Thyroid Hormone Changes Related to Growth Hormone Therapy in Growth Hormone Deficient Patients
Abstract
:1. Introduction
2. Research of Literature Data
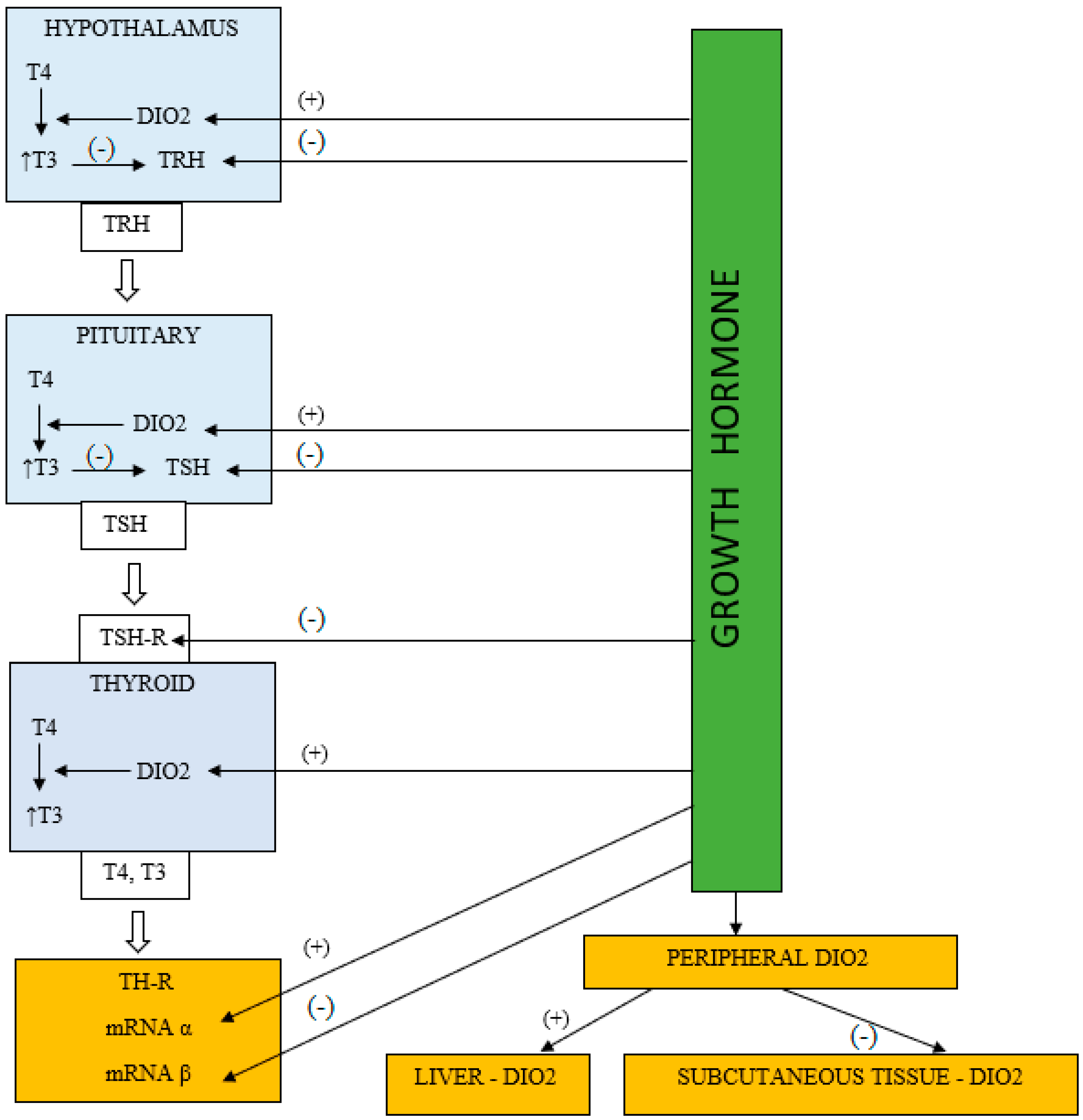
3. Mechanisms Explaining the GH/IGF-1 Effect on the HPT Axis
3.1. The HPT Axis Suppression in the Patients Treated with rhGH
3.2. Peripheral Thyroid Hormone Metabolism Alterations during rhGH Therapy
3.3. GH Impact on Thyroid Hormone Receptors
4. Therapeutic Implications of the rhGH Influence on Thyroid Function
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lippe, B.M.; Van Herle, A.J.; LaFranchi, S.H.; Uller, R.P.; Lavin, N.; Kaplan, S.A. Reversible hypothyroidism in growth hormone-deficient children treated with human growth hormone. J. Clin. Endocrinol. Metab. 1975, 40, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Porter, B.A.; Refetoff, S.; Rosenfield, R.L.; De Groot, L.J.; Fang, V.S.; Stark, V. Abnormal thyroxine metabolism in hyposomatotrophic dwarfism and inhibition of responsiveness to TRH during GH therapy. Pediatrics 1973, 51, 668–674. [Google Scholar] [PubMed]
- Cacciari, E.; Cicognani, A.; Pirazzoli, P.; Bernardi, F.; Zappulla, F.; Salardi, S.; Mazzanti, L.; Biasini, A.; Valenti, E. Effect of long-term GH administration on pituitary-thyroid function in idiopathic hypopituitarism. Acta Paediatr. Scand. 1979, 68, 405–409. [Google Scholar] [CrossRef]
- Demura, R.; Yamaguchi, H.; Wakabayashi, I.; Demura, H.; Shizume, K. The effect of hGH on hypothalamic-pituitary-thyroid function in patients with pituitary dwarfism. Acta Endocrinol. 1980, 93, 13–19. [Google Scholar] [CrossRef]
- Jørgensen, J.O.; Pedersen, S.A.; Laurberg, P.; Weeke, J.; Skakkebaek, N.E.; Christiansen, J.S. Effects of growth hormone therapy on thyroid function of growth hormone-deficient adults with and without concomitant thyroxine-substituted central hypothyroidism. J. Clin. Endocrinol. Metab. 1989, 69, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Pirazzoli, P.; Cacciari, E.; Mandini, M.; Sganga, T.; Capelli, M.; Cicognani, A.; Gualandi, S. Growth and thyroid function in children treated with growth hormone. J. Pediatr. 1992, 121, 210–213. [Google Scholar] [CrossRef]
- Rezvani, I.; DiGeorge, A.M.; Dowshen, S.A.; Bourdony, C.J. Action of Human Growth Hormone (hGH) on Extrathyroidal Conversion of Thyroxine (T4) to Triiodothyronine (T3) in Children with Hypopituitarism. Pediatr. Res. 1981, 15, 6–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jørgensen, J.O.; Møller, J.; Laursen, T.; Orskov, H.; Christiansen, J.S.; Weeke, J. Growth hormone administration stimulates energy expenditure and extrathyroidal conversion of thyroxine to triiodothyronine in a dose-dependent manner and suppresses circadian thyrotrophin levels: Studies in GH-deficient adults. Clin. Endocrinol. 1994, 41, 609–614. [Google Scholar] [CrossRef]
