Echocardiographic Advances in Dilated Cardiomyopathy
Abstract
:1. Introduction
2. Left Ventricular Dimensions, Geometry and Systolic Function
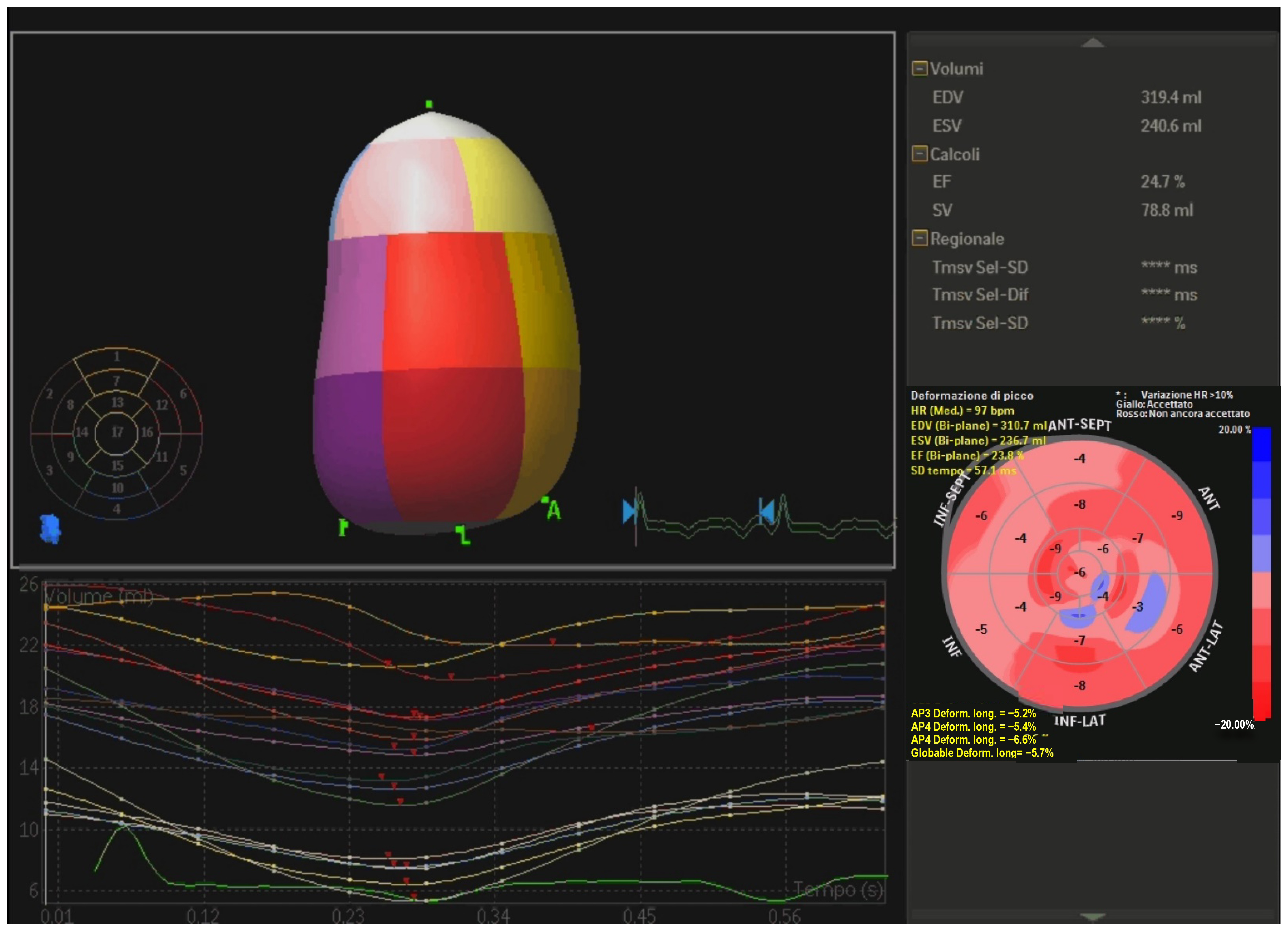
- Three-dimensional (3D) echocardiography. This technique improves reproducibility of LV volumes with an accuracy similar to CMR [28]. It is an ideal tool for measuring LV volumes because no geometric assumptions about shape are needed and is unaffected by foreshortening of the apex. Conversely, it is characterized by lower temporal resolution, high dependance on image quality and there are only a few data available on reference values. Real-time 3D echocardiographic systems have been developed [29,30,31]; these systems utilize fully samples matrix array transducers capable of acquiring volumetric data. 3D echocardiography (Figure 1) is more reproducible and superior to 2D in the accuracy of LV volumes and EF measurements [32], however, despite clear recommendations in the guidelines, its use is still limited probably due to the long learning curve [33]. Moreover, 3D-STE has been proven a reliable tool for the evaluation of LV systolic function in patients with non-ischemic DCM with a good interobserver, intraobserver and test-retest reliability [34] and a good correlation with data obtained from CMR [35].
- Non-invasive left ventricular pressure-strain loop (PSL) and global work index (GWI). Myocardial work (MW) is a new parameter that considers both myocardial deformation and after-load. It is calculated combining LV strain and non-invasively estimated LV pressure curves. The area within the PSL represent an index of MW, and the following parameters can be determined [36]: GWI, as the total work within the PSL area from mitral closure to opening; constructive MW, as the work performed by LV responsible for LV systolic ejection; wasted MW work, as work that does not contribute to ejection; MW efficiency, as constructive MW/constructive MW plus wasted MW. These equations permit to better understand the relationships between LV remodeling and increased after-load under different loading conditions. Moreover GWI and constructive work (GCW) are powerful and independent predictors of outcome in patients with DCM and advanced HFrEF [37]. GCW better predicts LV fibrosis than GLS, and could represent a surrogate marker for detection of fibrosis in addition to CMR [38].
- Reverse remodeling index. Although DCM is classically defined as above, conventional geometric parameters used have not been demonstrated to have a prognostic value [39,40,41,42]. Remodeling index is a novel geometric criterion calculated as the cubic root of LVEDV divided by mean LV wall thickness. It was shown to be an equivalent in evaluating ventricular maladaptive remodeling [43] in hypertensive patients. Xu et al. recently investigated the value of this new marker in DCM cohorts as an independent predictor of all-cause mortality, heart transplantation and HFrEF readmissions [44].
