Prognostic Value of LC3B and p62 Expression in Small Intestinal Adenocarcinoma
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. IHC
2.3. Evaluation of IHC
2.4. Microsatellite Instability (MSI) Analysis
2.5. Statistical Analysis
3. Results
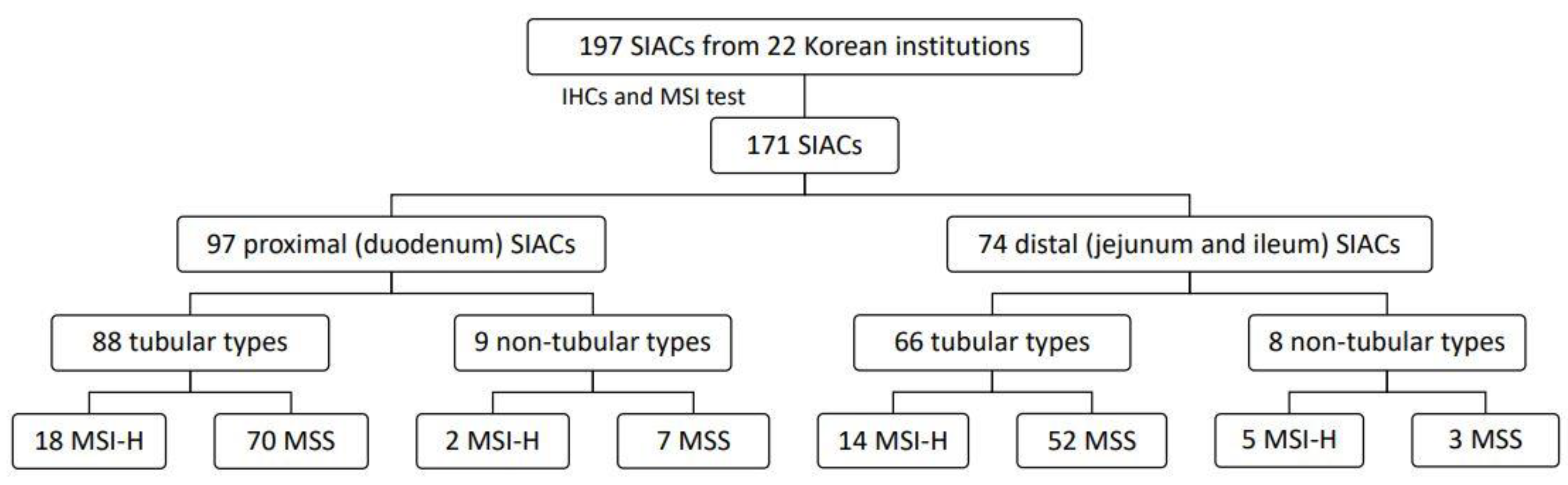
3.1. Study Population and Tumor Characteristics
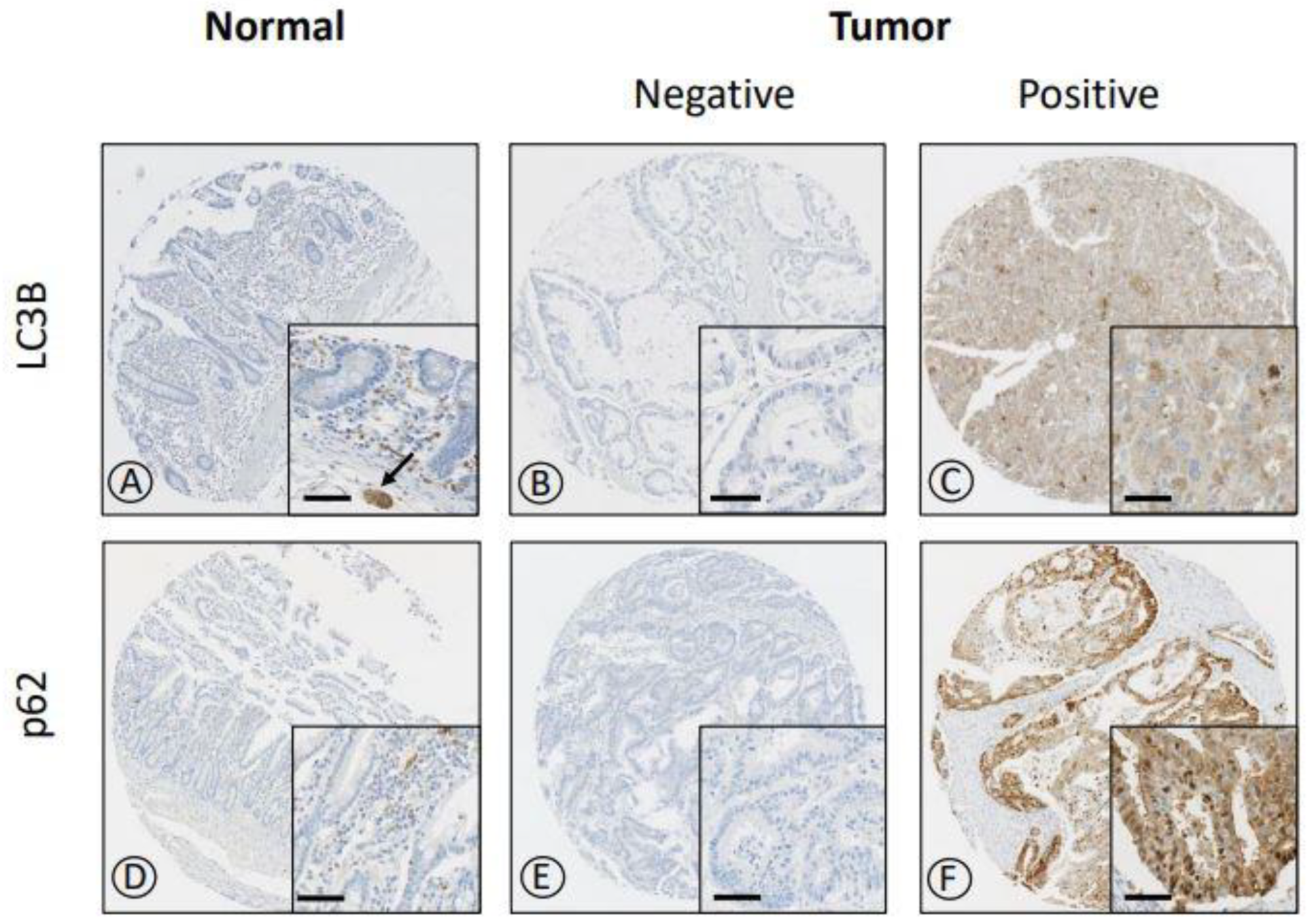
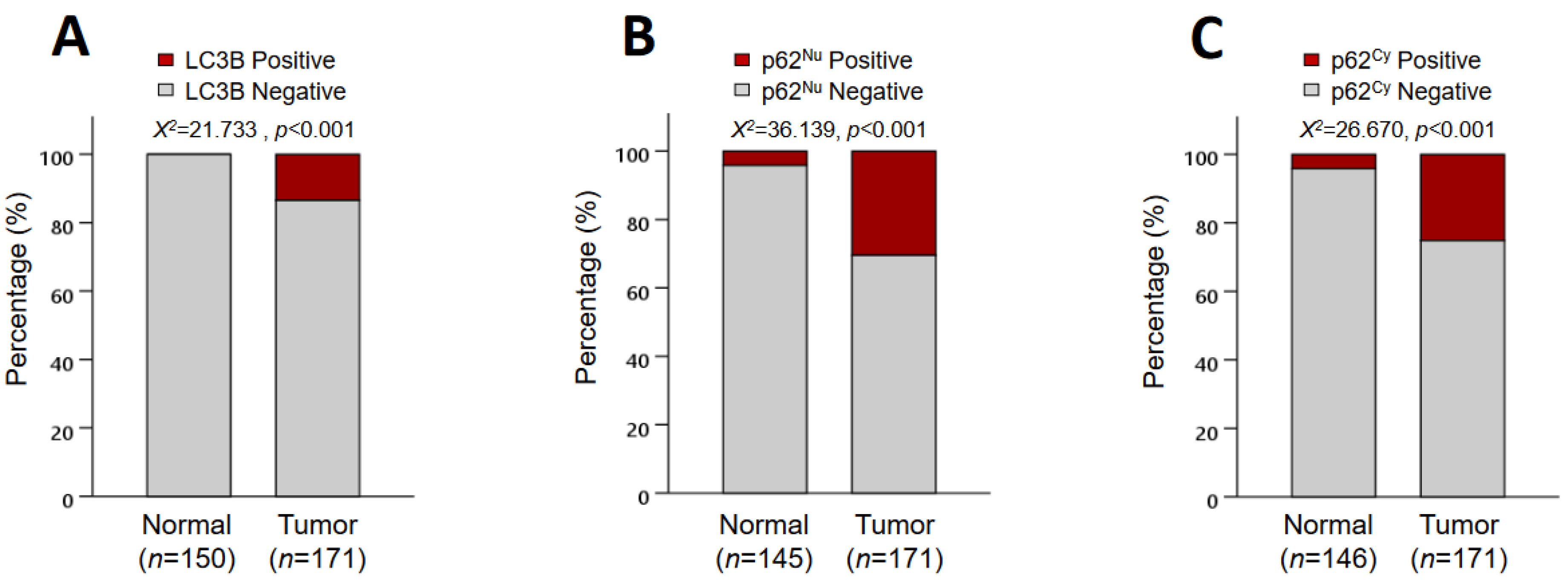
3.2. IHC Expression of LC3B and p62
3.3. Association between Clinicopathologic Features and p62 and LC3B Expression
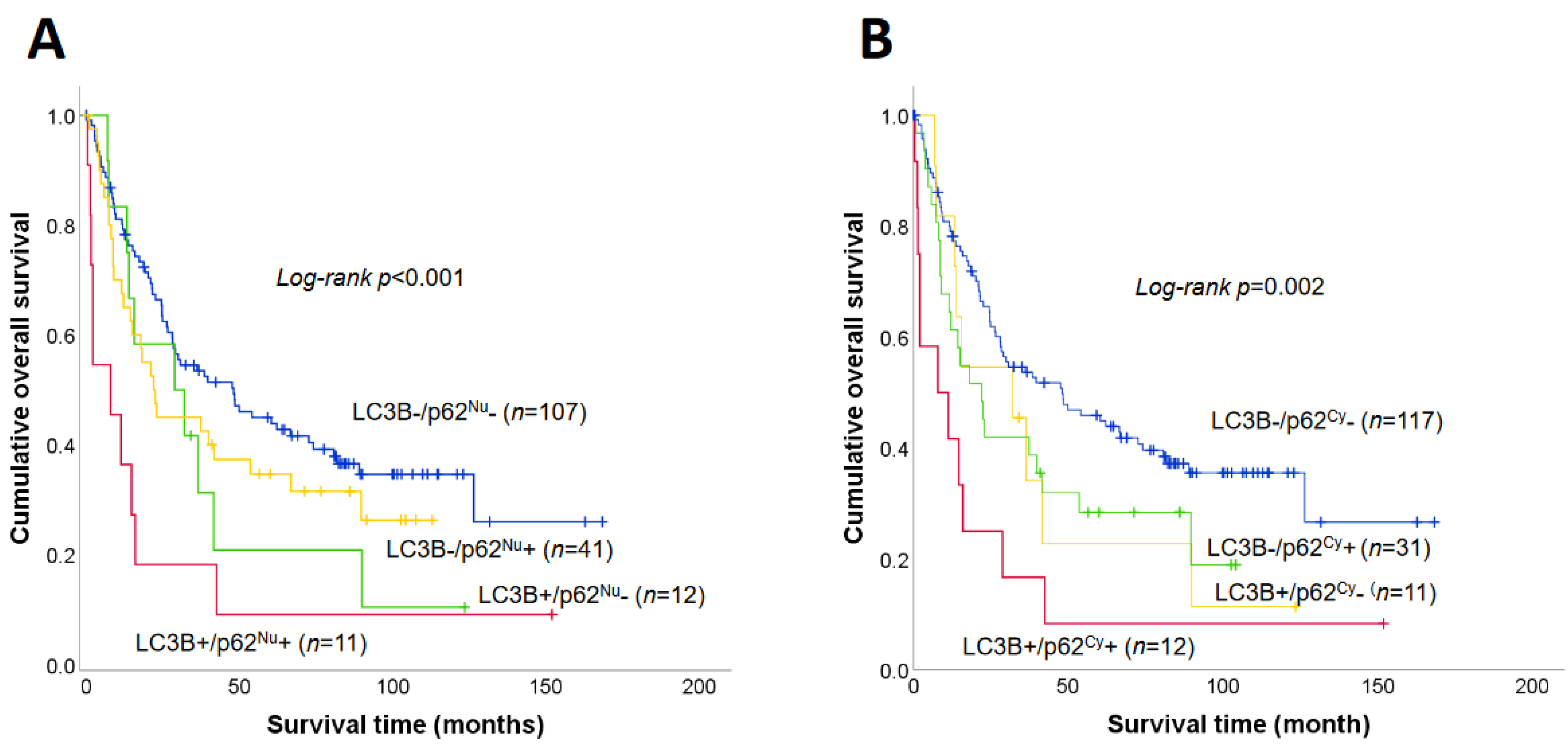
3.4. Prognostic Significance of LC3B and p62 Expression
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korea Central Cancer Registry. National Cancer Center, Korea Central Cancer Registry, Information. Annual Report of Cancer Statistics in Korea in 2018. Available online: http://ncc.re.kr/cancerStatsList.ncc (accessed on 30 April 2021).
- Korea Central Cancer Registry. National Cancer Center, Korea Central Cancer Registry, Information. Annual Report of National Cancer Registration 2003. Available online: http://ncc.re.kr/cancerStatsList.ncc (accessed on 7 January 2009).
- Bilimoria, K.Y.; Bentrem, D.J.; Wayne, J.D.; Ko, C.Y.; Bennett, C.L.; Talamonti, M.S. Small bowel cancer in the United States: Changes in epidemiology, treatment, and survival over the last 20 years. Ann. Surg. 2009, 249, 63–71. [Google Scholar] [CrossRef]
- Delaunoit, T.; Neczyporenko, F.; Limburg, P.J.; Erlichman, C. Pathogenesis and risk factors of small bowel adenocarcinoma: A colorectal cancer sibling? Off. J. Am. Coll. Gastroenterol. ACG 2005, 100, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Vanoli, A.; Balci, S.; Reid, M.M.; Saka, B.; Bagci, P.; Memis, B.; Choi, H.; Ohike, N.; Tajiri, T.; et al. Non-ampullary-duodenal carcinomas: Clinicopathologic analysis of 47 cases and comparison with ampullary and pancreatic adenocarcinomas. Mod. Pathol. 2017, 30, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.D.; Balci, S.; Ohike, N.; Xue, Y.; Kim, G.E.; Tajiri, T.; Memis, B.; Coban, I.; Dolgun, A.; Krasinskas, A.M.; et al. Ampullary carcinoma is often of mixed or hybrid histologic type: An analysis of reproducibility and clinical relevance of classification as pancreatobiliary versus intestinal in 232 cases. Mod. Pathol. 2016, 29, 1575–1585. [Google Scholar] [CrossRef] [Green Version]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.A.; Cooper, H.S.; Deming, D.A.; Garrido-Laguna, I. Small bowel adenocarcinoma, version 1.2020, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 1109–1133. [Google Scholar] [CrossRef] [PubMed]
