Lateralization in Reverse Shoulder Arthroplasty
Abstract
:1. Introduction
2. Traditional Grammont Design
3. Notching
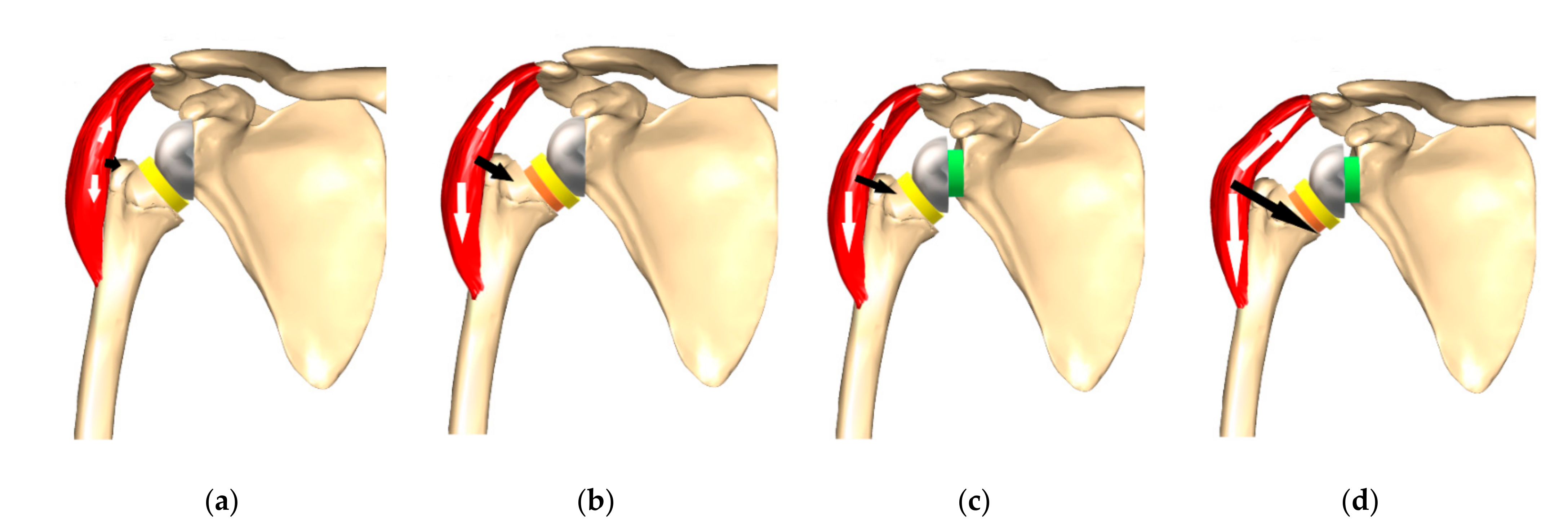
4. Lateralization (Global)
4.1. Glenoid Lateralization
4.1.1. Advantages of Glenoid-Sided Lateralization
4.1.2. Possible Negative Effects of Glenoid-Sided Lateralization
4.1.3. Technical Limitations to Metal-Augmented or Bony Lateralization
4.2. Humeral Lateralization
4.2.1. Advantages of Humeral Lateralization
4.2.2. Disadvantages of Isolated Humeral Lateralization
4.3. How Much to Lateralize?
5. Author’s Suggested Technique
- (1)
- The computed sphere that best fits the 3D model of the glenoid concavity is displayed with the glenoid best fit sphere radius (GBFSR), a marker of the glenohumeral size (observed range: 25–45 mm). Patients with GBFS of less than 30 mm are at higher risk of overstuffing.
- (2)
- Lateralization (variation 0 to +10 mm): The surgeon should be cautious with lateralization beyond +5–10 mm depending on the amount of loss of medial bone stock. Excessive superior migration (vertically decentered) but, more importantly, posterior (e.g., B2 glenoid) and anterior subluxation are signs that there will be increased tension when attempting to reduce the RSA.
- (3)
- Distalization (variation: 20 to +40 mm) depends on the amount of cranial humeral head migration. The surgeon should be cautious with distalization beyond 35–40 mm.
5.1. Glenoid Side
5.2. Gap Space Assessment
5.3. Humeral Side
5.4. Risks
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grammont, P.M.; Trouilloud, P.; Laffay, J.; Deries, X. Etude et Réalisation D’une Nouvelle Prothèse D’épaule. Available online: https://www.semanticscholar.org/paper/Etude-et-r%C3%A9alisation-d%27une-nouvelle-proth%C3%A8se-Grammont-Trouilloud/ce86490e649299d28303798d257d14c9b2642fbe (accessed on 28 July 2020).