- Post-systolic shortening (PSS) and early systolic lengthening (ESL). Objective measures of cardiac function are supplied by PSS and ESL [45], both parameters reflecting the paradoxical deformation of the myocardium. During systolic ejection approximately one third of segments display physiologic PSS and the same applies to ESL [46,47]. These indices are quantified by invasive and non-invasive methods, like strain-rate, tissue Doppler imaging, speckle tracking. Pathological deformation typically occurs during acute ischemia, and it is a predictor of recovery and ischemic memory, and, recently, the ability of PSS and ESL to predict adverse cardiac outcomes has been demonstrated in a wide range of special population [48,49,50,51,52].
- Mitral valve complex (MVC) tissue longitudinal elongation. It is clear that LV sphericity is associated with impaired LVEF, functional mitral regurgitation (MR) and poor prognosis [53,54] in DCM. LV shape becomes spherical and myocardial tissue elongates transversally [55]. The dynamic nature of MVC is quite different from myocardium [56] and so it may not be affected while myocardium spherically remodels. Hence, MVC may potentially deforms regionally and not uniformly leading to limited elongation of LV base and asymmetrically less elongation of the MVC. LV sphericity can be calculated via 2D echocardiography by the ratio of transverse cavity dimension to the longitudinal diameter at end diastole, so a higher sphericity index represents greater LV sphericity: therefore, remodeling is predominant at bases and not as clear at the apex leading to new promising diagnostic tools and interventions [57].
- Artificial intelligence and machine learning (ML). AI is historically defined as the ability of computer systems to perform tasks that would usually require human levels of intelligence [58]. AI has recently become a research hotspot in echocardiography [59], although this is yet not so advanced and in 1978 was already used to estimate the waveform of anterior mitral leaflet via M-mode. The progress of new technologies, such as deep learning and neural networks, has dramatically boosted the strength of echocardiography. Advantages of using ML models includes automated analysis, reducing observer variability, providing more consistent and reproducible data, allowing big data analysis, predicting future data, helping in therapeutic decision making, and nevertheless it is time and cost saving, reducing unnecessary further investigations [60]. Specifically, LV evaluation can be derived from algorithms built mimicking what a trained sonographer can do [61,62]. The same applies to GLS analysis: AI technique can help recognize standard views, perform timing of cardiac events, trace the myocardium, achieve motion estimation and measure GLS in <15 s [63]. In the context of DCM, a vector machine classifier was addressed to study the ventricular wall changes to discriminate between normal and dilated pattern [64]. The inclusion of ML models in echocardiography appears very promising, as they are able to precisely identify various echocardiographic features and predict outcomes, without the limitations related to human interpretation: a novel multicenter research shown that AI-based LV analysis were predictor of mortality [65]. There are still doubts about insufficient standardization of echocardiography, poor robustness and scant generalization of the models in clinical application. The continue advancement of AI technology will gradually remodel the forthcoming of echocardiography to grant practical auxiliary assistance for cardiologists [66].
3. Left Ventricular Diastolic Function
4. Right Ventricular Dilatation and Disfunction
5. Left Ventricular Dyssynchrony (LVD) and Left Ventricular Mechanical Dispersion
6. Secondary Mitral and Tricuspid Regurgitation
7. Left Ventricular Thrombus
8. Myocardial Scars and Fibrosis
- Contrast-enhanced (CE) 3D Echo. Montant et al. tested CE-3D echo versus CMR-LGE and found that second-harmonic imaging (with transmission/receive 1.6/3.2 Mhz) at a mechanical index of 0.5 was the best combination to differentiate normal myocardium from fibrotic scar [165].
- Three dimensional (3D) speckle tracking echocardiography. A study conducted in DCM patients prior to heart transplant comparing STE versus histological findings found that 3D GLS may be an optimal surrogate marker for reflecting MF (Area Under Curve, AUC 0.86) [166].
- Pulse cancellation ultrasound technique (eSCAR). The echo machine built-in setting for LV opacification used without CE, thanks to cancellation of “linear” signals back from normal myocardium, is incidentally very efficient to enhance signals from abnormal myocardial tissue, such as fibrotic [167] and calcific tissues [168], which on the contrary show “nonlinear” response. This technique is able not only to provide a semi-quantitative identification of MF (number of scarred segments), but also to simply define a binary response “scar: yes/no” by the use of the software binary filter (“default” thresholding method) [167]. Furthermore, eScar has been shown to be able to identify scar burden as a prognostic marker for ICD appropriate discharge [169].
- Radiomics-based texture analysis. Using a ML pipeline to integrate and process ultrasound images texture features the presence of MF is predictable with an AUC of 0.84 (sensitivity 86.4% and specificity 83.3%) [170].