- Akce, M.; Jiang, R.; Zakka, K.; Wu, C.; Alese, O.B.; Shaib, W.L.; Behera, M.; El-Rayes, B.F. Clinical Outcomes of Small Bowel Adenocarcinoma. Clin. Colorectal Cancer 2019, 18, 257–268. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galluzzi, L.; Pietrocola, F.; Bravo-San Pedro, J.M.; Amaravadi, R.K.; Baehrecke, E.H.; Cecconi, F.; Codogno, P.; Debnath, J.; Gewirtz, D.A.; Karantza, V. Autophagy in malignant transformation and cancer progression. EMBO J. 2015, 34, 856–880. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.M.; Mohamad Hanif, E.A.; Chin, S.-F. Is targeting autophagy mechanism in cancer a good approach? The possible double-edge sword effect. Cell Biosci. 2021, 11, 56. [Google Scholar] [CrossRef]
- Levy, J.M.M.; Thompson, J.C.; Griesinger, A.M.; Amani, V.; Donson, A.M.; Birks, D.K.; Morgan, M.J.; Mirsky, D.M.; Handler, M.H.; Foreman, N.K. Autophagy inhibition improves chemosensitivity in BRAFV600E brain tumors. Cancer Discov. 2014, 4, 773–780. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Zhang, J.; Li, K.; Deng, L.; Wang, H. Combination of an Autophagy Inducer and an Autophagy Inhibitor: A Smarter Strategy Emerging in Cancer Therapy. Front. Pharmacol. 2020, 11, 408. [Google Scholar] [CrossRef] [Green Version]
- Cheong, H.; Klionsky, D.J. Biochemical methods to monitor autophagy-related processes in yeast. Methods Enzymol. 2008, 451, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Kabeya, Y.; Mizushima, N.; Ueno, T.; Yamamoto, A.; Kirisako, T.; Noda, T.; Kominami, E.; Ohsumi, Y.; Yoshimori, T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000, 19, 5720–5728. [Google Scholar] [CrossRef] [PubMed]
- Schläfli, A.; Berezowska, S.; Adams, O.; Langer, R.; Tschan, M. Reliable LC3 and p62 autophagy marker detection in formalin fixed paraffin embedded human tissue by immunohistochemistry. Eur. J. Histochem. EJH 2015, 59, 2481. [Google Scholar] [CrossRef] [Green Version]
- Pankiv, S.; Clausen, T.H.; Lamark, T.; Brech, A.; Bruun, J.-A.; Outzen, H.; Øvervatn, A.; Bjørkøy, G.; Johansen, T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 2007, 282, 24131–24145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Arozena, A.A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adeli, K. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 2016, 12, 1–222. [Google Scholar] [CrossRef] [Green Version]
- Jiang, P.; Mizushima, N. LC3-and p62-based biochemical methods for the analysis of autophagy progression in mammalian cells. Methods 2015, 75, 13–18. [Google Scholar] [CrossRef]
- Ko, Y.H.; Cho, Y.S.; Won, H.S.; Jeon, E.K.; An, H.J.; Hong, S.U.; Park, J.H.; Lee, M.A. Prognostic significance of autophagy-related protein expression in resected pancreatic ductal adenocarcinoma. Pancreas 2013, 42, 829–835. [Google Scholar] [CrossRef]