- Monson, J.P.; Weaver, J.U.; Noonan, K.; Burrin, J.M. Effect of growth hormone on serum thyroid hormone concentrations and desmopressin requirements in adults with hypopituitarism and central diabetes insipidus: A preliminary report. Endocrinol. Metab. 1994, 1 (Suppl. A), 51–55. [Google Scholar]
- Sato, T.; Suzukui, Y.; Taketani, T.; Ishiguro, K.; Masuyama, T.; Takata, I.; Sano, M.; Kawashima, H.; Koizumi, S.; Nakajima, H. Enhanced peripheral conversion of thyroxine to triiodothyronine during hGH therapy in GH deficient children. J. Clin. Endocrinol. Metab. 1977, 45, 324–329. [Google Scholar] [CrossRef]
- Wyatt, D.T.; Gesundheit, N.; Sherman, B. Changes in thyroid hormone levels during growth hormone therapy in initially euthyroid patients: Lack of need for thyroxine supplementation. J. Clin. Endocrinol. Metab. 1998, 83, 3493–3497. [Google Scholar] [CrossRef] [PubMed]
- Portes, E.S.; Oliveira, J.H.; MacCagnan, P.; Abucham, J. Changes in serum thyroid hormones levels and their mechanisms during long-term growth hormone (GH) replacement therapy in GH deficient children. Clin. Endocrinol. 2000, 53, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Porretti, S.; Giavoli, C.; Ronchi, C.; Lombardi, G.; Zaccaria, M.; Valle, D.; Arosio, M.; Beck-Peccoz, P. Recombinant human GH replacement therapy and thyroid function in a large group of adult GH-deficient patients: When does L-T(4) therapy become mandatory? J. Clin. Endocrinol. Metab. 2002, 87, 2042–2045. [Google Scholar] [CrossRef] [PubMed]
- Giavoli, C.; Porretti, S.; Ferrante, E.; Cappiello, V.; Ronchi, C.L.; Travaglini, P.; Epaminonda, P.; Arosio, M.; Beck-Peccoz, P. Recombinant hGH replacement therapy and the hypothalamus-pituitary-thyroid axis in children with GH deficiency: When should we be concerned about the occurrence of central hypothyroidism? Clin. Endocrinol. 2003, 59, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Kalina-Faska, B.; Kalina, M.; Koehler, B. effects of recombinant growth hormone therapy on thyroid hormone concentrations. Int. J. Clin. Pharmacol. Ther. 2004, 42, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Hubina, E.; Mersebach, H.; Rasmussen, A.K.; Juul, A.; Sneppen, S.B.; Góth, M.I.; Feldt-Rasmussen, U. Effect of growth hormone replacement therapy on pituitary hormone secretion and hormone replacement therapies in GHD adults. Horm. Res. 2004, 61, 211–217. [Google Scholar] [CrossRef]
- Seminara, S.; Stagi, S.; Candura, L.; Scrivano, M.; Lenzi, L.; Nanni, L.; Pagliai, F.; Chiarelli, F. Changes of thyroid function during long-term hGH therapy in GHD children. A possible relationship with catchup growth? Horm. Metab. Res. 2005, 37, 751–756. [Google Scholar] [CrossRef]
