1), S73–S84. [Google Scholar] [CrossRef] [PubMed]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical targets for continuous glucose monitoring data interpretation: Recommendations from the international consensus on time in range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bornstein, S.R.; Rubino, F.; Khunti, K.; Mingrone, G.; Hopkins, D.; Birkenfeld, A.L.; Boehm, B.; Amiel, S.; Holt, R.I.; Skyler, J.S.; et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020, 8, 546–550. [Google Scholar] [CrossRef]
- Maahs, D.M.; DeSalvo, D.; Pyle, L.; Ly, T.; Messer, L.; Clinton, P.; Westfall, E.; Wadwa, R.P.; Buckingham, B. Effect of acetaminophen on CGM glucose in an outpatient setting. Diabetes Care 2015, 38, e158–e159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, B.; Huang, S.; Zhou, J. Perspectives of antidiabetic drugs in diabetes with coronavirus infections. Front. Pharmacol. 2021, 11, 592439. [Google Scholar] [CrossRef]
- Finan, B.; Yang, B.; Ottaway, N.; Smiley, D.L.; Ma, T.; Clemmensen, C.; Chabenne, J.; Zhang, L.; Habegger, K.M.; Fischer, K.; et al. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat. Med. 2015, 21, 27–36. [Google Scholar] [CrossRef]
- Cariou, B.; Hadjadj, S.; Wargny, M.; Pichelin, M.; Al-Salameh, A.; Allix, I.; Amadou, C.; Arnault, G.; Baudoux, F.; Bauduceau, B.; et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: The CORONADO study. Diabetologia 2020, 63, 1500–1515. [Google Scholar] [CrossRef]
- Pérez-Belmonte, L.M.; Torres-Peña, J.D.; López-Carmona, M.D.; Ayala-Gutiérrez, M.M.; Fuentes-Jiménez, F.; Huerta, L.J.; Muñoz, J.A.; Rubio-Rivas, M.; Madrazo, M.; Guzmán García, M.; et al. Mortality and other adverse outcomes in patients with type 2 diabetes mellitus admitted for COVID-19 in association with glucose-lowering drugs: A nationwide cohort study. BMC Med. 2020, 18, 359. [Google Scholar] [CrossRef]
- DeFronzo, R.; Fleming, G.A.; Chen, K.; Bicsak, T.A. Metformin-associated lactic acidosis: Current perspectives on causes and risk. Metabolism 2016, 65, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Vitale, R.J.; Valtis, Y.K.; McDonnell, M.E.; Palermo, N.E.; Fisher, N.D.L. Euglycemic diabetic ketoacidosis with COVID-19 infection in patients with type 2 diabetes taking SGLT2 inhibitors. AACE Clin. Case Rep. 2021, 7, 10–13. [Google Scholar] [CrossRef]
- Schwartz, S.; DeFronzo, R.A. Is incretin-based therapy ready for the care of hospitalized patients with type 2 diabetes? The time has come for GLP-1 receptor agonists! Diabetes Care 2013, 36, 2107–2111. [Google Scholar] [CrossRef] [Green Version]
- Longo, M.; Caruso, P.; Maiorino, M.I.; Bellastella, G.; Giugliano, D.; Esposito, K. Treating type 2 diabetes in COVID-19 patients: The potential benefits of injective therapies. Cardiovasc. Diabetol. 2020, 19, 115. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Vardeny, O.; Michel, T.; McMurray, J.J.V.; Pfeffer, M.A.; Solomon, S.D. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. N. Engl. J. Med. 2020, 382, 1653–1659. [Google Scholar] [CrossRef]
