Pregnancy as a Risk Factor of Severe COVID-19
Abstract
:1. Introduction
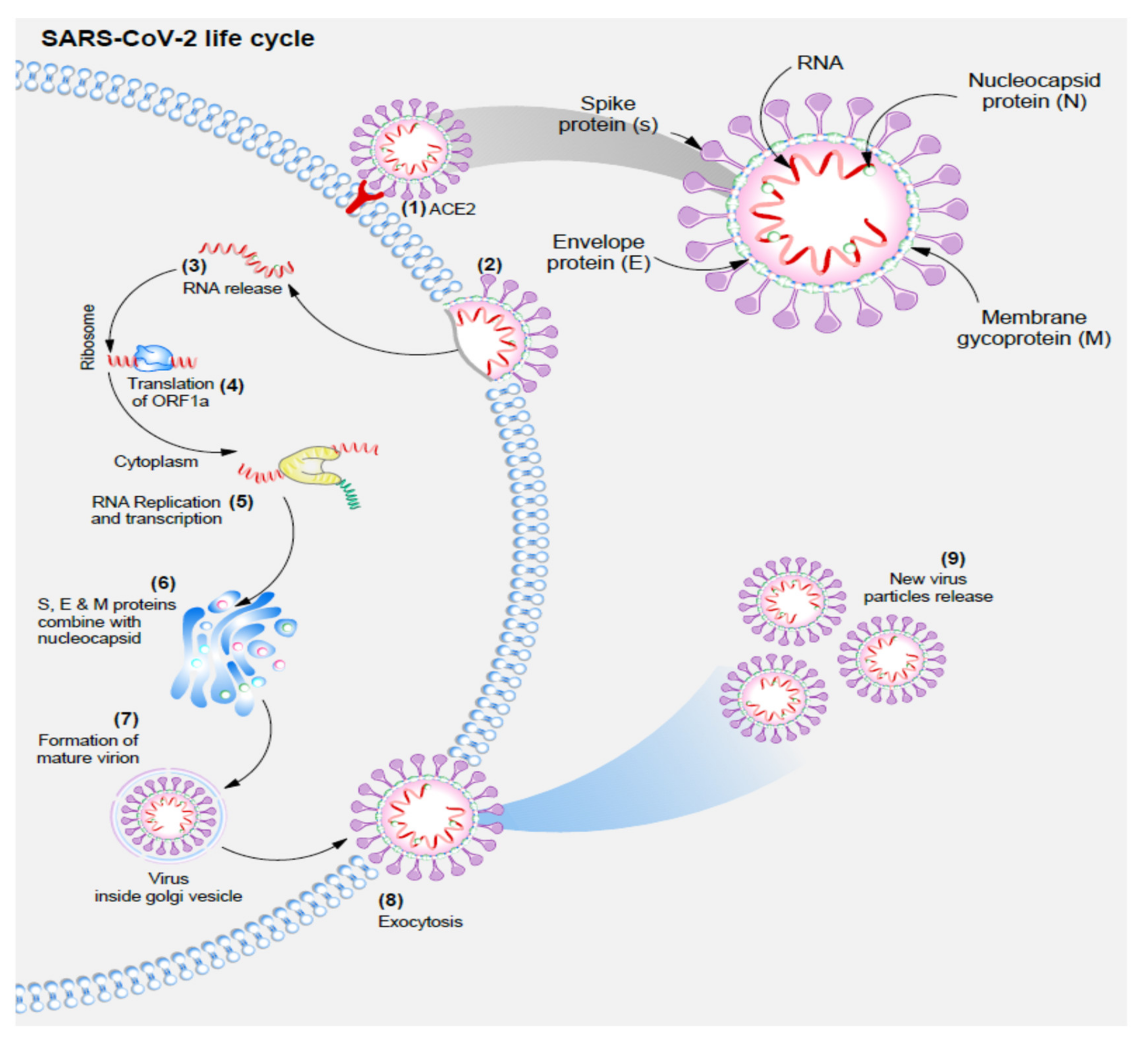
2. Coronaviridae and SARS-CoV-2 Lifecycles
- (1)
- SARS-CoV-2 enters the cell due to the strong tropism of S protein for ACE-2 receptor;
- (2)
- Fusion of SARS-CoV-2 and the hosts’ cell membrane;
- (3)
- Viral RNA is released into the cell;
- (4)
- Viral RNA is treated as a transcript allowing the translation of ORF1a, which causes frameshifting within 30% of ribosomes, and production of polyprotein pp1ab;
- (5)
- Genomic RNA is then used as a template for the synthesis of an antisense genome, and then a new full-length RNA genome of SARS-CoV-2;
- (6)
- Virion assembly in the ER-Golgi intermediate compartment (ERGIC) with the M protein attracting and attaching to other viral protein complexes M-S, M-N, and M-E;
- (7)
- Mature viral particles are transported by smooth-walled vesicles through the secretory pathway;
- (8)
- Exocytosis of new mature viral particles.