- Martins, M.R.A.; Doin, F.C.; Komatsu, W.R.; Barros-Neto, T.L.; Moises, V.A.; Abucham, J. Growth hormone replacement improves thyroxine biological effects: Implications for management of central hypothyroidism. J. Clin. Endocrinol. Metab. 2007, 92, 4144–4153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agha, A.; Walker, D.; Perry, L.; Drake, W.M.; Chew, S.L.; Jenkins, P.J.; Grossman, A.B.; Monson, J.P. Unmasking of central hypothyroidism following growth hormone replacement in adult hypopituitary patients. Clin. Endocrinol. 2007, 66, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Moayeri, H.; Hemati, A.; Bidad, K.; Dalili, H. Effects of growth hormone replacement therapy on thyroid function tests in growth hormone deficient children. Acta Med. Iran. 2008, 46, 473–476. [Google Scholar]
- Losa, M.; Scavini, M.; Gatti, E.; Rossini, A.; Madaschi, S.; Formenti, I.; Caumo, A.; Stidley, C.A.; Lanzi, R. Long-term effects of growth hormone replacement on thyroid function in adults with growth hormone deficiency. Thyroid 2008, 18, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Smyczyńska, J.; Hilczer, M.; Stawerska, R.; Lewiński, A. Thyroid function in children with growth hormone (GH) deficiency during the initial phase of GH replacement therapy—Clinical implications. Thyroid. Res. 2010, 3, 2. [Google Scholar] [CrossRef] [Green Version]
- Ciresi, A.; Guarnotta, V.; Amato, M.C.; Giordano, C. Correlation between severity of growth hormone deficiency and thyroid metabolism and effects of long-term growth hormone treatment on thyroid function in children with idiopathic growth hormone deficiency. Horm. Res. Paediatr. 2014, 81, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Glynn, N.; Kenny, H.; Salim, T.; Halsall, D.J.; Smith, D.; Tun, T.K.; McDermott, J.H.; Tormey, W.; Thompson, C.J.; McAdam, B.; et al. Alterations in thyroid hormone levels following growth hormone replacement exert complex biological effects. Endocr. Pract. 2018, 24, 342–350. [Google Scholar] [CrossRef]
- Keskin, M.; Bayramoglu, E.; Aycan, Z. Effects of 1-year growth hormone replacement therapy on thyroid volume and function of the children and adolescents with idiopathic growth hormone deficiency. J. Pediatr. Endocrinol Metab. 2017, 30, 1187–1190. [Google Scholar] [CrossRef] [PubMed]
- Witkowska-Sędek, E.; Borowiec, A.; Majcher, A.; Sobol, M.; Rumińska, M.; Pyrżak, B. Thyroid function in children with growth hormone deficiency during long-term growth hormone replacement therapy. Cent. Eur. J. Immunol. 2018, 43, 255–261. [Google Scholar] [CrossRef]