- Patel, A.B.; Verma, A. COVID-19 and angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: What is the evidence? JAMA 2020, 323, 1769–1770. [Google Scholar] [CrossRef]
- Bangash, M.N.; Patel, J.; Parekh, D. COVID-19 and the liver: Little cause for concern. Lancet Gastroenterol. Hepatol. 2020, 5, 529–530. [Google Scholar] [CrossRef] [Green Version]
- Ceriello, A.; Standl, E.; Catrinoiu, D.; Itzhak, B.; Lalic, N.M.; Rahelic, D.; Schnell, O.; Škrha, J.; Valensi, P. Diabetes and Cardiovascular Disease (D&CVD) EASD Study Group. Issues of cardiovascular risk management in people with diabetes in the COVID-19 era. Diabetes Care 2020, 43, 1427–1432. [Google Scholar]
- Rahimi, L.; Malek, M.; Ismail-Beigi, F.; Khamseh, M.E. Challenging issues in the management of cardiovascular risk factors in diabetes during the COVID-19 pandemic: A review of current literature. Adv. Ther. 2020, 37, 3450–3462. [Google Scholar] [CrossRef] [PubMed]
- Ebekozien, O.A.; Noor, N.; Gallagher, M.P.; Alonso, G.T. Type 1 diabetes and COVID-19: Preliminary findings from a multicenter surveillance study in the U.S. Diabetes Care 2020, 43, e83–e85. [Google Scholar] [CrossRef]
- Korytkowski, M.; Antinori-Lent, K.; Drincic, A.; Hirsch, I.B.; McDonnell, M.E.; Rushakoff, R.; Muniyappa, R. A pragmatic approach to inpatient diabetes management during the COVID-19 pandemic. J. Clin. Endocrinol. Metab. 2020, 105, 342. [Google Scholar] [CrossRef]
- Bellido, V.; Pérez, A. Inpatient hyperglycemia management and COVID-19. Diabetes Ther. 2021, 12, 121–132. [Google Scholar] [CrossRef]
- American Diabetes Association. 15 Diabetes care in the hospital-Standards of medical care in diabetes—2020. Diabetes Care 2020, 43 (Suppl. S1), S193–S202. [Google Scholar] [CrossRef] [Green Version]
- Umpiérrez, G.; Cardona, S.; Pasquel, F.; Jacobs, S.; Peng, L.; Unigwe, M.; Newton, C.A.; Smiley-Byrd, D.; Vellanki, P.; Halkos, M.; et al. Randomized controlled trial of intensive versus conservative glucose control in patients undergoing coronary artery bypass graft surgery: GLUCO-CABG Trial. Diabetes Care 2015, 38, 1665–1672. [Google Scholar] [CrossRef] [Green Version]
- Krinsley, J.S.; Preiser, J.C.; Hirsch, I.B. Safety and efficacy of personalized glycemic control in critically ill patients: A 2-year before and after intervention trial. Endocr. Pract. 2017, 23, 318–330. [Google Scholar] [CrossRef] [Green Version]
- Pérez, A.; Ramos, A.; Carreras, G. Insulin therapy in hospitalized patients. Am. J. Ther. 2020, 27, e71–e78. [Google Scholar] [CrossRef]
- Pasquel, F.J.; Umpiérrez, G.E. Individualizing inpatient diabetes management during the coronavirus disease 2019 pandemic. J. Diabetes Sci. Technol. 2020, 14, 705–707. [Google Scholar] [CrossRef]
- Hamdy, O.; Gabbay, R.A. Early observation and mitigation of challenges in diabetes management of COVID-19 patients in critical care units. Diabetes Care 2020, 43, e81–e82. [Google Scholar] [CrossRef]
- Moghissi, E.S.; Korytkowski, M.T.; DiNardo, M.; Einhorn, D.; Hellman, R.; Hirsch, I.B.; Inzucchi, S.E.; Ismail-Beigi, F.; Kirkman, M.S.; Umpierrez, G.E. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care 2009, 32, 1119–1131. [Google Scholar] [CrossRef] [Green Version]