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elliott, P.; Andersson, B.; Arbustini, E.; Bilinska, Z.; Cecchi, F.; Charron, P.; Dubourg, O.; Kühl, U.; Maisch, B.; McKenna, W.J.; et al. Classification of the cardiomyopathies: A position statement from the european society of cardiology working group on myocardial and pericardial diseases. Eur. Heart J. 2008, 29, 270–276. [Google Scholar] [CrossRef] [Green Version]
- Merlo, M.; Cannatà, A.; Gobbo, M.; Stolfo, D.; Elliott, P.M.; Sinagra, G. Evolving concepts in dilated cardiomyopathy. Eur. J. Heart Fail. 2018, 20, 228–239. [Google Scholar] [CrossRef] [Green Version]
- Sinagra, G.; Elliott, P.M.; Merlo, M. Dilated cardiomyopathy: So many cardiomyopathies! Eur. Heart J. 2020, 41, 784–3786. [Google Scholar] [CrossRef]
- Merlo, M.; Cannatà, A.; Sinagra, G. Dilated Cardiomyopathy: A Paradigm of Revolution in Medicine. J. Clin. Med. 2020, 9, 3385. [Google Scholar] [CrossRef]
- Merlo, M.; Pivetta, A.; Pinamonti, B.; Stolfo, D.; Zecchin, M.; Barbati, G.; Lenarda, A.D.; Sinagra, G. Long-term prognostic impact of therapeutic strategies in patients with idiopathic dilated cardiomyopathy: Changing mortality over the last 30 years. Eur. J. Heart Fail. 2014, 16, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Merlo, M.; Cannatà, A.; Loco, C.P.; Stolfo, D.; Barbati, G.; Artico, J.; Gentile, P.; Paris, V.D.; Ramani, F.; Zecchin, M.; et al. Contemporary survival trends and aetiological characterization in non-ischaemic dilated cardiomyopathy. Eur. J. Heart Fail. 2020, 22, 1111–1121. [Google Scholar] [CrossRef]
- Rosenbaum, A.N.; Agre, K.E.; Pereira, N.L. Genetics of dilated cardiomyopathy: Practical implications for heart failure management. Nat. Rev. Cardiol. 2020, 17, 286–297. [Google Scholar] [CrossRef]
- Sinagra, G.; Pinamonti, B.; Merlo, M. Dilated Cardiomyopathy: From Genetics to Clinical Management; Springer Nature: Basingstoke, UK, 2019. [Google Scholar]
- Singh, S.; Goyal, A. The origin of echocardiography: A tribute to Inge Edler. Tex. Heart Inst. J. 2007, 34, 431. [Google Scholar]
- Wang, C.L.; Hung, K.C. Recent Advances in Echocardiography. J. Med. Ultrasound 2017, 25, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.E. Cramming more components onto integrated circuits. Proc. IEEE 1998, 86, 82–85. [Google Scholar] [CrossRef]
- Mitropoulou, P.; Georgiopoulos, G.; Figliozzi, S.; Klettas, D.; Nicoli, F.; Masci, P.G. Multi-Modality Imaging in Dilated Cardiomyopathy: With a Focus on the Role of Cardiac Magnetic Resonance. Front. Cardiovasc. Med. 2020, 7, 97. [Google Scholar] [CrossRef]
- Elliott, P. Diagnosis and management of dilated cardiomyopathy. Heart 2000, 84, 106. [Google Scholar] [CrossRef] [Green Version]
- Pinto, Y.M.; Elliott, P.M.; Arbustini, E.; Adler, Y.; Anastasakis, A.; Böhm, M.; Duboc, D.; Gimeno, J.; Groote, P.D.; Imazio, M.; et al. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: A position statement of the ESC working group on myocardial and pericardial diseases. Eur. Heart J. 2016, 37, 1850–1858. [Google Scholar] [CrossRef] [Green Version]
- Amzulescu, M.S.; De Craene,, M.; Langet, H.; Pasquet, A.; Vancraeynest, D.; Pouleur, A.C.; Vanoverschelde, J.L.; Gerber, B.L. Myocardial strain imaging: Review of general principles, validation, and sources of discrepancies. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 605–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abtahi, F.; Abdi, A.; Jamshidi, S.; Karimi, M.; Babaei-Beigi, M.A.; Attar, A. Global longitudinal strain as an Indicator of cardiac Iron overload in thalassemia patients. Cardiovasc. Ultrasound. 2019, 17, 24. [Google Scholar] [CrossRef] [Green Version]
- Stokk, T.M.; Hasselberg, N.E.; Smedsrud, M.K.; Sarvari, S.I.; Haugaa, K.H.; Smiseth, A.O.; Edvardsen, T.; Remme, E.W. Geometry as a Confounder When Assessing Ventricular Systolic Function. J. Am. Coll. Cardiol. 2017, 70, 942–954. [Google Scholar] [CrossRef]
- Geyer, H.; Caracciolo, G.; Abe, H.; Wilansky, S.; Carerj, S.; Gentile, F.; Nesser, H.-J.; Khandheria, B.; Narula, J.; Sengupta, P.P. Assessment of myocardial mechanics using speckle tracking echocardiography: Fundamentals and clinical applications. J. Am. Soc. Echocardiogr. 2010, 23, 351–369. [Google Scholar] [CrossRef] [PubMed]
- Smiseth, O.A.; Torp, H.; Opdahl, A.; Haugaa, K.; Urheim, S. Myocardial strain imaging: How useful is it in clinical decision making? Eur. Heart J. 2016, 37, 1196–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamo, L.; Perry, A.; Novak, E.; Makan, M.; Lindman, B.R.; Mann, D.L. Abnormal Global Longitudinal Strain Predicts Future Deterioration of Left Ventricular Function in Heart Failure Patients with a Recovered Left Ventricular Ejection Fraction. Circ. Heart Fail. 2017, 10, e003788. [Google Scholar] [CrossRef]
- Nikoo, M.H.; Naeemi, R.; Moaref, A.; Attar, A. Global longitudinal strain for prediction of ventricular arrhythmia in patients with heart failure. ESC Heart Fail. 2020, 7, 2956–2961. [Google Scholar] [CrossRef]
- Kažukauskienė, I.; Balčiūnaitė, G.; Baltrūnienė, V.; Čelutkienė, J.; Maneikienė, V.V.; Čibiras, S.; Ručinskas, K.; Grabauskienė, V. Left ventricular global longitudinal strain predicts elevated cardiac pressures and poor clinical outcomes in patients with non-ischemic dilated cardiomyopathy. Cardiovasc. Ultrasound 2021, 19, 1–13. [Google Scholar] [CrossRef]