- Bauer, S.; Walch, G.; Athwal, G. Patient-Specific Lateralization, Glenoid Offset, Implications and Operative Strategy. Lead. Opin. Orthopädie Rheumatol. 2020, 4, 22–27. [Google Scholar]
- Gerber, C.; Canonica, S.; Catanzaro, S.; Ernstbrunner, L. Longitudinal Observational Study of Reverse Total Shoulder Arthroplasty for Irreparable Rotator Cuff Dysfunction: Results after 15 Years. J. Shoulder Elb. Surg. 2018, 27, 831–838. [Google Scholar] [CrossRef]
- Ernstbrunner, L.; Andronic, O.; Grubhofer, F.; Camenzind, R.S.; Wieser, K.; Gerber, C. Long-Term Results of Reverse Total Shoulder Arthroplasty for Rotator Cuff Dysfunction: A Systematic Review of Longitudinal Outcomes. J. Shoulder Elb. Surg. 2019, 28, 774–781. [Google Scholar] [CrossRef]
- Boileau, P.; Watkinson, D.J.; Hatzidakis, A.M.; Balg, F. Grammont Reverse Prosthesis: Design, Rationale, and Biomechanics. J. Shoulder Elb. Surg. 2005, 14, 147S–161S. [Google Scholar] [CrossRef]
- Wall, B.; Nové-Josserand, L.; O’Connor, D.P.; Edwards, T.B.; Walch, G. Reverse Total Shoulder Arthroplasty: A Review of Results According to Etiology. J. Bone Jt. Surg. Am. 2007, 89, 1476–1485. [Google Scholar] [CrossRef]
- Routman, H.D.; Flurin, P.-H.; Wright, T.W.; Zuckerman, J.D.; Hamilton, M.A.; Roche, C.P. Reverse Shoulder Arthroplasty Prosthesis Design Classification System. Bull. Hosp. Jt. Dis. 2015, 73 (Suppl. S1), S5–S14. [Google Scholar]
- Werthel, J.-D.; Walch, G.; Vegehan, E.; Deransart, P.; Sanchez-Sotelo, J.; Valenti, P. Lateralization in Reverse Shoulder Arthroplasty: A Descriptive Analysis of Different Implants in Current Practice. Int. Orthop. 2019, 43, 2349–2360. [Google Scholar] [CrossRef]
- Guarrella, V.; Chelli, M.; Domos, P.; Ascione, F.; Boileau, P.; Walch, G. Risk Factors for Instability after Reverse Shoulder Arthroplasty. Shoulder Elb. 2021, 13, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Henninger, H.B.; Barg, A.; Anderson, A.E.; Bachus, K.N.; Burks, R.T.; Tashjian, R.Z. Effect of Lateral Offset Center of Rotation in Reverse Total Shoulder Arthroplasty: A Biomechanical Study. J. Shoulder Elb. Surg. 2012, 21, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Werner, B.S.; Chaoui, J.; Walch, G. Glenosphere Design Affects Range of Movement and Risk of Friction-Type Scapular Impingement in Reverse Shoulder Arthroplasty. Bone Jt. J. 2018, 100, 1182–1186. [Google Scholar] [CrossRef]
- Nyffeler, R.W.; Werner, C.M.L.; Simmen, B.R.; Gerber, C. Analysis of a Retrieved Delta III Total Shoulder Prosthesis. J. Bone Jt. Surg. Br. 2004, 86, 1187–1191. [Google Scholar] [CrossRef] [Green Version]
- Simovitch, R.W.; Zumstein, M.A.; Lohri, E.; Helmy, N.; Gerber, C. Predictors of Scapular Notching in Patients Managed with the Delta III Reverse Total Shoulder Replacement. J. Bone Jt. Surg. Am. 2007, 89, 588–600. [Google Scholar] [CrossRef]
- Friedman, R.J.; Barcel, D.A.; Eichinger, J.K. Scapular Notching in Reverse Total Shoulder Arthroplasty. J. Am. Acad. Orthop. Surg. 2019, 27, 200–209. [Google Scholar] [CrossRef]