- Pérez Pérez, A.; Conthe Gutiérrez, P.; Aguilar Diosdado, M.; Bertomeu Martínez, V.; Galdos Anuncibay, P.; García de Casasola, G.; Gomis de Bárbara, R.; Palma Gamiz, J.L.; Puig Domingo, M.; Sánchez Rodríguez, A. Hospital management of hyperglycemia. Med. Clin. 2009, 132, 465–475. [Google Scholar] [CrossRef]
- Avanzini, F.; Marelli, G.; Donzelli, W.; Busi, G.; Carbone, S.; Bellato, L.; Colombo, E.L.; Foschi, R.; Riva, E.; Roncaglioni, M.C.; et al. Transition from intravenous to subcutaneous insulin: Effectiveness and safety of a standardized protocol and predictors of outcome in patients with acute coronary syndrome. Diabetes Care. 2011, 34, 1445–1450. [Google Scholar] [CrossRef] [Green Version]
- Ramos, A.; Zapata, L.; Vera, P.; Betbese, A.J.; Pérez, A. Transition from intravenous insulin to subcutaneous long-acting insulin in critical care patients on enteral or parenteral nutrition. Endocrinol. Diabetes Nutr. 2017, 64, 552–556. [Google Scholar] [CrossRef]
- Kosiborod, M.N.; Esterline, R.; Furtado, R.H.M.; Oscarsson, J.; Gasparyan, S.B.; Koch, G.G.; Martinez, F.; Mukhtar, O.; Verma, S.; Chopra, V.; et al. Dapagliflozin in patients with cardiometabolic risk factors hospitalized with COVID-19 (DARE-19): A randomized, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2021, 9, 586–594. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sánchez, E.; Tattersall, R.S.; Manson, J.J.; HLH Across Specialty Collaboration, UK. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- FDA. FAQs on Home-Use Blood Glucose Meters Utilized within Hospitals during the COVID-19 Pandemic. 2020. Available online: https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/using-home-use-blood-glucose-meters-hospitals-during-covid-19-pandemic (accessed on 2 March 2021).
- Welsh, J.B.; Hu, G.; Walker, T.C.; Sharma, N.; Cherñavvsky, D. Glucose monitoring and diabetes management in the time of coronavirus disease 2019. J. Diabetes Sci. Technol. 2020, 14, 809–810. [Google Scholar] [CrossRef]
- Dexcom. Fact Sheet for Healthcare Providers: Use of Dexcom Continuous Glucose Monitoring Systems during the COVID-19 Pandemic. 2020. Available online: https://www.dexcom.com/hospitalfacts (accessed on 2 March 2021).
- Abbott. Press release. FreeStyle Libre: Diabetes Care during COVID-19. 2020. Available online: https://www.abbott.com/corpnewsroom/product-and-innovation/freestyle-libre-diabetes-care-during-covid-19.html (accessed on 25 March 2020).
- Galindo, R.J.; Migdal, A.L.; Davis, G.M.; Urrutia, M.A.; Albury, B.; Zambrano, C.; Vellanki, P.; Pasquel, F.J.; Fayfman, M.; Peng, L.; et al. Comparison of the FreeStyle Libre pro flash continuous glucose monitoring (CGM) system and point-of-care capillary glucose testing in hospitalized patients with type 2 diabetes treated with basal-bolus insulin regimen. Diabetes Care 2020, 43, 2730–2735. [Google Scholar] [CrossRef]