- Hayashi, T.; Yamada, S.; Iwano, H.; Nakabachi, M.; Sakakibara, M.; Okada, K.; Murai, D.; Nishino, H.; Kusunose, K.; Watanabe, K.; et al. Left Ventricular Global Strain for Estimating Relaxation and Filling Pressure—A Multicenter Study. Circ. J. 2016, 80, 1163–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, I.H.; Park, J.H.; Lee, J.-A.; Kim, G.S.; Lee, H.Y.; Byun, Y.S.; Kim, B.O. Left Ventricular Global Longitudinal Strain as a Predictor for Left Ventricular Reverse Remodeling in Dilated Cardiomyopathy. J. Cardiovasc. Imaging 2020, 28, 137–149. [Google Scholar] [CrossRef] [Green Version]
- Merlo, M.; Pyxaras, S.A.; Pinamonti, B.; Barbati, G.; di Lenarda, A.; Sinagra, G. Prevalence and prognostic significance of left ventricular reverse remodeling in dilated cardiomyopathy receiving tailored medical treatment. J. Am. Coll. Cardiol. 2011, 57, 1468–1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waddingham, P.H.; Bhattacharyya, S.; van Zalen, J.; Lloyd, G. Contractile reserve as a predictor of prognosis in patients with non-ischaemic systolic heart failure and dilated cardiomyopathy: A systematic review and meta-analysis. Echo Res. Pract. 2018, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Morimoto, R.; Okumura, T.; Hirashiki, A.; Ishii, H.; Ichii, T.; Aoki, S.; Furusawa, K.; Hiraiwa, H.; Kondo, T.; Watanabe, N.; et al. Myocardial contractile reserve predicts left ventricular reverse remodeling and cardiac events in dilated cardiomyopathy. J. Cardiol. 2017, 70, 303–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, C.; Bricknell, K.; Hanekom, L.; Marwick, T.H. Reproducibility and accuracy of echocardiographic measurements of left ventricular parameters using real-time three-dimensional echocardiography. J. Am. Coll. Cardiol. 2004, 44, 878–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kühl, H.P.; Schreckenberg, M.; Rulands, D.; Katoh, M.; Schäfer, W.; Bücker, A.; Hanrath, P.; Franke, A. High-Resolution Transthoracic Real-Time Three-Dimensional Echocardiography Quantitation of Cardiac Volumes and Function Using Semi-Automatic Border Detection and Comparison With Cardiac Magnetic Resonance Imaging. J. Am. Coll. Cardiol. 2004, 43, 2083–2090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mor-Avi, V.; Sugeng, L.; Weinert, L.; MacEneaney, P.; Caiani, E.; Koch, R.; Salgo, I.S.; Lang, R.M. Fast measurement of left ventricular mass with real-time three-dimensional echocardiography: Comparison with magnetic resonance imaging. Circulation 2004, 110, 1814–1818. [Google Scholar] [CrossRef] [Green Version]
- Ota, T.; Kisslo, J.; Von Ramm, O.T.; Yoshikawa, J. Real-time, volumetric echocardiography: Usefulness of volumetric scanning for the assessment of cardiac volume and function. J. Cardiol. 2001, 37 (Suppl. S1), 93–101. [Google Scholar] [PubMed]
- Ruddox, V.; Mathisen, M.; Bækkevar, M.; Aune, E.; Edvardsen, T.; Otterstad, J.E. Is 3D echocardiography superior to 2D echocardiography in general practice? A systematic review of studies published between 2007 and 2012. Int. J. Cardiol. 2013, 168, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Victor, M.A.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [Green Version]
- Duan, F.; Xie, M.; Wang, X.; Li, Y.; He, L.; Jiang, L.; Fu, Q. Preliminary clinical study of left ventricular myocardial strain in patients with non-ischemic dilated cardiomyopathy by three-dimensional speckle tracking imaging. Cardiovasc. Ultrasound 2012, 10, 8. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Yang, L. Value of three-dimensional speckle-tracking imaging in detecting left ventricular systolic function in patients with dilated cardiomyopathy. Echocardiography 2019, 36, 1492–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, J.; Edwards, N.F.A.; Khandheria, B.K.; Shiino, K.; Sabapathy, S.; Anderson, B.; Chamberlain, R.; Scalia, G.M. A new approach to assess myocardial work by non-invasive left ventricular pressure-strain relations in hypertension and dilated cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 31–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedwig, F.; Nemchyna, O.; Stein, J.; Knosalla, C.; Merke, N.; Knebel, F.; Hagendorff, A.; Schoenrath, F.; Falk, V.; Knierim, J. Myocardial Work Assessment for the Prediction of Prognosis in Advanced Heart Failure. Front. Cardiovasc. Med. 2021, 8, 622. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Li, Y.; Liu, Y.; Huang, D.; Hu, Y.; Wang, Y.; Ma, L.; Liu, L. Association between Echocardiographic Non-invasive Myocardial Work Indices and Myocardial Fibrosis in Patients with Dilated Cardiomyopathy. Front. Cardiovasc. Med. 2021, 8, 704251. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, P.; Bahl, A. Left ventricular size as a predictor of outcome in patients of non-ischemic dilated cardiomyopathy with severe left ventricular systolic dysfunction. Int. J. Cardiol. 2016, 221, 310–313. [Google Scholar] [CrossRef]
- Zhong, L.; Su, Y.; Yeo, S.-Y.; Tan, R.S.; Ghista, D.N.; Kassab, G. Left ventricular regional wall curvedness and wall stress in patients with ischemic dilated cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H573–H584. [Google Scholar] [CrossRef] [Green Version]
- Marume, K.; Noguchi, T.; Tateishi, E.; Morita, Y.; Kamakura, T.; Ishibashi, K.; Noda, T.; Miura, H.; Nishimura, K.; Nakai, M.; et al. Mortality and Sudden Cardiac Death Risk Stratification Using the Noninvasive Combination of Wide QRS Duration and Late Gadolinium Enhancement in Idiopathic Dilated Cardiomyopathy. Circ. Arrhythm. Electrophysiol. 2018, 11, e006233. [Google Scholar] [CrossRef]