- Berhouet, J.; Garaud, P.; Favard, L. Evaluation of the Role of Glenosphere Design and Humeral Component Retroversion in Avoiding Scapular Notching during Reverse Shoulder Arthroplasty. J Shoulder Elb. Surg. 2014, 23, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Sirveaux, F.; Favard, L.; Oudet, D.; Huquet, D.; Walch, G.; Molé, D. Grammont Inverted Total Shoulder Arthroplasty in the Treatment of Glenohumeral Osteoarthritis with Massive Rupture of the Cuff. Results of a Multicentre Study of 80 Shoulders. J. Bone Jt. Surg. Br. 2004, 86, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Mollon, B.; Mahure, S.A.; Roche, C.P.; Zuckerman, J.D. Impact of Scapular Notching on Clinical Outcomes after Reverse Total Shoulder Arthroplasty: An Analysis of 476 Shoulders. J. Shoulder Elb. Surg. 2017, 26, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Wellmann, M.; Struck, M.; Pastor, M.F.; Gettmann, A.; Windhagen, H.; Smith, T. Short and Midterm Results of Reverse Shoulder Arthroplasty According to the Preoperative Etiology. Arch. Orthop. Trauma Surg. 2013, 133, 463–471. [Google Scholar] [CrossRef]
- Simovitch, R.; Flurin, P.-H.; Wright, T.W.; Zuckerman, J.D.; Roche, C. Impact of Scapular Notching on Reverse Total Shoulder Arthroplasty Midterm Outcomes: 5-Year Minimum Follow-Up. J. Shoulder Elb. Surg. 2019, 28, 2301–2307. [Google Scholar] [CrossRef]
- Boileau, P.; Morin-Salvo, N.; Bessière, C.; Chelli, M.; Gauci, M.-O.; Lemmex, D.B. Bony Increased-Offset-Reverse Shoulder Arthroplasty: 5 to 10 Years’ Follow-Up. J. Shoulder Elb. Surg. 2020, 29, 2111–2122. [Google Scholar] [CrossRef]
- Erickson, B.J.; Frank, R.M.; Harris, J.D.; Mall, N.; Romeo, A.A. The Influence of Humeral Head Inclination in Reverse Total Shoulder Arthroplasty: A Systematic Review. J. Shoulder Elb. Surg. 2015, 24, 988–993. [Google Scholar] [CrossRef]
- Teissier, P.; Teissier, J.; Kouyoumdjian, P.; Asencio, G. The TESS Reverse Shoulder Arthroplasty without a Stem in the Treatment of Cuff-Deficient Shoulder Conditions: Clinical and Radiographic Results. J. Shoulder Elb. Surg. 2015, 24, 45–51. [Google Scholar] [CrossRef]
- Beck, S.; Patsalis, T.; Busch, A.; Dittrich, F.; Dudda, M.; Jäger, M.; Wegner, A. Long-Term Results of the Reverse Total Evolutive Shoulder System (TESS). Arch. Orthop. Trauma Surg. 2019, 139, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.A.; Diep, P.; Roche, C.; Flurin, P.H.; Wright, T.W.; Zuckerman, J.D.; Routman, H. Effect of Reverse Shoulder Design Philosophy on Muscle Moment Arms. J. Orthop. Res. 2015, 33, 605–613. [Google Scholar] [CrossRef]
- Frankle, M.; Levy, J.C.; Pupello, D.; Siegal, S.; Saleem, A.; Mighell, M.; Vasey, M. The Reverse Shoulder Prosthesis for Glenohumeral Arthritis Associated with Severe Rotator Cuff Deficiency. A Minimum Two-Year Follow-up Study of Sixty Patients Surgical Technique. J. Bone Jt. Surg. Am. 2006, 88 Pt 2, 178–190. [Google Scholar] [CrossRef]
- Mulieri, P.; Dunning, P.; Klein, S.; Pupello, D.; Frankle, M. Reverse Shoulder Arthroplasty for the Treatment of Irreparable Rotator Cuff Tear without Glenohumeral Arthritis. J. Bone Jt. Surg. Am. 2010, 92, 2544–2556. [Google Scholar] [CrossRef] [PubMed]
- Harman, M.; Frankle, M.; Vasey, M.; Banks, S. Initial Glenoid Component Fixation in “Reverse” Total Shoulder Arthroplasty: A Biomechanical Evaluation. J. Shoulder Elb. Surg. 2005, 14, 162S–167S. [Google Scholar] [CrossRef] [PubMed]
- Boileau, P.; Moineau, G.; Roussanne, Y.; O’Shea, K. Bony Increased-Offset Reversed Shoulder Arthroplasty: Minimizing Scapular Impingement While Maximizing Glenoid Fixation. Clin. Orthop. Relat. Res. 2011, 469, 2558–2567. [Google Scholar] [CrossRef] [Green Version]