- Fortmann, A.L.; Spierling Bagsic, S.R.; Talavera, L.; García, I.M.; Sandoval, H.; Hottinger, A.; Philis-Tsimikas, A. Glucose as the fifth vital sign: A randomized controlled trial of continuous glucose monitoring in a non-ICU hospital setting. Diabetes Care 2020, 43, 2873–2877. [Google Scholar] [CrossRef]
- Singh, L.G.; Satyarengga, M.; Marcano, I.; Scott, W.H.; Pinault, L.F.; Feng, Z.; Sorkin, J.D.; Umpierrez, G.E.; Spanakis, E.K. Reducing inpatient hypoglycemia in the general wards using real-time continuous glucose monitoring: The glucose telemetry system, a randomized clinical trial. Diabetes Care 2020, 43, 2736–2743. [Google Scholar] [CrossRef]
- Reutrakul, S.; Genco, M.; Salinas, H.; Sargis, R.M.; Paul, C.; Eisenberg, Y.; Fang, J.; Caskey, R.N.; Henkle, S.; Fatoorehchi, S.; et al. Feasibility of inpatient continuous glucose monitoring during the COVID-19 pandemic: Early experience. Diabetes Care 2020, 43, e137–e138. [Google Scholar] [CrossRef]
- Agarwal, S.; Mathew, J.; Davis, G.M.; Shephardson, A.; Levine, A.; Louard, R.; Urrutia, A.; Perez-Guzman, C.; Umpierrez, G.E.; Peng, L.; et al. Continuous glucose monitoring in the intensive care unit during the COVID-19 pandemic. Diabetes Care 2021, 44, 847–849. [Google Scholar] [CrossRef] [PubMed]
- Galindo, R.J.; Aleppo, G.; Klonoff, D.C.; Spanakis, E.K.; Agarwal, S.; Vellanki, P.; Olson, D.E.; Umpierrez, G.E.; Davis, G.M.; Pasquel, F.J. Implementation of continuous glucose monitoring in the hospital: Emergent considerations for remote glucose monitoring during the COVID-19 pandemic. J. Diabetes Sci. Technol. 2020, 14, 822–832. [Google Scholar] [CrossRef]
- Galindo, R.J.; Umpiérrez, G.E.; Rushakoff, R.J.; Basu, A.; Lohnes, S.; Nichols, J.H.; Spanakis, E.K.; Espinoza, J.; Palermo, N.E.; Awadjie, D.G. Continuous glucose monitors and automated insulin dosing systems in the Hospital Consensus Guideline. J. Diabetes Sci. Technol. 2020, 14, 1035–1064. [Google Scholar] [CrossRef] [PubMed]
- Alexander, G.C.; Tajanlangit, M.; Heyward, J.; Mansour, O.; Qato, D.M.; Stafford, R.S. Use and content of primary care office-based vs telemedicine care visits during the COVID-19 pandemic in the US. JAMA Netw. Open 2020, 3, e2021476. [Google Scholar] [CrossRef]
- Faruque, L.I.; Wiebe, N.; Ehteshami-Afshar, A.; Liu, Y.; Dianati-Maleki, N.; Hemmelgarn, B.R.; Manns, B.J.; Tonelli, M.; Alberta Kidney Disease Network. Effect of telemedicine on glycated hemoglobin in diabetes: A systematic review and meta-analysis of randomized trials. CMAJ. 2017, 189, E341–E364. [Google Scholar] [CrossRef] [Green Version]
- Tchero, H.; Kangambega, P.; Briatte, C.; Brunet-Houdard, S.; Retali, G.-R.; Rusch, E. Clinical effectiveness of telemedicine in diabetes mellitus: A meta-analysis of 42 randomized controlled trials. Telemed. J. E Health 2019, 25, 569–583. [Google Scholar] [CrossRef]
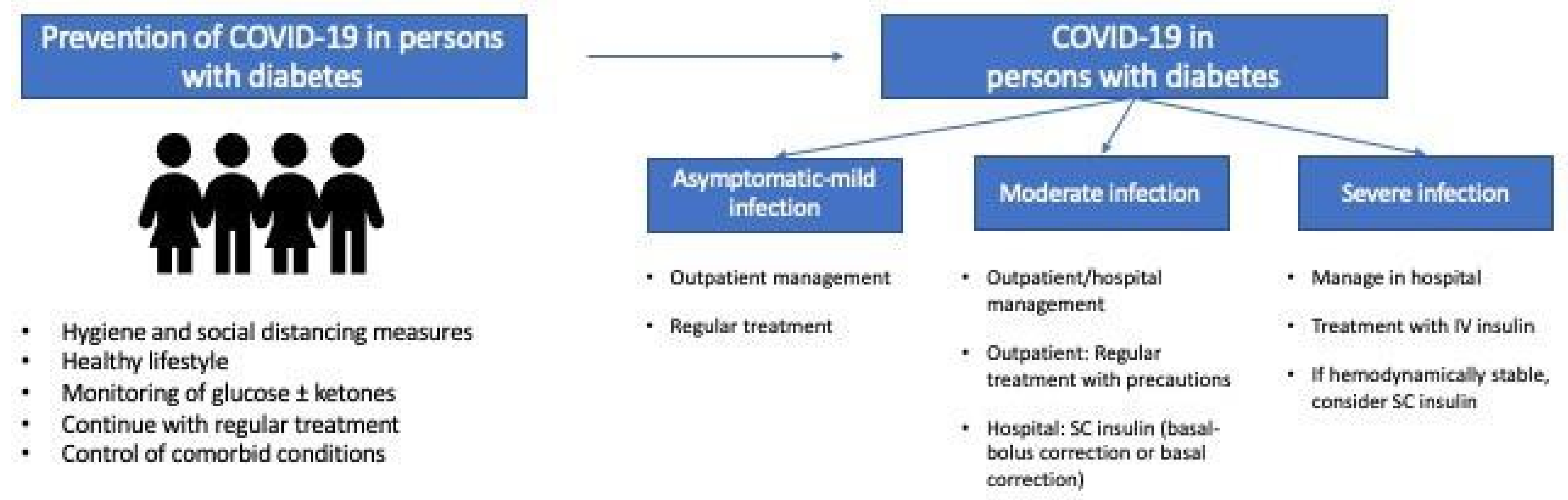
| Hygiene and social Distancing Measures |
| Lifestyle |
|
| Glycemic control |
|
| Control of comorbid conditions (obesity, blood pressure, dyslipidemia, etc.) |
| Vaccination (routine vaccination against pneumococcal and seasonal influenza) |
| Minimize exposure to SARS-CoV-2 (prioritize telemedicine) |
| Treatment | Clinical Recommendation | Special Considerations in COVID-19 |