- Van Iersel, L.; Santen, H.M.; Zandwijken, G.R.J.; Zwaveling-Soonawala, N.; Hokken-Koelega, A.C.S.; Trotsenburg, A.S.P. Low FT4 Concentrations around the Start of Recombinant Human Growth Hormone Treatment: Predictor of Congenital Structural Hypothalamic-Pituitary Abnormalities? Horm. Res. Paediatr. 2018, 89, 98–107. [Google Scholar] [CrossRef]
- Yamauchi, I.; Sakane, Y.; Yamashita, T.; Hirota, K.; Ueda, Y.; Kanai, Y.; Yamashita, Y.; Kondo, F.; Fujii, T.; Taura, D.; et al. Effects of growth hormone on thyroid function are mediated by type 2 iodothyronine deiodinase in humans. Endocrine 2018, 59, 353–363. [Google Scholar] [CrossRef] [Green Version]
- Ebuchi, Y.; Kubo, T.; Furujo, M.; Higuchi, Y.; Fujinaga, S.; Tsuchiya, H.; Urata, N.; Ochi, M.; Namba, T.; Hara, N.; et al. Effect of growth hormone therapy on thyroid function in isolated growth hormone deficient and short small for gestational age children: A two-year study, including on assessment of the usefulness of the thyrotropin-releasing hormone (TRH) stimulation test. J. Pediatr. Endocrinol. Metab. 2020, 33, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Zheng, D.; Liang, Y.; Hou, L.; Ying, Y.Q.; Luo, X.P.; Wu, W. The effects of recombinant human growth hormone therapy on thyroid function in pediatric patients with growth hormone deficiency. Transl. Pediatr. 2021, 10, 851–859. [Google Scholar] [CrossRef]
- Møller, J.; Jørgensen, J.O.; Møller, N.; Christiansen, J.S.; Weeke, J. Effects of growth hormone administration on fuel oxidation and thyroid function in normal man. Metabolism 1992, 41, 728–731. [Google Scholar] [CrossRef]
- Leong, G.M.; Rose, S.R.; Barnes, K.M.; Cutler, G.B. Thyroid function in non-GH-deficient short children during a placebo-controlled double-blind trail of GH therapy. Pediatr. Res. 1993, 5, 33–41. [Google Scholar]
- Rose, S.R.; Leong, G.M.; Yanovski, J.A.; Blum, D.; Heavner, G.; Bartnes, K.M.; Chipman, J.J.; Dichek, H.L.; Jacobsen, J.; Oertner, K.E.; et al. Thyroid function in non-growth hormone deficient short children during a placebo controlled double blind trial of recombinant growth hormone therapy. J. Clin. Endocrinol. Metab. 1995, 80, 320–324. [Google Scholar] [PubMed]
- de Kort, S.W.; Willemsen, R.H.; van der Kaay, D.C.; van Dijk, M.; Visser, T.J.; Hokken-Koelega, A.C. Thyroid function in short children born small-for-gestational age (SGA) before and during GH treatment. Clin. Endocrinol. 2008, 69, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Susperreguy, S.; Miras, M.B.; Montesinos, M.M.; Mascanfroni, I.D.; Muñoz, L.; Sobrero, G.; Silvano, L.; Masini-Repiso, A.M.; Coleoni, A.H.; Targovnik, H.M.; et al. Growth hormone (GH) treatment reduces peripheral thyroid hormone action in girls with Turner syndrome. Clin. Endocrinol. 2007, 67, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Grunfeld, C.; Sherman, B.M.; Cavalieri, R.R. The acute effects of human growth hormone administration on thyroid function in normal men. J. Clin. Endocrinol. Metab. 1988, 67, 1111–1114. [Google Scholar] [CrossRef]