- Denard, P.J.; Lederman, E.; Parsons, B.O.; Romeo, A.A. Finite Element Analysis of Glenoid-Sided Lateralization in Reverse Shoulder Arthroplasty. J. Orthop. Res. 2017, 35, 1548–1555. [Google Scholar] [CrossRef]
- Valenti, P.; Sauzières, P.; Katz, D.; Kalouche, I.; Kilinc, A.S. Do Less Medialized Reverse Shoulder Prostheses Increase Motion and Reduce Notching? Clin. Orthop. Relat. Res. 2011, 469, 2550–2557. [Google Scholar] [CrossRef] [Green Version]
- Katz, D.; Valenti, P.; Kany, J.; Elkholti, K.; Werthel, J.-D. Does Lateralisation of the Centre of Rotation in Reverse Shoulder Arthroplasty Avoid Scapular Notching? Clinical and Radiological Review of One Hundred and Forty Cases with Forty Five Months of Follow-Up. Int. Orthop. 2016, 40, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Hansen, M.L.; Routman, H. The Biomechanics of Current Reverse Shoulder Replacement Options. Ann. Jt. 2019, 4, 17. [Google Scholar] [CrossRef]
- Helmkamp, J.K.; Bullock, G.S.; Amilo, N.R.; Guerrero, E.M.; Ledbetter, L.S.; Sell, T.C.; Garrigues, G.E. The Clinical and Radiographic Impact of Center of Rotation Lateralization in Reverse Shoulder Arthroplasty: A Systematic Review. J. Shoulder Elb. Surg. 2018, 27, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, C.; Williams, G.R.; Namdari, S. Influence of Glenosphere Design on Outcomes and Complications of Reverse Arthroplasty: A Systematic Review. Clin. Orthop. Surg. 2016, 8, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Boileau, P.; Rumian, A.P.; Zumstein, M.A. Reversed Shoulder Arthroplasty with Modified L’Episcopo for Combined Loss of Active Elevation and External Rotation. J. Shoulder Elb. Surg. 2010, 19, 20–30. [Google Scholar] [CrossRef]
- Berglund, D.D.; Rosas, S.; Triplet, J.J.; Kurowicki, J.; Horn, B.; Levy, J.C. Restoration of External Rotation Following Reverse Shoulder Arthroplasty without Latissimus Dorsi Transfer. JBJS Open Access 2018, 3, e0054. [Google Scholar] [CrossRef]
- Giles, J.W.; Langohr, G.D.G.; Johnson, J.A.; Athwal, G.S. Implant Design Variations in Reverse Total Shoulder Arthroplasty Influence the Required Deltoid Force and Resultant Joint Load. Clin. Orthop. Relat. Res. 2015, 473, 3615–3626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zumstein, M.A.; Pinedo, M.; Old, J.; Boileau, P. Problems, Complications, Reoperations, and Revisions in Reverse Total Shoulder Arthroplasty: A Systematic Review. J. Shoulder Elb. Surg. 2011, 20, 146–157. [Google Scholar] [CrossRef]
- Rojas, J.; Choi, K.; Joseph, J.; Srikumaran, U.; McFarland, E.G. Aseptic Glenoid Baseplate Loosening After Reverse Total Shoulder Arthroplasty: A Systematic Review and Meta-Analysis. JBJS Rev. 2019, 7, e7. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.T.; Langohr, G.D.G.; Athwal, G.S.; Johnson, J.A. Implant Positioning in Reverse Shoulder Arthroplasty Has an Impact on Acromial Stresses. J. Shoulder Elb. Surg. 2016, 25, 1889–1895. [Google Scholar] [CrossRef] [PubMed]
- Haidamous, G.; Lädermann, A.; Frankle, M.A.; Gorman, R.A.; Denard, P.J. The Risk of Postoperative Scapular Spine Fracture Following Reverse Shoulder Arthroplasty Is Increased with an Onlay Humeral Stem. J. Shoulder Elb. Surg. 2020, 29, 2556–2563. [Google Scholar] [CrossRef]