- Wu, D.H.; Jia, C.C.; Chen, J.; Lin, Z.X.; Ruan, D.Y.; Li, X.; Lin, Q.; Min, D.; Ma, X.K.; Wan, X.B.; et al. Autophagic LC3B overexpression correlates with malignant progression and predicts a poor prognosis in hepatocellular carcinoma. Tumor Biol. 2014, 35, 12225–12233. [Google Scholar] [CrossRef]
- Sakanashi, F.; Shintani, M.; Tsuneyoshi, M.; Ohsaki, H.; Kamoshida, S. Apoptosis, necroptosis and autophagy in colorectal cancer: Associations with tumor aggressiveness and p53 status. Pathol. Res. Pract. 2019, 215, 152425. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Z.; Zhuang, J.; Wu, X.; Wang, Z.; Zhang, B.; Gao, G.; Zhang, Y.; Guo, C.; Xia, Q. Prognostic Value of Autophagy, Microsatellite Instability, and KRAS Mutations in Colorectal Cancer. J. Cancer 2021, 12, 3515. [Google Scholar] [CrossRef]
- Iwadate, R.; Inoue, J.; Tsuda, H.; Takano, M.; Furuya, K.; Hirasawa, A.; Aoki, D.; Inazawa, J. High Expression of p62 Protein Is Associated with Poor Prognosis and Aggressive Phenotypes in Endometrial Cancer. Am. J. Pathol. 2015, 185, 2523–2533. [Google Scholar] [CrossRef]
- Thompson, H.G.; Harris, J.W.; Wold, B.J.; Lin, F.; Brody, J.P. p62 overexpression in breast tumors and regulation by prostate-derived Ets factor in breast cancer cells. Oncogene 2003, 22, 2322–2333. [Google Scholar] [CrossRef] [Green Version]
- Lei, C.; Zhao, B.; Liu, L.; Zeng, X.; Yu, Z.; Wang, X. Expression and clinical significance of p62 protein in colon cancer. Medicine 2020, 99, e18791. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-K.; Yu, E.; Kim, J.; Bae, Y.K.; Jang, K.-T.; Jung, E.S.; Yoon, G.S.; Kim, J.M.; Oh, Y.-H.; Bae, H.-I. Adenocarcinoma of the small intestine: A multi-institutional study of 197 surgically resected cases. Hum. Pathol. 2010, 41, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.Y.; Lee, E.J.; Kim, M.J.; Chun, S.M.; Bae, Y.K.; Hong, S.U.; Choi, J.; Kim, J.M.; Jang, K.T.; Kim, J.Y.; et al. Lynch syndrome-related small intestinal adenocarcinomas. Oncotarget 2017, 8, 21483–21500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coit, D.G.; Kelsen, D.; Tang, L.H.; Erasmus, J.J.; Gerdes, H.; Hostetter, W.L. Small Intestine. In AJCC Cancer Staging Manual, 8th ed.; Amin, M.B., Edge, S.B., Greene, F.L., Eds.; American Joint Committee on Cancer: Chicago, IL, USA, 2017; pp. 221–234. [Google Scholar]
- Nagtegaal, I.D.; Arends, M.J.; Salto-Tellez, M. Colorectal Adenocarcinoma. In WHO Classification of Tumors Editorial Board. Digestive System Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2019; pp. 177–187. [Google Scholar]
- Lee, H.J.; Lee, O.-J.; Jang, K.-T.; Bae, Y.K.; Chung, J.-Y.; Eom, D.W.; Kim, J.M.; Yu, E.; Hong, S.-M. Combined Loss of E-cadherin and Aberrant β-Catenin Protein Expression Correlates With a Poor Prognosis for Small Intestinal Adenocarcinomas. Am. J. Clin. Pathol. 2013, 139, 167–176. [Google Scholar] [CrossRef]