- Zaman, S.; Goldberger, J.J.; Kovoor, P. Sudden Death Risk-Stratification in 2018–2019: The Old and the New. Heart. Lung Circ. 2019, 28, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Goh, V.J.; Le, T.-T.; Bryant, J.; Wong, J.I.; Su, B.; Lee, C.-H.; Pua, C.J.; Sim, C.P.; Ang, B.; Aw, T.C.; et al. Novel Index of Maladaptive Myocardial Remodeling in Hypertension. Circ. Cardiovasc. Imaging 2017, 10, e006840. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Lin, J.; Liang, Y.; Wan, K.; Li, W.; Wang, J.; Zhu, Y.; Mui, D.; Wang, L.; Li, Y.; et al. Prognostic value of left ventricular remodelling index in idiopathic dilated cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2020, 22, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, T.; Nakatani, S. Myocardial ischaemia and post-systolic shortening. Heart 2015, 101, 509–516. [Google Scholar] [CrossRef]
- Voigt, J.; Lindenmeier, G.; Exner, B.; Regenfus, M.; Werner, D.; Reulbach, D.; Nixdorff, U.; Flachskampf, F.S.; Daniel, G.A. Incidence and characteristics of segmental postsystolic longitudinal shortening in normal, acutely ischemic, and scarred myocardium. J. Am. Soc. Echocardiogr. 2003, 16, 415–423. [Google Scholar] [CrossRef]
- Weidemann, F.; Broscheit, J.A.; Bijnens, B.; Claus, P.; Sutherland, G.R.; Voelker, W.; Ertl, G.; Strotmann, J.M. How to distinguish between ischemic and nonischemic postsystolic thickening: A strain rate imaging study. Ultrasound Med. Biol. 2006, 32, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Brainin, P. Myocardial Postsystolic Shortening and Early Systolic Lengthening: Current Status and Future Directions. Diagnostics 2021, 11, 1428. [Google Scholar] [CrossRef] [PubMed]
- Brainin, P.; Skaarup, K.G.; Iversen, A.Z.; Jørgensen, P.G.; Platz, E.; Jensen, J.S.; Biering-Sørensen, T. Post-systolic Shortening Predicts Heart Failure following Acute Coronary Syndrome. Int. J. Cardiol. 2019, 276, 191. [Google Scholar] [CrossRef]
- Brainin, P.; Jensen, M.T.; Biering-Sørensen, T.; Møgelvang, R.; Fritz-Hansen, T.; Vilsbøll, T.; Rossing, P.; Jørgensen, P.G. Post-Systolic Shortening by Speckle Tracking Echocardiography Predicts Cardiac Events in Type 2 Diabetes. JACC Cardiovasc. Imaging 2020, 13, 1289–1291. [Google Scholar] [CrossRef]
- Brainin, P.; Haahr-Pedersen, S.; Sengeløv, M.; Olsen, F.J.; Fritz-Hansen, T.; Jensen, J.S.; Biering-Sørensen, T. Presence of post-systolic shortening is an independent predictor of heart failure in patients following ST-segment elevation myocardial infarction. Int. J. Cardiovasc. Imaging 2018, 34, 751–760. [Google Scholar] [CrossRef]
- Hsiao, J.-F.; Pan, K.-L.; Chu, C.-M.; Chang, S.-T.; Chung, C.-M.; Hsu, J.-T. Usefulness of serial post-systolic shortening by speckle tracking echocardiography to predict major adverse cardiovascular events and segmental function improvement after acute myocardial infarction. PLoS ONE 2020, 15, e0244589. [Google Scholar] [CrossRef]
- Cohn, J.N.; Ferrari, R.; Sharpe, N. Cardiac remodeling—Concepts and clinical implications: A consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J. Am. Coll. Cardiol. 2000, 35, 569–582. [Google Scholar] [CrossRef] [Green Version]
- Yiu, S.F.; Enriquez-Sarano, M.; Tribouilloy, C.; Seward, J.B.; Tajik, A.J. Determinants of the degree of functional mitral regurgitation in patients with systolic left ventricular dysfunction: A quantitative clinical study. Circulation 2000, 102, 1400–1406. [Google Scholar] [CrossRef] [Green Version]
- Reddy, H.K.; Tjahja, I.E.; Campbell, S.E.; Janicki, J.S.; Hayden, M.R.; Tyagi, S.C. Expression of matrix metalloproteinase activity in idiopathic dilated cardiomyopathy: A marker of cardiac dilatation|Semantic Scholar. Mol. Cell. Biochem. 2004, 264, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.A.; Network, F.T.L.M.T.; Hagege, A.; Judge, D.; Padala, M.; Dal-Bianco, J.P.; Aikawa, E.; Beaudoin, J.; Bischoff, J.; Bouatia-Naji, N.; et al. Mitral valve disease—Morphology and mechanisms. Nat. Rev. Cardiol. 2015, 12, 689–710. [Google Scholar] [CrossRef] [Green Version]
- Nagata, Y.; Iwataki, M.; Nabeshima, Y.; Hei, S.; Onoue, T.; Hayashi, A.; Otani, K.; Tsuda, Y.; Araki, M.; Kim, D.-H.; et al. Potential mechanism of left ventricular spherical remodeling: Association of mitral valve complex-myocardium longitudinal tissue remodeling mismatch. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H694–H704. [Google Scholar] [CrossRef] [PubMed]
- Shameer, K.; Johnson, K.W.; Glicksberg, B.S.; Dudley, J.T.; Sengupta, P.P. Machine learning in cardiovascular medicine: Are we there yet? Heart 2018, 104, 1156–1164. [Google Scholar] [CrossRef]
- Sanders, W.E.; Burton, T.; Khosousi, A.; Ramchandani, S. Machine learning: At the heart of failure diagnosis. Curr. Opin. Cardiol. 2021, 36, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Alsharqi, M.; Woodward, W.; Mumith, J.A.; Markham, D.C.; Upton, R.; Leeson, P. Artificial intelligence and echocardiography. Echo Res. Pract. 2018, 5, R115–R125. [Google Scholar] [CrossRef] [Green Version]
- Asch, F.M.; Poilvert, N.; Abraham, T.; Jankowski, M.; Cleve, J.; Adams, M.; Romano, N.; Hong, H.; Mor-Avi, V.; Martin, R.P.; et al. Automated Echocardiographic Quantification of Left Ventricular Ejection Fraction Without Volume Measurements Using a Machine Learning Algorithm Mimicking a Human Expert. Circ. Cardiovasc. Imaging 2019, 12, e009303. [Google Scholar] [CrossRef]