3. Physiological Changes Occurring in Pregnancy That May Contribute to COVID-19 Course
3.1. Cardiovascular System
3.2. Respiratory System
3.3. Immune System
4. Manifestation and Outcomes of COVID-19 in Pregnancy
4.1. Clinical Manifestations
4.2. Vaccination and Pregnancy
5. Conclusions and Further Study Directions
Funding
Conflicts of Interest
References
- COVID-19 Coronavirus Pandemic. Available online: https://www.worldometers.info/coronavirus/?utm_campaign=homeAdvegas1 (accessed on 10 September 2021).
- Machhi, J.; Herskovitz, J.; Senan, A.M.; Dutta, D.; Nath, B.; Oleynikov, M.D.; Blomberg, W.R.; Meigs, D.D.; Hasan, M.; Patel, M.; et al. The Natural History, Pathobiology, and Clinical Manifestations of SARS-CoV-2 Infections. J. Neuroimm. Pharmacol. 2020, 15, 359–386. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, A.K.; Noureldin, A.; Othman, M. COVID-19 susceptibility in pregnancy: Immune/inflammatory considerations, the role of placental ACE-2 and research considerations. Reprod. Biol. 2020, 20, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, S.O. A review on currently available potential therapeutic options for covid-19. Int. J. Gen. Med. 2020, 13, 443–467. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.A.; Smulian, J.C.; Lednicky, J.A.; Wen, T.S.; Jamieson, D.J. Coronavirus Disease 2019 (COVID-19) and pregnancy: What obstetricians need to know. Am. J. Obstet. Gynecol. 2020, 222, 415–426. [Google Scholar] [CrossRef] [PubMed]
- DeBolt, C.A.; Bianco, A.; Limaye, M.A.; Silverstein, J.; Penfield, C.A.; Roman, A.S.; Rosenberg, H.M.; Ferrara, L.; Lambert, C.; Khoury, R.; et al. Pregnant women with severe or critical COVID-19 have increased composite morbidity compared to non-pregnant matched controls. Am. J. Obstet. Gynecol. 2020, 24, P510.E1–P510.E12. [Google Scholar] [CrossRef]
- Sadeghi, A.; Moghadam, A.D.; Pirsalehi, A.; Shojaee, S.; Sanadgol, G.; Vahidi, M.; Mojarad, E.N. The characteristics of cancerous patients infected with COVID-19 in hospital setting. Acta Biomed. 2020, 91, e2020145. [Google Scholar] [CrossRef]
- Wong, S.F.; Chow, K.M.; Leung, T.N.; Ng, W.F.; Ng, T.K.; Shek, C.C.; Ng, P.C.; Lam, P.W.; Ho, L.C.; To, W.W.; et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am. J. Obstet. Gynecol. 2004, 191, P292–P297. [Google Scholar] [CrossRef] [Green Version]
- Dashraath, P.; Wong, J.L.J.; Lim, M.X.K.; Lim, L.M.; Li, S.; Biswas, A.; Choolani, M.; Mattar, C.; Su, L.L. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am. J. Obstet. Gynecol. 2020, 222, P521–P531. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [Green Version]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, P565–P574. [Google Scholar] [CrossRef] [Green Version]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. bioRxiv 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.; Li, F.; Shi, Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef] [PubMed]
- Schoeman, D.; Fielding, B.C. Coronavirus envelope protein: Current knowledge. Virol. J. 2019, 16, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khailany, R.A.; Safdar, M.; Ozaslan, M. Genomic characterization of a novel SARS-CoV-2. Gene Rep. 2020, 19, 100682. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448, Published online. [Google Scholar] [CrossRef] [Green Version]
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19. N. Engl. J. Med. 2020, 382, 1787–1799. [Google Scholar] [CrossRef]