- Kim, J.W.; Chung, J.-Y.; Ylaya, K.; Park, Y.; Jun, S.-Y.; Hong, S.-M.; Hewitt, S.M. Prognostic implication of SOX2 expression in small intestinal adenocarcinoma. Virchows Arch. 2021, 478, 1049–1060. [Google Scholar] [CrossRef]
- Masuda, G.; Yashiro, M.; Kitayama, K.; Miki, Y.; Kasashima, H.; Kinoshita, H.; Morisaki, T.; Fukuoka, T.; Hasegawa, T.; Sakurai, K. Clinicopathological correlations of autophagy-related proteins LC3, Beclin 1 and p62 in gastric cancer. Anticancer. Res. 2016, 36, 129–136. [Google Scholar]
- Niklaus, M.; Adams, O.; Berezowska, S.; Zlobec, I.; Graber, F.; Slotta-Huspenina, J.; Nitsche, U.; Rosenberg, R.; Tschan, M.P.; Langer, R. Expression analysis of LC3B and p62 indicates intact activated autophagy is associated with an unfavorable prognosis in colon cancer. Oncotarget 2017, 8, 54604–54615. [Google Scholar] [CrossRef] [PubMed]
- Myung Park, J.; Huang, S.; Wu, T.-T.; Foster, N.R.; Sinicrope, F.A. Prognostic impact of Beclin 1, p62/sequestosome 1 and LC3 protein expression in colon carcinomas from patients receiving 5-fluorouracil as adjuvant chemotherapy. Cancer Biol. Ther. 2013, 14, 100–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Chen, F.; Lung, J.; Lo, C.; Lee, F.; Lu, Y.; Hung, C. Prognostic significance of p62/SQSTM1 subcellular localization and LC3B in oral squamous cell carcinoma. Br. J. Cancer 2014, 111, 944–954. [Google Scholar] [CrossRef] [Green Version]
- Adams, O.; Dislich, B.; Berezowska, S.; Schläfli, A.M.; Seiler, C.A.; Kroell, D.; Tschan, M.P.; Langer, R. Prognostic relevance of autophagy markers LC3B and p62 in esophageal adenocarcinomas. Oncotarget 2016, 7, 39241. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.S.; Bae, G.E.; Kim, K.-H.; Lee, S.-I.; Chung, C.; Lee, D.; Lee, T.H.; Kwon, I.S.; Yeo, M.-K. Prognostic significance of LC3B and p62/SQSTM1 expression in gastric adenocarcinoma. Anticancer Res. 2019, 39, 6711–6722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitz, K.J.; Ademi, C.; Bertram, S.; Schmid, K.W.; Baba, H.A. Prognostic relevance of autophagy-related markers LC3, p62/sequestosome 1, Beclin-1 and ULK1 in colorectal cancer patients with respect to KRAS mutational status. World J. Surg. Oncol. 2016, 14, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pankiv, S.; Lamark, T.; Bruun, J.-A.; Øvervatn, A.; Bjørkøy, G.; Johansen, T. Nucleocytoplasmic shuttling of p62/SQSTM1 and its role in recruitment of nuclear polyubiquitinated proteins to promyelocytic leukemia bodies. J. Biol. Chem. 2010, 285, 5941–5953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, J.-Y.; Brezden-Masley, C.; Streutker, C.J. Autophagic Heterogeneity in Gastric Adenocarcinoma. Front. Oncol. 2021, 11, 903. [Google Scholar] [CrossRef]