- Kusunose, K.; Haga, A.; Yamaguchi, N.; Abe, T.; Fukuda, D.; Yamada, H.; Harada, M.; Sata, M. Deep Learning for Assessment of Left Ventricular Ejection Fraction from Echocardiographic Images. J. Am. Soc. Echocardiogr. 2020, 33, 632–635.e1. [Google Scholar] [CrossRef] [PubMed]
- Salte, I.M.; Østvik, A.; Smistad, E.; Melichova, D.; Nguyen, T.M.; Karlsen, S.; Brunvand, H.; Haugaa, K.H.; Edvardsen, T.; Lovstakken, L.; et al. Artificial Intelligence for Automatic Measurement of Left Ventricular Strain in Echocardiography. JACC. Cardiovasc. Imaging 2021, 14, 1918–1928. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, R.; Syeda-Mahmood, T. Automatic detection of dilated cardiomyopathy in cardiac ultrasound videos. AMIA Annu. Symp. Proc. 2014, 2014, 865–871. [Google Scholar]
- Asch, F.M. Human vs AI-Based Echocardiography Analysis as Predictor of Mortality in Acute COVID-19 Patients: WASE-COVID Study; MedStar Health Research Institute: Washington, DC, USA, 2021. [Google Scholar]
- Zhou, J.; Du, M.; Chang, S.; Chen, Z. Artificial intelligence in echocardiography: Detection, functional evaluation, and disease diagnosis. Cardiovasc. Ultrasound. 2021, 19, 1–11. [Google Scholar] [CrossRef]
- Sato, K.; Grant, A.D.M.; Negishi, K.; Cremer, P.C.; Negishi, T.; Kumar, A.; Collier, P.; Kapadia, S.R.; Grimm, R.A.; Desai, M.Y.; et al. Reliability of updated left ventricular diastolic function recommendations in predicting elevated left ventricular filling pressure and prognosis. Am. Heart J. 2017, 189, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Playford, D.; Strange, G.; Celermajer, D.S.; Evans, G.; Scalia, G.M.; Stewart, S.; Prior, D.; NEDA Investigators. Diastolic dysfunction and mortality in 436 360 men and women: The National Echo Database Australia (NEDA). Eur. Heart J. Cardiovasc. Imaging. 2021, 22, 505–515. [Google Scholar] [CrossRef]
- Keogh, A.M.; Baron, D.W.; Hickie, J.B. Prognostic guides in patients with idiopathic or ischemic dilated cardiomyopathy assessed for cardiac transplantation. Am. J. Cardiol. 1990, 65, 903–908. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeung, D.F.; Jiang, R.; Behnami, D.; Jue, J.; Sharma, R.; Turaga, M.; Luong, C.L.; Tsang, M.Y.C.; Gin, K.G.; Girgis, H.; et al. Impact of the updated diastolic function guidelines in the real world. Int. J. Cardiol. 2021, 326, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.G.; Fontes-Carvalho, R.; Sampaio, F.; Ribeiro, J.; Bettencourt, P.; Flachskampf, F.A.; Leite-Moreira, A.; Azevedo, A. Impact of the 2016 ASE/EACVI recommendations on the prevalence of diastolic dysfunction in the general population. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 380–386. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Khoury, D.S.; Thohan, V.; Torre-Amione, G.; Nagueh, S.F. Global diastolic strain rate for the assessment of left ventricular relaxation and filling pressures. Circulation 2007, 115, 1376–1383. [Google Scholar] [CrossRef] [Green Version]
- Dokainish, H.; Sengupta, R.; Pillai, M.; Bobek, J.; Lakkis, N. Usefulness of New Diastolic Strain and Strain Rate Indexes for the Estimation of Left Ventricular Filling Pressure. Am. J. Cardiol. 2008, 101, 1504–1509. [Google Scholar] [CrossRef] [PubMed]
- Shanks, M.; Ng, A.C.T.; Veire, N.R.L.; Antoni, M.L.; Bertini, M.; Delgado, V.; Nucifora, G.; Holman, E.R.; Choy, J.B.; Leung, D.Y.; et al. Incremental Prognostic Value of Novel Left Ventricular Diastolic Indexes for Prediction of Clinical Outcome in Patients with ST-Elevation Myocardial Infarction. Am. J. Cardiol. 2010, 105, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.C.; Lee, W.H.; Chu, C.Y.; Lee, C.S.; Yen, H.W.; Su, H.M.; Lin, T.H.; Voon, W.C.; Lai, W.T.; Sheu, S.H. The ratio of early mitral inflow velocity to global diastolic strain rate as a useful predictor of cardiac outcomes in patients with atrial fibrillation. J. Am. Soc. Echocardiogr. 2014, 27, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Tossavainen, E.; Henein, M.Y.; Grönlund, C.; Lindqvist, P. Left Atrial Intrinsic Strain Rate Correcting for Pulmonary Wedge Pressure Is Accurate in Estimating Pulmonary Vascular Resistance in Breathless Patients. Echocardiography 2016, 33, 1156–1165. [Google Scholar] [CrossRef]
- Cameli, M.; Mandoli, G.E.; Loiacono, F.; Dini, F.L.; Henein, M.; Mondillo, S. Left atrial strain: A new parameter for assessment of left ventricular filling pressure. Heart Fail. Rev. 2016, 21, 65–76. [Google Scholar] [CrossRef]
- Guler, A.; Tigen, K.M.; Dundar, C.; Karaahmet, T.; Karabay, C.Y.; Aung, S.M.; Akgun, T.; Bulut, M.; Kirma, C. Left atrial deformation and nonischemic dilated cardiomyopathy: A 2D speckle-tracking imaging study. Herz. 2014, 39, 251–257. [Google Scholar] [CrossRef]
- Bytyçi, I.; Bajraktari, G.; Lindqvist, P.; Henein, M.Y. Compromised left atrial function and increased size predict raised cavity pressure: A systematic review and meta-analysis. Clin. Physiol. Funct. Imaging 2019, 39, 297–307. [Google Scholar] [CrossRef]
- D’Andrea, A.; Caso, P.; Romano, S.; Scarafile, R.; Cuomo, S.; Salerno, G.; Riegler,, L.; Limongelli, G.; Di Salvo, G.; Romano, M.; et al. Association between left atrial myocardial function and exercise capacity in patients with either idiopathic or ischemic dilated cardiomyopathy: A two-dimensional speckle strain study. Int. J. Cardiol. 2009, 132, 354–363. [Google Scholar] [CrossRef]