- Shang, J.; Ye, G.; Shi, K.; Wan, Y.; Luo, C.; Aihara, H.; Geng, Q.; Auerbach, A.; Li, F. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020, 581, 221–224. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Zhang, C.; Sui, J.; Kuhn, J.H.; Moore, M.J.; Luo, S.; Wong, S.-K.; Huang, I.-C.; Xu, K.; Vasilieva, N.; et al. Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J. 2005, 24, 1634–1643. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Du, R.-H.; Li, B.; Zheng, X.-S.; Yang, X.-L.; Hu, B.; Wang, Y.-Y.; Xiao, G.-F.; Yan, B.; Shi, Z.-L.; et al. Molecular and serological investigation of 2019-nCoV infected patients: Implication of multiple shedding routes. Emerg. Microbes Infect. 2020, 9, 386–389. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, P271–P280.e8. [Google Scholar] [CrossRef] [PubMed]
- Rey, F.A.; Lok, S.M. Common Features of Enveloped Viruses and Implications for Immunogen Design for Next-Generation Vaccines. Cell 2018, 172, P1319–P1334. [Google Scholar] [CrossRef]
- Li, F. Structure, Function, and Evolution of Coronavirus Spike Proteins. Annu. Rev. Virol. 2016, 3, 237–261. [Google Scholar] [CrossRef] [Green Version]
- Masters, P.S. The Molecular Biology of Coronaviruses. Adv. Virus Res. 2006, 65, 193–292. [Google Scholar] [CrossRef]
- Gao, Y.; Yan, L.; Huang, Y.; Liu, F.; Zhao, Y.; Cao, L.; Wang, T.; Sun, Q.; Ming, Z.; Zhang, L.; et al. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science 2020, 368, 779–782. [Google Scholar] [CrossRef] [Green Version]
- Kono, M.; Tatsumi, K.; Imai, A.M.; Saito, K.; Kuriyama, T.; Shirasawa, H. Inhibition of human coronavirus 229E infection in human epithelial lung cells (L132) by chloroquine: Involvement of p38 MAPK and ERK. Antiviral Res. 2008, 77, 150–152. [Google Scholar] [CrossRef]
- Siu, Y.L.; Teoh, K.T.; Lo, J.; Chan, C.M.; Kien, F.; Escriou, N.; Tsao, S.W.; Nicholls, J.M.; Altmeyer, R.; Peiris, J.S.M.; et al. The M, E, and N Structural Proteins of the Severe Acute Respiratory Syndrome Coronavirus Are Required for Efficient Assembly, Trafficking, and Release of Virus-Like Particles. J. Virol. 2008, 82, 11318–11330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, K.P.; Liu, D.X. The missing link in coronavirus assembly. Retention of the avian coronavirus infectious bronchitis virus envelope protein in the pre-Golgi compartments and physical interaction between the envelope and membrane proteins. J. Biol. Chem. 2001, 276, 17515–17523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakauchi, M.; Kariwa, H.; Kon, Y.; Yoshii, K.; Maeda, A.; Takashima, I. Analysis of severe acute respiratory syndrome coronavirus structural proteins in virus-like particle assembly. Microbiol. Immunol. 2008, 52, 625–630. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Wu, Z.; Li, J.W.; Zhao, H.; Wang, G.Q. Cytokine release syndrome in severe COVID-19: Interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int. J. Antimicrob. Agents 2020, 55, 05954. [Google Scholar] [CrossRef]
- Moore, J.B.; June, C.H. Cytokine release syndrome in severe COVID-19. Science 2020, 368, 473–474. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Zhou, X.; Zhu, C.; Song, Y.; Feng, F.; Qiu, Y.; Feng, J.; Jia, Q.; Song, Q.; Zhu, B.; et al. Immune Phenotyping Based on the Neutrophil-to-Lymphocyte Ratio and IgG Level Predicts Disease Severity and Outcome for Patients With COVID-19. Front. Mol. Biosci. 2020, 7, 157. [Google Scholar] [CrossRef] [PubMed]
- Park, M.D. Macrophages: A Trojan horse in COVID-19? Nat. Rev. Immunol. 2020, 20, 351. [Google Scholar] [CrossRef] [PubMed]