- Sgrò, P.; Sansone, M.; Parisi, A.; Sartorio, A.; Sansone, A.; Romanelli, F.; Lenzi, A.; Di Luigi, L. Supra-physiological rhGH administration induces gender-related differences in the hypothalamus-pituitary-thyroid (HPT) axis in healthy individuals. J. Endocrinol. Investig. 2016, 39, 1383–1390. [Google Scholar] [CrossRef]
- Lania, A.; Giavoli, C.; Ferrante, E.; Beck-Peccoz, P. Central hypothyroidism and growth hormone treatment: Clinical care. J. Endocrinol. Investig. 2008, 31, 66–70. [Google Scholar]
- Dąbrowska, A.M.; Tarach, J.S.; Kurowska, M.; Nowakowski, A. Thyroid diseases in patients with acromegaly. Arch. Med. Sci. 2014, 29, 837–845. [Google Scholar] [CrossRef]
- Natchev, E.; Vandeva, S.; Kovatcheva, R.; Kirilov, G.; Kalinov, K.; Zacharieva, S. Thyroid gland changes in patients with acromegaly. Arch. Endocrinol. Metab. 2020, 64, 269–275. [Google Scholar] [PubMed]
- Dagdelen, S.; Cinar, N.; Erbas, T. Increased thyroid cancer risk in acromegaly. Pituitary 2014, 17, 299–306. [Google Scholar] [CrossRef]
- Wolinski, K.; Stangierski, A.; Dyrda, K.; Nowicka, K.; Pelka, M.; Iqbal, A.; Car, A.; Lazizi, M.; Bednarek, N.; Czarnywojtek, A.; et al. Risk of malignant neoplasms in acromegaly: A case-control study. J. Endocrinol. Investig. 2017, 40, 319–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danilowicz, K.; Sosa, S.; Gonzalez Pernas, M.S.; Bamberger, E.; Dies, S.M.; Fainstein-Day, P.; Furioso, A.; Glerean, M.; Guitelman, M.; Katz, D.; et al. Acromegaly and thyroid cancer: Analysis of evolution in a series of patients. Clin. Diabetes Endocrinol. 2020, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Roelfsema, F.; Biermasz, N.R.; Frolich, M.; Keenan, D.M.; Veldhuis, J.D.; Romijn, J.A. Diminished and irregular thyrotropin secretion with preserved diurnal rhythm in patients with active acromegaly. J. Clin. Endocrinol. Metab. 2009, 94, 1945–1950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fliers, E.; Unmehopa, U.A.; Alkemade, A. Functional neuroanatomy of thyroid hormone feedback in the human hypothalamus and pituitary gland. Mol. Cell Endocrinol. 2006, 251, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Municchi, G.; Malozowski, S.; Nisula, B.C.; Cristiano, A.; Rose, S.R. Nocturnal thyrotropin surge in growth hormone-deficient children. J. Pediatr. 1992, 121, 214–220. [Google Scholar] [CrossRef]
- Edén Engström, B.; Burman, P.; Holdstock, C.; Karlsson, F.A. Effects of growth hormone (GH) on ghrelin, leptin, and adiponectin in GH-deficient patients. J. Clin. Endocrinol. Metab. 2003, 88, 5193–5198. [Google Scholar] [CrossRef] [Green Version]