- Boileau, P.; Morin-Salvo, N.; Gauci, M.-O.; Seeto, B.L.; Chalmers, P.N.; Holzer, N.; Walch, G. Angled BIO-RSA (Bony-Increased Offset-Reverse Shoulder Arthroplasty): A Solution for the Management of Glenoid Bone Loss and Erosion. J. Shoulder Elb. Surg. 2017, 26, 2133–2142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Biase, C.F.; Delcogliano, M.; Borroni, M.; Castagna, A. Reverse Total Shoulder Arthroplasty: Radiological and Clinical Result Using an Eccentric Glenosphere. Musculoskelet Surg. 2012, 96 (Suppl. S1), S27–S34. [Google Scholar] [CrossRef]
- Martinez, A.A.; Bejarano, C.; Carbonel, I.; Iglesias, D.; Gil-Albarova, J.; Herrera, A. The Treatment of Proximal Humerus Nonunions in Older Patients with Reverse Shoulder Arthroplasty. Injury 2012, 43 (Suppl. S2), S3–S6. [Google Scholar] [CrossRef]
- Randelli, P.; Randelli, F.; Arrigoni, P.; Ragone, V.; D’Ambrosi, R.; Masuzzo, P.; Cabitza, P.; Banfi, G. Optimal Glenoid Component Inclination in Reverse Shoulder Arthroplasty. How to Improve Implant Stability. Musculoskelet. Surg. 2014, 98, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Sayana, M.K.; Kakarala, G.; Bandi, S.; Wynn-Jones, C. Medium Term Results of Reverse Total Shoulder Replacement in Patients with Rotator Cuff Arthropathy. Ir. J. Med. Sci. 2009, 178, 147–150. [Google Scholar] [CrossRef]
- Young, A.A.; Smith, M.M.; Bacle, G.; Moraga, C.; Walch, G. Early Results of Reverse Shoulder Arthroplasty in Patients with Rheumatoid Arthritis. J. Bone Jt. Surg. 2011, 93, 1915–1923. [Google Scholar] [CrossRef]
- Franceschetti, E.; de Sanctis, E.G.; Ranieri, R.; Palumbo, A.; Paciotti, M.; Franceschi, F. The Role of the Subscapularis Tendon in a Lateralized Reverse Total Shoulder Arthroplasty: Repair versus Nonrepair. Int. Orthop. 2019, 43, 2579–2586. [Google Scholar] [CrossRef]
- Langohr, G.D.G.; Giles, J.W.; Athwal, G.S.; Johnson, J.A. The Effect of Glenosphere Diameter in Reverse Shoulder Arthroplasty on Muscle Force, Joint Load, and Range of Motion. J. Shoulder Elb. Surg. 2015, 24, 972–979. [Google Scholar] [CrossRef]
- Hamilton, M.A.; Roche, C.P.; Diep, P.; Flurin, P.-H.; Routman, H.D. Effect of Prosthesis Design on Muscle Length and Moment Arms in Reverse Total Shoulder Arthroplasty. Bull. Hosp. Jt. Dis. 2013, 71 (Suppl. S2), S31–S35. [Google Scholar]
- Lädermann, A.; Denard, P.J.; Collin, P.; Zbinden, O.; Chiu, J.C.-H.; Boileau, P.; Olivier, F.; Walch, G. Effect of Humeral Stem and Glenosphere Designs on Range of Motion and Muscle Length in Reverse Shoulder Arthroplasty. Int. Orthop. 2020, 44, 519–530. [Google Scholar] [CrossRef]
- Oh, J.H.; Shin, S.-J.; McGarry, M.H.; Scott, J.H.; Heckmann, N.; Lee, T.Q. Biomechanical Effects of Humeral Neck-Shaft Angle and Subscapularis Integrity in Reverse Total Shoulder Arthroplasty. J. Shoulder Elb. Surg. 2014, 23, 1091–1098. [Google Scholar] [CrossRef]
- Beltrame, A.; Di Benedetto, P.; Cicuto, C.; Cainero, V.; Gisonni, R.; Causero, A. Onlay versus Inlay Humeral Steam in Reverse Shoulder Arthroplasty (RSA): Clinical and Biomechanical Study. Acta Biomed. 2019, 90, 54–63. [Google Scholar] [CrossRef]
- Roche, C.P.; Diep, P.; Hamilton, M.; Crosby, L.A.; Flurin, P.-H.; Wright, T.W.; Zuckerman, J.D.; Routman, H.D. Impact of Inferior Glenoid Tilt, Humeral Retroversion, Bone Grafting, and Design Parameters on Muscle Length and Deltoid Wrapping in Reverse Shoulder Arthroplasty. Bull. Hosp. Jt. Dis. 2013, 71, 284–293. [Google Scholar]