- Yuki, K.; Fujiogi, M.; Koutsogiannaki, S. COVID-19 pathophysiology: A review. Clin. Immunol. 2020, 215, 108427. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501.e15. [Google Scholar] [CrossRef] [PubMed]
- Almeida, F.A.; Pavan, M.V.; Rodrigues, C.I.S. The haemodynamic, renal excretory and hormonal changes induced by resting in the left lateral position in normal pregnant women during late gestation. BJOG 2009, 116, 1749–1754. [Google Scholar] [CrossRef]
- Clark, S.L.; Cotton, D.B.; Lee, W.; Bishop, C.; Hill, T.; Southwick, J.; Pivarnik, J.; Spillman, T.; DeVore, G.R.; Phelan, J.; et al. Central hemodynamic assessment of normal term pregnancy. Am. J. Obstet. Gynecol. 1989, 161, 1439–1442. [Google Scholar] [CrossRef]
- Bernstein, I.M.; Ziegler, W.; Badger, G.J. Plasma volume expansion in early pregnancy. Obstet. Gynecol. 2001, 97, 669–672. [Google Scholar] [CrossRef]
- Capeless, E.L.; Clapp, J.F. Cardiovascular changes in early phase of pregnancy. Am. J. Obstet. Gynecol. 1989, 161, 1449–1453. [Google Scholar] [CrossRef]
- Capeless, E.L.; Clapp, J.F. When do cardiovascular parameters return to their preconception values? Am. J. Obstet. Gynecol. 1991, 165, 883–886. [Google Scholar] [CrossRef]
- Flo, K.; Wilsgaard, T.; Vårtun, A.; Acharya, G. A longitudinal study of the relationship between maternal cardiac output measured by impedance cardiography and uterine artery blood flow in the second half of pregnancy. BJOG 2010, 117, 837–844. [Google Scholar] [CrossRef]
- Campos, O.; Andrade, J.L.; Bocanegra, J.; Ambrose, J.A.; Carvalho, A.C.; Harada, K.; Martinez, E.E. Physiologic multivalvular regurgitation during pregnancy: A longitudinal Doppler echocardiographic study. Int. J. Cardiol. 1993, 40, 265–272. [Google Scholar] [CrossRef]
- Evans, P.J.; Rajappan, K.; Stocks, G.M. Cardiorespiratory symptoms during pregnancy--not always pulmonary embolism. Int. J. Obstet. Anesth. 2006, 15, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Irani, R.A.; Xia, Y. Renin Angiotensin Signaling in Normal Pregnancy and Preeclampsia. Semin. Nephrol. 2011, 31, 47–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brosnihan, K.B.; Neves, L.A.A.; Anton, L.; Joyner, J.; Valdes, G.; Merrill, D.C. Enhanced expression of Ang-(1-7) during pregnancy. Braz. J. Med. Biol. Res. 2004, 37, 1255–1262. [Google Scholar] [CrossRef] [Green Version]
- Hsueh, W.A.; Luetscher, J.A.; Carlson, E.J.; Grislis, G.; Fraze, E.; McHargue, A. Changes in active and inactive renin throughout pregnancy. J. Clin. Endocrinol. Metab. 1982, 54, 1010–1016. [Google Scholar] [CrossRef]
- Brown, M.A.; Gallery, E.D.M.; Ross, M.R.; Esber, R.P. Sodium excretion in normal and hypertensive pregnancy: A prospective study. Am. J. Obstet. Gynecol. 1988, 159, 297–307. [Google Scholar] [CrossRef]
- Kangussu, L.M.; Melo-Braga, M.N.; de Souza Lima, B.S.; Santos, R.A.S.; de Andrade, H.M.; Campagnole-Santos, M.J. Angiotensin-(1-7) Central Mechanisms After ICV Infusion in Hypertensive Transgenic (mRen2)27 Rats. Front. Neurosci. 2021, 15, 624249. [Google Scholar] [CrossRef]
- Merrill, D.C.; Karoly, M.; Chen, K.; Ferrario, C.M.; Brosnihan, K.B. Angiotensin-(1-7) in Normal and Preeclamptic Pregnancy. Endocrine 2002, 18, 239–246. [Google Scholar] [CrossRef]
- LoMauro, A.; Aliverti, A. Respiratory physiology of pregnancy: Physiology masterclass. Breathe 2015, 11, 297–301. [Google Scholar] [CrossRef] [Green Version]