- Takeuchi, M.; Borden, W.B.; Nakai, H.; Nishikage, T.; Kokumai, M.; Nagakura, T.; Otani, S.; Lang, R.M. Reduced and delayed untwisting of the left ventricle in patients with hypertension and left ventricular hypertrophy: A study using two-dimensional speckle tracking imaging. Eur. Heart J. 2007, 28, 2756–2762. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.J.; Lim, H.S.; Choi, B.J.; Choi, S.Y.; Hwang, G.S.; Yoon, M.H.; Tahk, S.J.; Shin, J.H. Longitudinal Strain and Torsion Assessed by Two-Dimensional Speckle Tracking Correlate with the Serum Level of Tissue Inhibitor of Matrix Metalloproteinase-1, a Marker of Myocardial Fibrosis, in Patients with Hypertension. J. Am. Soc. Echocardiogr. 2008, 21, 907–911. [Google Scholar] [CrossRef]
- Park, S.J.; Miyazaki, C.; Bruce, C.J.; Ommen, S.; Miller, F.A.; Oh, J.K. Left Ventricular Torsion by Two-Dimensional Speckle Tracking Echocardiography in Patients with Diastolic Dysfunction and Normal Ejection Fraction. J. Am. Soc. Echocardiogr. 2008, 21, 1129–1137. [Google Scholar] [CrossRef]
- Takeuchi, M.; Otani, K.; Otsuji, Y.; Lang, R.M. Assessment of left ventricular torsion using speckle tracking echocardiography. Curr. Cardiovasc. Imaging Rep. 2009, 2, 356–362. [Google Scholar] [CrossRef]
- Yoon, Y.E.; Kim, S.; Chang, H.J. Artificial intelligence and echocardiography. J. Cardiovasc. Imaging 2021, 29, 193–204. [Google Scholar] [CrossRef]
- Baloch, Z.Q.; Raza, S.A.; Pathak, R.; Marone, L.; Ali, A. Machine learning confirms nonlinear relationship between severity of peripheral arterial disease, functional limitation and symptom severity. Diagnostics 2020, 10, 515. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.P.; Huang, Y.M.; Bansal, M.; Ashrafi, A.; Fisher, M.; Shameer, K.; Gall, W.; Dudley, J.T. Cognitive Machine-Learning Algorithm for Cardiac Imaging: Pilot Study for Differentiating Constrictive Pericarditis from Restrictive Cardiomyopathy. Circ. Cardiovasc. Imaging 2017, 9, 1–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, D.J.; Park, J.J.; Ali, T.; Lee, S. Artificial intelligence for the diagnosis of heart failure. NPJ Digit. Med. 2020, 3, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lancaster, M.C.; Omar, A.M.S.; Narula, S.; Kulkarni, H.; Narula, J.; Sengupta, P.P. Phenotypic Clustering of Left Ventricular Diastolic Function Parameters: Patterns and Prognostic Relevance. JACC Cardiovasc. Imaging 2019, 12, 1149–1161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gajjala, S.; Agrawal, P.; Tison, G.H.; Hallock, L.A.; Beussink-Nelson, L.; Lassen, M.H.; Fan, E.; Aras, M.A.; Jordan, C.R.; et al. Fully automated echocardiogram interpretation in clinical practice: Feasibility and diagnostic accuracy. Circulation 2018, 138, 1623–1635. [Google Scholar] [CrossRef]
- Stone, M. Cross-Validatory Choice and Assessment of Statistical Predictions. J. R. Stat. Soc. Ser. B 1974, 36, 111–133. [Google Scholar] [CrossRef]
- Venner, C.; Selton-Suty, C.; Huttin, O.; Erpelding, M.L.; Aliot, E.; Juillière, Y. Right ventricular dysfunction in patients with idiopathic dilated cardiomyopathy: Prognostic value and predictive factors. Arch. Cardiovasc. Dis. 2016, 109, 231–241. [Google Scholar] [CrossRef]
- Gulati, A.; Ismail, T.F.; Jabbour, A.; Alpendurada, F.; Guha, K.; Ismail, N.A.; Raza, S.; Khwaja, J.; Brown, T.D.H.; Morarji, K.; et al. The prevalence and prognostic significance of right ventricular systolic dysfunction in nonischemic dilated cardiomyopathy. Circulation 2013, 128, 1623–1633. [Google Scholar] [CrossRef] [Green Version]
- Zairi, I.; Mzoughi, K.; Jabeur, M.; Jnifene, Z.; Kamoun, S.; Fennira, S.; Kraiem, S. Valeur pronostique des paramètres de la fonction systolique du ventricule droit dans les cardiomyopathies dilatées (Right ventricular systolic echocardiographic parameters in dilated cardiomyopathy and prognosis). Tunis. Med. 2017, 95, 87–91. [Google Scholar]
- Kawata, T.; Daimon, M.; Kimura, K.; Nakao, T.; Lee, S.L.; Hirokawa, M.; Kato, T.S.; Watanabe, M.; Yatomi, Y.; Komuro, I. Echocardiographic assessment of right ventricular function in routine practice: Which parameters are useful to predict one-year outcome in advanced heart failure patients with dilated cardiomyopathy? J. Cardiol. 2017, 70, 316–322. [Google Scholar] [CrossRef] [Green Version]
- Merlo, M.; Gobbo, M.; Stolfo, D.; Losurdo, P.; Ramani, F.; Barbati, G.; Pivetta, A.; Lenarda, A.D.; Anzini, M.; Gigli, M.; et al. The Prognostic Impact of the Evolution of RV Function in Idiopathic DCM. JACC Cardiovasc. Imaging 2016, 9, 1034–1042. [Google Scholar] [CrossRef]