- López-Siguero, J.P.; López-Canti, L.F.; Espino, R.; Caro, E.; Fernández-García, J.M.; Gutiérrez-Macías, A.; Rial, J.M.; Lechuga, J.L.; Macías, F.; Martínez-Aedo, M.J.; et al. Effect of recombinant growth hormone on leptin, adiponectin, resistin, interleukin-6, tumor necrosis factor-α and ghrelin levels in growth hormone-deficient children. J. Endocrinol. Investig. 2011, 34, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, H.; Fors, H.; Bosaeus, I.; Rosberg, S.; Albertsson-Wikland, K.; Bjarnason, R. Changes in body composition and leptin levels during growth hormone (GH) treatment in short children with various GH secretory capacities. Eur. J. Endocrinol. 1999, 140, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Glynn, N.; Kenny, H.; Quisenberry, L.; Halsall, D.J.; Cook, P.; Kyaw Tun, T. The effect of growth hormone replacement on the thyroid axis in patients with hypopituitarism: In vivo and ex vivo studies. Clin. Endocrinol. 2017, 86, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Hocquette, J.F.; Postel-Vinay, M.C.; Kayser, C.; De Hemptinne, B.; Amar-Costeses, A. The Human Liver Growth Hormone Receptor. Endocrinology 1989, 125, 2167–2174. [Google Scholar] [CrossRef] [PubMed]
- MacLatchy, D.L.; Kawauchi, H.; Eales, J.G. Stimulation of hepatic thyroxine 5′-deiodinase activity in rainbow trout (Oncorhynchus mykiss) by Pacific salmon growth hormone. Comp. Biochem. Physiol. Comp. Physiol. 1992, 101, 689–691. [Google Scholar] [CrossRef]
- Darras, V.M.; Rudas, P.; Visser, T.J.; Hall, T.R.; Huybrechts, L.M.; Vanderpooten, A.; Berghman, L.R.; Decuypere, E.; Kuhn, E.R. Endogenous growth hormone controls high plasma levels of 3,3′,5-triiodothyronine (T3) in growing chickens by decreasing the T3-degrading type III deiodinase activity. Domest. Anim. Endocrinol. 1993, 10, 55–65. [Google Scholar] [CrossRef]
- Salvatore, D.; Tu, H.; Harney, J.W.; Larsen, P.R. Type 2 iodothyronine deiodinase is highly expressed in human thyroid. J. Clin. Investig. 1996, 98, 962–968. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.A.; Schmitz, O.; Jorgensen, J.O.; Christiansen, J.S.; Weeke, J.; Schmid, C.; Froesch, E.R. Insulin-like growth factor I alters peripheral thyroid hormone metabolism in humans: Comparison with growth hormone. Eur. J. Endocrinol. 1996, 134, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Klinger, B.; Ionesco, A.; Anin, S.; Laron, Z. Effects of insulin-like growth factor I on the thyroid axis in patients with Laron-type dwarfism and healthy subjects. Acta Endocrinol. 1992, 127, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Van der Lan, B.F.; Freeman, J.L.; Asa, S.L. Expression of growth factors and growth factors receptors in normal and tumorous human thyroid tissues. Thyroid 1995, 5, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, R.; Frasca, F.; Garozzo, A.; Gianı, F.; Pandini, G.; Vella, V.; Vigneri, R.; Belfiore, A. Insulin receptor isoforms and insulin-like growth factor receptor in human follicular cell precursors from papillary thyroid cancer and normal thyroid. J. Clin. Endocrinol. Metab. 2011, 96, 766–774. [Google Scholar] [CrossRef] [Green Version]