- Lyons, H.; Antonio, R. The sensitivity of the respiratory center in pregnancy and after administration of progesterone. Trans. Assoc. Am. Physicians 1959, 72, 173–180. [Google Scholar]
- Liu, H.; Wang, L.L.; Zhao, S.J.; Kwak-Kim, J.; Mor, G.; Liao, A.H. Why are pregnant women susceptible to COVID-19? An immunological viewpoint. J. Reprod. Immunol. 2020, 139, 103122. [Google Scholar] [CrossRef]
- Jensen, D.; Webb, K.A.; Davies, G.A.L.; O’Donnell, D.E. Mechanical ventilatory constraints during incremental cycle exercise in human pregnancy: Implications for respiratory sensation. J. Physiol. 2018, 586, 4735–4750. [Google Scholar] [CrossRef]
- McAuliffe, F.; Kametas, N.; Costello, J.; Rafferty, G.F.; Greenough, A.; Nicolaides, K. Respiratory function in singleton and twin pregnancy. BJOG An. Int. J. Obstet. Gynaecol. 2002, 109, 765–769. [Google Scholar] [CrossRef]
- Aghaeepour, N.; Ganio, E.A.; Mcilwain, D.; Tsai, A.S.; Tingle, M.; Van Gassen, S.; Gaudilliere, D.K.; Baca, Q.; McNeil, L.; Okada, R.; et al. An immune clock of human pregnancy. Sci. Immunol. 2017, 2, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kay, A.W.; Fukuyama, J.; Aziz, N.; Dekker, C.L.; Mackey, S.; Swan, G.E.; Davis, M.M.; Holmes, S.; Blish, C.A. Enhanced natural killer-cell and T-cell responses to influenza A virus during pregnancy. Proc. Natl. Acad. Sci. USA 2014, 111, 14506–14511. [Google Scholar] [CrossRef] [Green Version]
- Le Gars, M.; Kay, A.W.; Bayless, N.L.; Aziz, N.; Dekker, C.L.; Swan, G.E.; Davis, M.M.; Blish, C.A. Increased proinflammatory responses of monocytes and plasmacytoid dendritic cells to influenza a virus infection during pregnancy. J. Infect. Dis. 2016, 214, 1666–1671. [Google Scholar] [CrossRef] [Green Version]
- Ghi, T.; di Pasquo, E.; Mekinian, A.; Calza, L.; Frusca, T. Sars-CoV-2 in pregnancy: Why is it better than expected? Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 252, 476–478. [Google Scholar] [CrossRef] [PubMed]
- Shevyrev, D.; Tereshchenko, V. Treg Heterogeneity, Function, and Homeostasis. Front. Immunol. 2020, 10, 3100. [Google Scholar] [CrossRef] [Green Version]
- Allotey, J.; Stallings, E.; Bonet, M.; Yap, M.; Chatterjee, S.; Kew, T.; Debenham, L.; Llavall, A.C.; Dixit, A.; Zhou, D.; et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ 2020, 370, 1641–1647. [Google Scholar] [CrossRef]
- Narang, K.; Enninga, E.A.L.; Gunaratne, M.D.; Ibirogba, E.R.; Trad, A.T.A.; Elrefaei, A.; Theiler, R.N.; Ruano, R.; Szymanski, L.M.; Chakraborty, R.; et al. SARS-CoV-2 Infection and COVID-19 During Pregnancy: A Multidisciplinary Review. Mayo Clin. Proc. 2020, 95, 1750–1765. [Google Scholar] [CrossRef]
- Lokken, E.M.; Huebner, E.M.; Taylor, G.G.; Hendrickson, S.; Vanderhoeven, J.; Kachikis, A.; Coler, B.; Walker, C.L.; Sheng, J.S.; Al-Haddad, B.J.; et al. Disease severity, pregnancy outcomes, and maternal deaths among pregnant patients with severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am. J. Obstet. Gynecol. 2021, 225, 77.e1–77.e14. [Google Scholar] [CrossRef] [PubMed]
- Villar, J.; Ariff, S.; Gunier, R.B. Maternal and Neonatal Morbidity and Mortality Among Pregnant Women With and Without COVID-19 Infection: The INTERCOVID Multinational Cohort Study. JAMA Pediatr. 2021, 175, 817–826. [Google Scholar] [CrossRef]
- ACOG Vaccinating Pregnant and Lactating Patients against COVID-19. 24 March 2021. Available online: https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/vaccinating-pregnant-and-lactating-patients-against-covid-19 (accessed on 17 November 2021).