- Yoshioka, A.; Miyata, H.; Doki, Y.; Yamasaki, M.; Sohma, I.; Gotoh, K.; Takiguchi, S.; Fujiwara, Y.; Uchiyama, Y.; Monden, M. LC3, an autophagosome marker, is highly expressed in gastrointestinal cancers. Int. J. Oncol. 2008, 33, 461–468. [Google Scholar] [CrossRef] [Green Version]
- Sena, P.; Mariani, F.; Mancini, S.; Benincasa, M.; Magnani, G.; Pedroni, M.; Palumbo, C.; Roncucci, L. Autophagy is upregulated during colorectal carcinogenesis, and in DNA microsatellite stable carcinomas. Oncol. Rep. 2015, 34, 3222–3230. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Lapaquette, P.; Bringer, M.-A.; Darfeuille-Michaud, A. Autophagy and Crohn’s disease. J. Innate Immun. 2013, 5, 434–443. [Google Scholar] [CrossRef]

| Category | N | % |
|---|---|---|
| Age | ||
| <60 years | 95 | 55.6 |
| ≥60 years | 76 | 44.4 |
| Sex | ||
| Male | 106 | 62.0 |
| Female | 65 | 38.0 |
| Location | ||
| Proximal (duodenum) | 97 | 56.7 |
| Distal (jejunum and ileum) | 74 | 43.3 |
| Type of growth a | ||
| Polypoid | 31 | 19.0 |
| Flat | 11 | 6.8 |
| Ulceroinfiltrative | 121 | 74.2 |
| Histological subtype | ||
| Adenocarcinoma | 154 | 90.1 |
| Mucinous carcinoma | 9 | 5.3 |
| Signet ring cell carcinoma | 4 | 2.3 |
| Undifferentiated carcinoma | 4 | 2.3 |
| Tumor grade | ||
| Low | 131 | 76.6 |
| High | 40 | 23.4 |
| Lymphovascular invasion | ||
| Absent | 78 | 45.6 |
| Present | 93 | 54.4 |
| Predisposing condition | ||
| Absent | 147 | 86.0 |
| Present | 24 | 14.0 |
| MSI status | ||
| MSS | 132 | 77.2 |
| MSI-H | 39 | 22.8 |
| pT classification | ||
| pTis − pT1 | 9 | 5.3 |
| pT2 | 8 | 4.7 |
| pT3 | 58 | 33.9 |
| pT4 | 96 | 56.1 |
| pN classification b | ||
| pN0 | 77 | 46.4 |
| pN1 + pN2 | 89 | 53.6 |
| AJCC staging c | ||
| 0–I | 14 | 8.4 |
| II | 63 | 38.0 |
| III | 89 | 53.6 |
| Status | ||
| Alive | 58 | 33.9 |
| Expire | 113 | 66.1 |
| LC3B, N (%) | p62Nu, N (%) | |||||
|---|---|---|---|---|---|---|
| Negative | Positive | p-Value | Negative | Positive | p-Value | |
| p62Cy | 0.003 * | <0.001 * | ||||
| Negative | 117 (79.1) | 11 (47.8) | 118 (99.2) | 10 (19.2) | ||
| Positive | 31 (20.9) | 12 (52.2) | 1 (0.8) | 42 (80.8) | ||
| p62Nu | 0.088 | |||||
| Negative | 107 (72.3) | 12 (52.2) | ||||
| Positive | 41 (27.7) | 11 (47.8) | ||||
| LC3B, N (%) | p62Nu, N (%) | p62Cy, N (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Negative N = 148 | Positive N = 23 | p-Value | Negative N = 119 | Positive N = 52 | p-Value | Negative N = 128 | Positive N = 43 | p-Value | |
| Gender | 0.300 | 0.438 | 0.503 | ||||||
| Male | 89 (60.1) | 17 (73.9) | 71 (59.7) | 35 (67.3) | 77 (60.2) | 29 (67.4) | |||
| Female | 59 (39.9) | 6 (26.1) | 48 (40.3) | 17 (32.7) | 51 (39.8) | 14 (32.6) | |||
| Age | 0.139 | 0.071 | 0.056 | ||||||