- Merolla, G.; Walch, G.; Ascione, F.; Paladini, P.; Fabbri, E.; Padolino, A.; Porcellini, G. Grammont Humeral Design versus Onlay Curved-Stem Reverse Shoulder Arthroplasty: Comparison of Clinical and Radiographic Outcomes with Minimum 2-Year Follow-Up. J. Shoulder Elb. Surg. 2018, 27, 701–710. [Google Scholar] [CrossRef]
- Ascione, F.; Kilian, C.M.; Laughlin, M.S.; Bugelli, G.; Domos, P.; Neyton, L.; Godeneche, A.; Edwards, T.B.; Walch, G. Increased Scapular Spine Fractures after Reverse Shoulder Arthroplasty with a Humeral Onlay Short Stem: An Analysis of 485 Consecutive Cases. J. Shoulder Elb. Surg. 2018, 27, 2183–2190. [Google Scholar] [CrossRef]
- Mayne, I.P.; Bell, S.N.; Wright, W.; Coghlan, J.A. Acromial and Scapular Spine Fractures after Reverse Total Shoulder Arthroplasty. Shoulder Elb. 2016, 8, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Boileau, P.; Walch, G. The Three-Dimensional Geometry of the Proximal Humerus. Implications for Surgical Technique and Prosthetic Design. J. Bone Jt. Surg. Br. 1997, 79, 857–865. [Google Scholar] [CrossRef]
- Kim, H.J.; Kwon, T.Y.; Jeon, Y.S.; Kang, S.G.; Rhee, Y.G.; Rhee, S.-M. Neurologic Deficit after Reverse Total Shoulder Arthroplasty: Correlation with Distalization. J. Shoulder Elb. Surg. 2020, 29, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Vajapey, S.P.; Contreras, E.S.; Cvetanovich, G.L.; Neviaser, A.S. Neurologic Complications in Primary Anatomic and Reverse Total Shoulder Arthroplasty: A Review. J. Clin. Orthop. Trauma 2021, 20, 101475. [Google Scholar] [CrossRef]
- Moroder, P.; Akgün, D.; Plachel, F.; Baur, A.D.J.; Siegert, P. The Influence of Posture and Scapulothoracic Orientation on the Choice of Humeral Component Retrotorsion in Reverse Total Shoulder Arthroplasty. J. Shoulder Elb. Surg. 2020, 29, 1992–2001. [Google Scholar] [CrossRef]
- Arenas-Miquelez, A.; Murphy, R.J.; Rosa, A.; Caironi, D.; Zumstein, M.A. Impact of Humeral and Glenoid Component Variations on Range of Motion in Reverse Geometry Total Shoulder Arthroplasty: A Standardized Computer Model Study. J. Shoulder Elb. Surg. 2021, 30, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Lädermann, A.; Tay, E.; Collin, P.; Piotton, S.; Chiu, C.-H.; Michelet, A.; Charbonnier, C. Effect of Critical Shoulder Angle, Glenoid Lateralization, and Humeral Inclination on Range of Movement in Reverse Shoulder Arthroplasty. Bone Jt. Res. 2019, 8, 378–386. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauer, S.; Corbaz, J.; Athwal, G.S.; Walch, G.; Blakeney, W.G. Lateralization in Reverse Shoulder Arthroplasty. J. Clin. Med. 2021, 10, 5380. https://doi.org/10.3390/jcm10225380
Bauer S, Corbaz J, Athwal GS, Walch G, Blakeney WG. Lateralization in Reverse Shoulder Arthroplasty. Journal of Clinical Medicine. 2021; 10(22):5380. https://doi.org/10.3390/jcm10225380
Chicago/Turabian StyleBauer, Stefan, Jocelyn Corbaz, George S. Athwal, Gilles Walch, and William G. Blakeney. 2021. "Lateralization in Reverse Shoulder Arthroplasty" Journal of Clinical Medicine 10, no. 22: 5380. https://doi.org/10.3390/jcm10225380
APA StyleBauer, S., Corbaz, J., Athwal, G. S., Walch, G., & Blakeney, W. G. (2021). Lateralization in Reverse Shoulder Arthroplasty. Journal of Clinical Medicine, 10(22), 5380. https://doi.org/10.3390/jcm10225380