- Kimura, T.; van Keymeulen, A.; Golstein, J.; Fusco, A.; Dumont, J.E.; Roger, P.P. Regulation of thyroid cell proliferation by TSH and other factors: A critical evaluation of in vitro models. Endocr. Rev. 2001, 22, 631–656. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.Y.; Wang, L.; Ballock, R.T. Thyroid hormone and the growth plate. Rev. Endocr. Metab. Disord. 2006, 7, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Susperreguy, S.; Muñoz, L.; Tkalenko, N.Y.; Mascanfroni, I.; Alamino, V.A.; Montesinos, M.; Masini-Repiso, A.M.; Miras, M.; Pellizas, C. Growth hormone treatment in children with idiopathic short stature: Correlation of growth response with peripheral thyroid hormone action. Clin. Endocrinol. 2011, 74, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jiang, S.; Cui, Z.; Luo, X.; Shi, L.; Zheng, H. Sensitivity of supplementation of thyroid hormone on treatment of idiopathic short-stature children during therapy with recombinant human growth hormone. Front Med. 2018, 5, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Ocaranza, P.; Morales, F.; Matamala, Á.; Gaete, X.; Román, R.; Lammoglia, J.; Cassorla, F. Growth hormone signaling in fibroblasts from newborn boys and prepubertal boys. Growth Horm. IGF Res. 2016, 27, 18–27. [Google Scholar] [CrossRef]
- Binder, G.; Schnabel, D.; Reinehr, T.; Pfäffle, R.; Dörr, H.G.; Bettendorf, M.; Hauffa, B.; Woelfle, J. Evolving pituitary hormone deficits in primarily isolated GHD: A review and experts’ consensus. Mol. Cell. Pediatr. 2020, 7, 16. [Google Scholar] [CrossRef] [PubMed]
| Authors | Year | Diagnosis | No of Patients | Study Population | T4 and/or fT4 | T3 and/or fT3 | rT3 | TSH |
|---|---|---|---|---|---|---|---|---|
| Porter et al. [2] | 1973 | IGHD/MPHD | 6 | Children | ↔ | n.a. | n.a. | ↓ |
| Lippe et al. [1] | 1975 | IGHD/MPHD | 6 | Children | ↓ | ↓ | n.a. | ↓ |
| Cacciari et al. [3] | 1979 | IGHD/MPHD | 24 | Children | ↔ | ↔ | n.a. | ↔ |
| Demura et al. [4] | 1980 | IGHD/MPHD | 26 | Children | ↓ | ↔ | n.a. | ↔ |
| Jørgensen et al. [5] | 1989 | IGHD/MPHD | 22 | Adults | ↓ | ↑ | ↓ | ↔ |
| Pirazolli et al. [6] | 1992 | IGHD | 57 | Children | ↓ | ↑ | n.a. | ↔ |
| Rezvani et al. [7] | 1992 | IGHD/MPHD | 7 | Children | ↔ | ↑ | ↓ | ↔ |
| Jørgensen et al. [8] | 1994 | IGHD/MPHD | 10 | Adults | ↔ | ↑ | ↓ | ↓ |