| <60 years | 86 (58.1) | 9 (39.1) | 72 (60.5) | 23 (44.2) | 77 (60.2) | 18 (41.9) | |||
| ≥60 years | 62 (41.9) | 14 (60.9) | 47 (39.5) | 29 (55.8) | 51 (39.8) | 25 (58.1) | |||
| Tumor location | 0.805 | 1.000 | 0.693 | ||||||
| Proximal (duodenum) | 85 (57.4) | 12 (52.2) | 68 (57.1) | 29 (55.8) | 71 (55.5) | 26 (60.5) | |||
| Distal (jejunum and ileum) | 63 (42.6) | 11 (47.8) | 51 (42.9) | 23 (44.2) | 57 (44.5) | 17 (39.5) | |||
| Histological subtype | <0.001 * | 0.068 | 0.056 | ||||||
| Adenocarcinoma | 136 (91.9) | 18 (78.3) | 106 (89.1) | 48 (92.3) | 115 (89.8) | 39 (90.7) | |||
| Mucinous carcinoma | 8 (5.4) | 1 (4.3) | 8 (6.7) | 1 (1.9) | 8 (6.3) | 1 (2.3) | |||
| Signet ring cell carcinoma | 4 (2.7) | 0 (0.0) | 4 (3.4) | 0 (0.0) | 4 (3.1) | 0 | |||
| Undifferentiated carcinoma | 0 (0.0) | 4 (17.4) | 1 (0.8) | 3 (5.8) | 1 (0.8) | 3 (7.0) | |||
| Tumor grade | 0.029 * | 0.600 | 0.854 | ||||||
| Low | 118 (79.7) | 13 (56.5) | 93 (78.2) | 38 (73.1) | 99 (77.3) | 32 (74.4) | |||
| High | 30 (20.3) | 10 (43.5) | 26 (21.8) | 14 (26.9) | 29 (22.7) | 11 (25.6) | |||
| Lymphovascular invasion | 0.178 | 0.684 | 0.454 | ||||||
| Absent | 71 (48.0) | 7 (30.4) | 56 (47.1) | 22 (42.3) | 61 (47.7) | 17 (39.5) | |||
| Present | 77 (52.0) | 16 (69.6) | 63 (52.9) | 30 (57.7) | 67 (52.3) | 26 (60.5) | |||
| Predisposing condition | 1.000 | 0.044 * | 0.023 * | ||||||
| Absent | 127 (85.8) | 20 (87.0) | 107 (89.9) | 40 (76.9) | 115 (89.8) | 32 (74.4) | |||
| Present | 21 (14.2) | 3 (13.0) | 12 (10.1) | 12 (23.1) | 13 (10.1) | 11 (25.6) | |||
| MSI status | 0.690 | 0.590 | 0.583 | ||||||
| MSS | 113 (76.4) | 19 (82.6) | 90 (75.6) | 42 (80.8) | 97 (75.8) | 35 (81.4) | |||
| MSI-H | 35 (23.6) | 4 (17.4) | 29 (24.4) | 10 (19.2) | 31 (24.2) | 8 (18.6) | |||
| pT classification | 0.372 | 0.585 | 0.351 | ||||||
| pTis − pT1 | 8 (5.4) | 1 (4.4) | 6 (5.0) | 3 (5.8) | 7 (5.5) | 2 (4.7) | |||
| pT2 | 8 (5.4) | 0 (0.0) | 6 (5.0) | 2 (3.8) | 6 (4.7) | 2 (4.7) | |||
| pT3 | 47 (31.8) | 11 (47.8) | 44 (37.0) | 14 (26.9) | 48 (37.5) | 10 (23.3) | |||
| pT4 | 85 (57.4) | 11 (47.8) | 63 (53.0) | 33 (63.5) | 67 (52.3) | 29 (67.3) | |||
| pN classification a | 0.129 | 0.917 | 0.852 | ||||||
| pN0 | 71 (49.0) | 6 (28.6) | 53 (45.7) | 24 (48.0) | 59 (47.2) | 18 (43.9) | |||
| pN1 + pN2 | 74 (51.0) | 15 (71.4) | 63 (54.3) | 26 (52.0) | 66 (52.8) | 23 (56.1) | |||
| AJCC staging b | 0.215 | 0.886 | 0.826 | ||||||
| 0–I | 13 (9.0) | 1 (4.8) | 9 (7.8) | 5 (10.0) | 10 (8.0) | 4 (9.8) | |||
| II | 58 (40.0) | 5 (23.8) | 44 (37.9) | 19 (38.0) | 49 (39.2) | 14 (34.1) | |||
| III | 74 (51.0) | 15 (71.4) | 63 (54.3) | 26 (52.0) | 66 (52.8) | 23 (56.1) | |||
| Case N | LC3B+/p62Nu+, N (%) | p-Value | LC3B+/p62Cy+, N (%) | p-Value | |
|---|---|---|---|---|---|