- CDC–COVID-19 Vaccine Safety Update Advisory Committee on Immunization Practices (ACIP). Tom Shimabukuro, MD, MPH, MBACDC COVID19 Vaccine Task Force Vaccine. 1 March 2021. Available online: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-02/28-03-01/05-covid-Shimabukuro.pdf (accessed on 17 November 2021).
- SMFM—Provider Considerations for Engaging in COVID-19 Vaccine Counseling with Pregnant and Lactating Patients. Available online: https://www.smfm.org/covidclinical (accessed on 17 November 2021).
- RCOG—COVID Guidelines Updated 16 April 2021. Available online: https://www.rcog.org.uk/globalassets/documents/guidelines/2021-02-24-combined-info-sheet-and-decision-aid.pdf (accessed on 17 November 2021).
- Shimabukuro, T.T.; Kim, S.Y.; Myers, T.R. Preliminary Findings of mRNA Covid-19 Vaccine Safety in Pregnant Persons. N. Engl. J. Med. 2021, 384, 2273–2282. [Google Scholar] [CrossRef]
- Peretz, S.B.; Regev, N.; Novick, L.; Nachshol, M.; Goffer, E.; Ben-David, A.; Asraf, K.; Doolman, R.; Levin, E.G.; Yochay, G.R.; et al. Short-term outcome of pregnant women vaccinated with BNT162b2 mRNA COVID-19 vaccine. Ultrasound Obstet. Gynecol. 2021, 58, 450–456. [Google Scholar] [CrossRef]
- Yan, Z.; Yang, M.; Lai, C.L. COVID-19 Vaccinations: A Comprehensive Review of Their Safety and Efficacy in Special Populations. Vaccines 2021, 9, 1097. [Google Scholar] [CrossRef]
- Magnus, M.C.; Gjessing, H.K.; Eide, H.N.; Wilcox, A.J.; Fell, D.B.; Håberg, S.E. Covid-19 Vaccination during Pregnancy and First-Trimester Miscarriage. N. Engl. J. Med. 2021, 385, 2008–2010. [Google Scholar] [CrossRef]
- Skjefte, M.; Ngirbabul, M.; Akeju, O.; Escudero, D.; Hernandez-Diaz, S.; Wyszynski, D.F.; Wu, J.W. COVID-19 vaccine acceptance among pregnant women and mothers of young children: Results of a survey in 16 countries. Eur. J. Epidemiol. 2021, 36, 197. [Google Scholar] [CrossRef]
- CDC COVID Data Tracker. Available online: https://covid.cdc.gov/covid-data-tracker/#vaccinations-pregnant-women (accessed on 17 November 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celewicz, A.; Celewicz, M.; Michalczyk, M.; Woźniakowska-Gondek, P.; Krejczy, K.; Misiek, M.; Rzepka, R. Pregnancy as a Risk Factor of Severe COVID-19. J. Clin. Med. 2021, 10, 5458. https://doi.org/10.3390/jcm10225458
Celewicz A, Celewicz M, Michalczyk M, Woźniakowska-Gondek P, Krejczy K, Misiek M, Rzepka R. Pregnancy as a Risk Factor of Severe COVID-19. Journal of Clinical Medicine. 2021; 10(22):5458. https://doi.org/10.3390/jcm10225458
Chicago/Turabian StyleCelewicz, Aleksander, Marta Celewicz, Michał Michalczyk, Paula Woźniakowska-Gondek, Kamila Krejczy, Marcin Misiek, and Rafał Rzepka. 2021. "Pregnancy as a Risk Factor of Severe COVID-19" Journal of Clinical Medicine 10, no. 22: 5458. https://doi.org/10.3390/jcm10225458
APA StyleCelewicz, A., Celewicz, M., Michalczyk, M., Woźniakowska-Gondek, P., Krejczy, K., Misiek, M., & Rzepka, R. (2021). Pregnancy as a Risk Factor of Severe COVID-19. Journal of Clinical Medicine, 10(22), 5458. https://doi.org/10.3390/jcm10225458