| Monson et al. [9] | 1994 | MPHD | 21 | Adults | ↓/↔ | ↑/↔ | n.a. | n.a. |
| Sato et al. [10] | 1977 | IGHD/MPHD | 8 | Children | ↓ | ↑/↔ | n.a. | ↑/↔ |
| Wyatt et al. [11] | 1998 | IGHD/MPHD | 15 | Children | ↓ | ↑ | ↓ | ↔ |
| Portes et al. [12] | 2000 | IGHD/MPHD | 20 | Children | ↓ | ↑ | ↓ | ↔ |
| Porretti et al. [13] | 2002 | IGHD/MPHD | 66 | Adults | ↓ | ↔ | ↓ | ↔ |
| Giavoli et al. [14] | 2003 | IGHD/MPHD | 26 | Children | ↓ | ↔ | n.a. | ↔ |
| Kalina-Faska et al. [15] | 2004 | IGHD | 32 | Children | n.a. | n.a. | n.a. | ↓ |
| Hubina et al. [16] | 2004 | MPHD | 112 | Adults | ↔ | ↑/↔ | n.a. | ↔ |
| Seminara et al. [17] | 2005 | IGHD | 19 | Children | ↓ | ↑ | n.a. | ↔ |
| Martins et al. [18] | 2007 | IGHD/MPHD | 30 | Children/adults | ↓ | ↑ | n.a. | n.a. |
| Agha et al. [19] | 2007 | MPHD | 243 | Adults | ↓ | ↔ | n.a. | ↔ |
| Moayeri et al. [20] | 2008 | IGHD | 21 | Children | ↓ | ↔ | n.a. | ↔ |
| Losa et al. [21] | 2008 | IGHD/MPHD | 49 | Adults | ↓ | ↔ | n.a. | ↔ |
| Smyczynska et al. [22] | 2010 | IGHD/NSD/inactGH | 75 | Children | ↓ | n.a. | n.a. | ↔ |
| Ciresi et al. [23] | 2014 | IGHD | 105 | Children | ↔ | ↑ | n.a. | ↔ |
| Glynn et al. [24] | 2017 | IGHD/MPHD | 20 | Adults | ↓ | ↑ | ↓ | ↔ |
| Keskin et al. [25] | 2017 | IGHD | 29 | Children | ↓ | 2194 | n.a. | ↓ |
| Witkowska-Sędek et al. [26] | 2018 | IGHD | 117 | Children | ↓ | n.a. | n.a. | ↔ |
| Van Iersel et al. [27] | 2018 | IGHD/MPHD | 456 | Children | ↓ | n.a. | n.a. | ↔ |
| Yamauchi et al. [28] | 2018 | MPHD | 20 | Adults | ↔ | ↑ | n.a. | ↓ |
| Ebuchi et al. [29] | 2020 | IGHD/SGA | 203 | Children | ↓GHD/↑SGA | n.a. | n.a. | ↓ |
| Yao et al. [30] | 2021 | IGHD | 55 | Children | ↓ | n.a. | n.a. | ↑ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kucharska, A.M.; Witkowska-Sędek, E.; Rumińska, M.; Pyrżak, B. Thyroid Hormone Changes Related to Growth Hormone Therapy in Growth Hormone Deficient Patients. J. Clin. Med. 2021, 10, 5354. https://doi.org/10.3390/jcm10225354
Kucharska AM, Witkowska-Sędek E, Rumińska M, Pyrżak B. Thyroid Hormone Changes Related to Growth Hormone Therapy in Growth Hormone Deficient Patients. Journal of Clinical Medicine. 2021; 10(22):5354. https://doi.org/10.3390/jcm10225354
Chicago/Turabian StyleKucharska, Anna Małgorzata, Ewelina Witkowska-Sędek, Małgorzata Rumińska, and Beata Pyrżak. 2021. "Thyroid Hormone Changes Related to Growth Hormone Therapy in Growth Hormone Deficient Patients" Journal of Clinical Medicine 10, no. 22: 5354. https://doi.org/10.3390/jcm10225354
APA StyleKucharska, A. M., Witkowska-Sędek, E., Rumińska, M., & Pyrżak, B. (2021). Thyroid Hormone Changes Related to Growth Hormone Therapy in Growth Hormone Deficient Patients. Journal of Clinical Medicine, 10(22), 5354. https://doi.org/10.3390/jcm10225354