| Gender | 0.662 | 0.970 | |||
| Male | 95 | 8 (72.7) | 8 (66.7) | ||
| Female | 76 | 3 (27.3) | 4 (33.3) | ||
| Age | 0.017 * | 0.056 | |||
| <60 years | 106 | 2 (18.2) | 3 (25.0) | ||
| ≥60 years | 65 | 9 (81.8) | 9 (75.0) | ||
| Tumor location | 0.642 | 0.430 | |||
| Proximal (duodenum) | 97 | 5 (45.5) | 5 (41.7) | ||
| Distal (jejunum and ileum) | 74 | 6 (54.5) | 7 (58.3) | ||
| Histological subtype | <0.001 * | <0.001 * | |||
| Adenocarcinoma | 154 | 8 (72.7) | 9 (75.0) | ||
| Mucinous carcinoma | 9 | 0 (0.0) | 0 (0.0) | ||
| Signet ring cell carcinoma | 4 | 0 (0.0) | 0 (0.0) | ||
| Undifferentiated carcinoma | 4 | 3 (27.3) | 3 (25.0) | ||
| Tumor grade | 0.031 * | 0.057 | |||
| Low | 131 | 5 (45.5) | 6 (50.0) | ||
| High | 40 | 6 (54.5) | 6 (50.0) | ||
| Lymphovascular invasion | 0.342 | 0.236 | |||
| Absent | 78 | 3 (27.3) | 3 (25.0) | ||
| Present | 93 | 8 (72.7) | 9 (75.0) | ||
| Predisposing condition | 1.000 | 1.000 | |||
| Absent | 147 | 9 (81.8) | 10 (83.3) | ||
| Present | 24 | 2 (18.2) | 2 (16.7) | ||
| MSI status | 1.000 | 1.000 | |||
| MSS | 132 | 8 (72.7) | 9 (75.0) | ||
| MSI-H | 39 | 3 (27.3) | 3 (25.0) | ||
| pT classification | 0.492 | 0.493 | |||
| pTis − pT1 | 9 | 0 (0.0) | 0 (0.0) | ||
| pT2 | 8 | 0 (0.0) | 0 (0.0) | ||
| pT3 | 58 | 2 (18.2) | 3 (25.0) | ||
| pT4 | 96 | 9 (81.8) | 9 (75.0) | ||
| pN classification a | 0.250 | 0.162 | |||
| pN0 | 77 | 2 (22.2) | 2 (20.0) | ||
| pN1 + pN2 | 89 | 7 (77.8) | 8 (80.0) | ||
| AJCC staging b | 0.292 | ||||
| 0–I | 14 | 0 (0.0) | 0 (0.0) | 0.204 | |
| II | 63 | 2 (22.2) | 2 (20.0) | ||
| III | 89 | 7 (77.8) | 8 (80.0) |
| Variable | Median Survival (95% CI) | Hazard Ratio (95% CI) | p-Value |
|---|---|---|---|
| Age | |||
| <60 years | 39.7 (20.5–58.9) | 1- | 0.116 |
| ≥60 years | 24.9 (10.5–39.3) | 1.35 (0.93–1.95) | |
| Sex | |||
| Male | 36.5 (24.0–49.0) | 1- | 0.793 |
| Female | 28.8 (13.2–44.4) | 1.05 (0.72–1.54) | |
| Location | |||
| Proximal (duodenum) | 39.9 (22.7–57.1) | 1- | 0.153 |
| Distal (jejunum and ileum) | 24.5 (15.9–33.2) | 1.31 (0.90–1.91) | |
| Histological subtype | |||
| Adenocarcinoma | 36.5 (20.4–52.6) | 1- | 0.036 * |
| Mucinous carcinoma | 21.0 (5.4–36.6) | 1.71 (0.79–3.70) | 0.171 |
| Signet ring cell carcinoma | 4.2 (NA) | 0.95 (0.23–3.85) | 0.941 |
| Undifferentiated carcinoma | 2.1 (NA) | 3.89 (1.42–10.66) | 0.008* |
| Tumor grade | |||
| Low | 37.4 (20.0–54.8) | 1- | 0.172 |
| High | 24.5 (16.1–32.9) | 1.34 (0.88–2.05) | |
| Lymphovascular invasion | |||
| Absent | 80.8 (40.7–120.9) | 1- | <0.001 * |
| Present | 21.1 (14.9–42.7) | 2.32 (1.57–3.44) | |
| Predisposing condition | |||
| Absent | 36.5 (21.84–51.16) | 1 | 0.269 |
| Present | 18.2 (7.68–28.72) | 1.34 (0.80–2.24) | |
| MSI status | |||