|---|---|---|
| Metformin | Suspend in severe cases, with hemodynamic instability or hypoxia | Risk of lactic acidosis in hypoxia and acute disease Monitor kidney function. Suspend if glomerular filtration <30 mL/min/1.73 m2 |
| Sulfonylureas | Suspend in cases where food intake is not guaranteed owing to the risk of hypoglycemia | The risk of hypoglycemia may be greater with concomitant use of treatments such as hydroxychloroquine |
| DPP4i | Continue in outpatients Potential option in hospitalized patients with mild hyperglycemia, combined with basal insulin | Favorable safety profile and possible use in kidney failure |
| GLP1ra | Suspend in severe cases | Risk of dehydration in the case of severe gastrointestinal adverse effects (nausea, vomiting, etc.) Maintain a regular diet and ensure good hydration |
| SGLT2i | Suspend in severe cases, or if food/fluid intake cannot be guaranteed | Risk of euglycemic diabetic ketoacidosis induced by dehydration and insulin deficiency Preserving cardiovascular and kidney function is critical for ensuring favorable progress of COVID-19 in persons with diabetes |
| Glitazones | Suspend in severe cases with hemodynamic instability or heart of liver dysfunction | Risk of fluid retention and heart failure |
| Insulin | Continue treatment of choice in hospitalized patients. Adjust dose depending on glycemic control, risk of hypoglycemia, severity of infection, and concomitant treatment Requires frequent monitoring of blood glucose (capillary glycemia or CGM/FGM) | Insulin requirements may be very high in hospitalized patients with severe infection |
| Blood Glucose Target | Clinical Situation | Insulin Regimen | Glucose Monitoring | ||
|---|---|---|---|---|---|
| Critically ill patients | 140–180 mg/dL * | Hemodynamically unstable Parenteral nutrition Varying insulin requirements Treatment with corticosteroids | Continuous intravenous insulin infusion | Every hour | |
| Hemodynamically stable Stable insulin requirements | Subcutaneous insulin Basal + correction or basal-bolus + correction | Every 4–6 h | |||
| Non-critically ill patients | 110–180 mg/dL ** | T1D T2D with OAD ± insulin | No oral intake | Basal insulin + correction | Every 4–6 h **** |
| Oral intake | Basal-bolus insulin + correction | Before meals and at bedtime **** | |||
| T2D with diet DM not known | Glycemia on admission <180 mg/dL | Correction insulin dose before meals or every 6 h *** | Before meals and at bedtime or every 6 h **** | ||
| Glycemia on admission >180 mg/dL | Basal-bolus insulin + correction | Before meals and at bedtime **** | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellido, V.; Pérez, A. COVID-19 and Diabetes. J. Clin. Med. 2021, 10, 5341. https://doi.org/10.3390/jcm10225341
Bellido V, Pérez A. COVID-19 and Diabetes. Journal of Clinical Medicine. 2021; 10(22):5341. https://doi.org/10.3390/jcm10225341
Chicago/Turabian StyleBellido, Virginia, and Antonio Pérez. 2021. "COVID-19 and Diabetes" Journal of Clinical Medicine 10, no. 22: 5341. https://doi.org/10.3390/jcm10225341