| MSS | 28.2 (18.9–37.5) | 1 | 0.029 * |
| MSI-H | 81.7 (6.2–157.2) | 0.58 (0.36–0.94) | |
| pT classification | |||
| pTis − pT1 | NA | 1- | 0.025 * |
| pT2 | 62 (NA) | 6.32 (0.71–56.55) | 0.099 |
| pT3 | 36.5 (22.3–50.7) | 7.96 (1.10–58.16) | 0.041 * |
| pT4 | 22.0 (15.4–28.6) | 11.52 (1.60–83.00) | 0.015 * |
| pN classification a | |||
| pN0 | 62.2 (28.4–96.0) | 1- | <0.001 * |
| pN1 + pN2 | 22.6 (17.0–28.2) | 2.01 (1.36–2.98) | |
| AJCC staging b | |||
| 0-I | NA | 1- | 0.001 * |
| II | 48.4 (17.9–78.9) | 2.76 (0.98–7.76) | 0.054 |
| III | 22.6 (17.0–28.2) | 4.74 (1.72–13.02) | 0.003 * |
| LC3B expression | |||
| Negative | 38.5 (23.3–53.7) | 1- | 0.006 * |
| Positive | 14.7 (11.1–18.3) | 1.97 (1.21–3.21) | |
| p62Nu expression | |||
| Negative | 39.7 (23.8–55.6) | 1- | 0.041 * |
| Positive | 17.8 (10.2–25.4) | 1.50 (1.02–2.22) | |
| p62Cy expression | 0.006 * | ||
| Negative | 41.6 (24.8–58.4) | 1 | |
| Positive | 15.1 (7.3–22.9) | 1.77 (1.18–2.65) | |
| LC3B+/p62Nu+ | 0.001 * | ||
| Absent | 48.1 (25.8–70.4) | 1 | |
| Present | 7.9 (0.0–15.8) | 3.32 (1.69–6.53) | |
| LC3B+/p62Cy+ | 0.002 * | ||
| Absent | 37.4 (22.9–51.9) | 1 | |
| Present | 7.9 (0.0–23.5) | 2.74 (1.46–5.13) |
| Variables | Hazard Ratio (95% CI) | Se (Coef) | z | p-Value |
|---|---|---|---|---|
| MSI-H | 0.471 (0.283–0.783) | 0.260 | −2.899 | 0.004 * |
| pT classification (≥pT2) | 1.534 (1.136–2.071) | 0.153 | 2.792 | 0.005 * |
| Nodal metastasis | 1.672 (1.112–2.514) | 0.208 | 2.472 | 0.013 * |
| LC3B+ expression | 1.817 (1.077–3.064) | 0.267 | 2.239 | 0.025 * |
| P62Nu+ expression | 1.334 (0.893–1.991) | 0.204 | 1.409 | 0.160 |
| P62Cy+ expression | 0.889 (0.576–1.370) | 0.221 | −0.535 | 0.592 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-W.; Jun, S.-Y.; Kim, J.-M.; Oh, Y.-H.; Yoon, G.; Hong, S.-M.; Chung, J.-Y. Prognostic Value of LC3B and p62 Expression in Small Intestinal Adenocarcinoma. J. Clin. Med. 2021, 10, 5398. https://doi.org/10.3390/jcm10225398
Kim J-W, Jun S-Y, Kim J-M, Oh Y-H, Yoon G, Hong S-M, Chung J-Y. Prognostic Value of LC3B and p62 Expression in Small Intestinal Adenocarcinoma. Journal of Clinical Medicine. 2021; 10(22):5398. https://doi.org/10.3390/jcm10225398
Chicago/Turabian StyleKim, Jeong-Won, Sun-Young Jun, Joon-Mee Kim, Young-Ha Oh, Ghilsuk Yoon, Seung-Mo Hong, and Joon-Yong Chung. 2021. "Prognostic Value of LC3B and p62 Expression in Small Intestinal Adenocarcinoma" Journal of Clinical Medicine 10, no. 22: 5398. https://doi.org/10.3390/jcm10225398
APA StyleKim, J. -W., Jun, S. -Y., Kim, J. -M., Oh, Y. -H., Yoon, G., Hong, S. -M., & Chung, J. -Y. (2021). Prognostic Value of LC3B and p62 Expression in Small Intestinal Adenocarcinoma. Journal of Clinical Medicine, 10(22), 5398. https://doi.org/10.3390/jcm10225398