- Bistola, V.; Parissis, J.T.; Paraskevaidis, I.; Panou, F.; Nikolaou, M.; Ikonomidis, I.; Flessas, N.; Filippatos, G.; Iliodromitis, E.; Kremastinos, D.T. Prognostic Value of Tissue Doppler Right Ventricular Systolic and Diastolic Function Indexes Combined with Plasma B-Type Natriuretic Peptide in Patients with Advanced Heart Failure Secondary to Ischemic or Idiopathic Dilated Cardiomyopathy. Am. J. Cardiol. 2010, 105, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Kawata, T.; Daimon, M.; Kimura, K.; Nakao, T.; Lee, S.C.; Hirokawa, M.; Yoshinaga, A.; Watanabe, M.; Yatomi, Y.; et al. Prognostic value of a simple echocardiographic parameter, the right ventricular systolic to diastolic duration ratio, in patients with advanced heart failure with non-ischemic dilated cardiomyopathy. Int. Heart J. 2018, 59, 968–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Focardi, M.; Cameli, M.; Carbone, S.F.; Massoni, A.; Vito, R.D.; Lisi, M.; Mondillo, S. Traditional and innovative echocardiographic parameters for the analysis of right ventricular performance in comparison with cardiac magnetic resonance. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Suarez, D.F.; López-Candales, A. Strain Imaging Echocardiography: What Imaging Cardiologists Should Know. Curr. Cardiol. Rev. 2017, 13, 118–129. [Google Scholar] [CrossRef] [Green Version]
- Hirasawa, K.; van Rosendael, P.J.; Dietz, M.F.; Marsan, N.A.; Delgado, V.; Bax, J.J. Comparison of the Usefulness of Strain Imaging by Echocardiography Versus Computed Tomography to Detect Right Ventricular Systolic Dysfunction in Patients with Significant Secondary Tricuspid Regurgitation. Am. J. Cardiol. 2020, 134, 116–122. [Google Scholar] [CrossRef]
- Motoki, H.; Borowski, A.G.; Shrestha, K.; Hu, B.; Kusunose, K.; Troughton, R.W.; Tang, W.H.W.; Klein, A.L. Right ventricular global longitudinal strain provides prognostic value incremental to left ventricular ejection fraction in patients with heart failure. J. Am. Soc. Echocardiogr. 2014, 27, 726–732. [Google Scholar] [CrossRef]
- Iacoviello, M.; Citarelli, G.; Antoncecchi, V.; Romito, R.; Monitillo, F.; Leone, M.; Puzzovivo, A.; Lattarulo, M.S.; Rizzo, C.; Caldarola, P.; et al. Right Ventricular Longitudinal Strain Measures Independently Predict Chronic Heart Failure Mortality. Echocardiography 2016, 33, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Houard, L.; Benaets, M.B.; Ravenstein, C.M.; Rousseau, M.F.; Ahn, S.A.; Amzulescu, M.S.; Roy, C.; Slimani, A.; Vancraeynest, D.; Pasquet, A.; et al. Additional Prognostic Value of 2D Right Ventricular Speckle-Tracking Strain for Prediction of Survival in Heart Failure and Reduced Ejection Fraction: A Comparative Study With Cardiac Magnetic Resonance. JACC Cardiovasc. Imaging 2019, 12, 2373–2385. [Google Scholar] [CrossRef] [PubMed]
- Ishiwata, J.; Daimon, M.; Nakanishi, K.; Sugimoto, T.; Kawata, T.; Shinozaki, T.; Nakao, T.; Hirokawa, M.; Sawada, N.; Yoshida, Y.; et al. Combined evaluation of right ventricular function using echocardiography in non-ischaemic dilated cardiomyopathy. ESC Heart Fail. 2021, 8, 3947–3956. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, E.S.; Travers, C.; Border, W.L.; Deshpande, S.; Sachdeva, R. Tricuspid annular plane systolic excursion as a marker of right ventricular dysfunction in pediatric patients with dilated cardiomyopathy. Echocardiography 2017, 34, 102–107. [Google Scholar] [CrossRef]
- Gopal, A.S.; Chukwu, E.O.; Iwuchukwu, C.J.; Katz, A.S.; Toole, R.S.; Schapiro, W.; Reichek, N. Normal Values of Right Ventricular Size and Function by Real-time 3-Dimensional Echocardiography: Comparison with Cardiac Magnetic Resonance Imaging. J. Am. Soc. Echocardiogr. 2007, 20, 445–455. [Google Scholar] [CrossRef]
- Fusini, L.; Tamborini, G.; Gripari, P.; Maffessanti, F.; Mazzanti, V.; Muratori, M.; Salvi, L.; Sisillo, E.; Caiani, E.G.; Alamanni, F.; et al. Feasibility of intraoperative three-dimensional transesophageal echocardiography in the evaluation of right ventricular volumes and function in patients undergoing cardiac surgery. J. Am. Soc. Echocardiogr. 2011, 24, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Vîjîiac, A.; Onciul, S.; Guzu, C.; Verinceanu, V.; Bătăilă, V.; Deaconu, S.; Scărlătescu, A.; Zamfir, D.; Petre, I.; Onuţ, R.; et al. The prognostic value of right ventricular longitudinal strain and 3D ejection fraction in patients with dilated cardiomyopathy. Int. J. Cardiovasc. Imaging 2021, 37, 3233–3244. [Google Scholar] [CrossRef]
- Merlo, M.; Caiffa, T.; Gobbo, M.; Adamo, L.; Sinagra, G. Reverse remodeling in Dilated Cardiomyopathy: Insights and future perspectives. IJC Heart Vasc. 2018, 18, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Prinzen, F.W.; Vernooy, K.; Auricchio, A. Cardiac resynchronization therapy: State-of-the-art of current applications, guidelines, ongoing trials, and areas of controversy. Circulation 2013, 128, 2407–2418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, G.S.; Curry, C.W.; Wyman, B.T.; Kramer, A.; Declerck, J.; Talbot, M.; Douglas, M.R.; Berger, R.D.; McVeigh, E.R.; Kass, D.A. Predictors of systolic augmentation from left ventricular preexcitation in patients with dilated cardiomyopathy and intraventricular conduction delay. Circulation 2000, 101, 2703–2709. [Google Scholar] [CrossRef] [Green Version]
- Nesser, H.-J.; Winter, S. Speckle tracking in the evaluation of left ventricular dyssynchrony. Echocardiography 2009, 26, 324–336. [Google Scholar] [CrossRef]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef]
- Bleeker, G.B.; Mollema, S.A.; Holman, E.R.; Van De Veire, N.; Ypenburg, C.; Boersma, E.; van der Wall, E.E.; Schalij, M.J.; Bax, J.J. Left ventricular resynchronization is mandatory for response to cardiac resynchronization therapy: Analysis in patients with echocardiographic evidence of left ventricular dyssynchrony at baseline. Circulation 2007, 116, 1440–1448. [Google Scholar] [CrossRef] [Green Version]
- Sassone, B.; Capecchi, A.; Boggian, G.; Gabrieli, L.; Saccà, S.; Vandelli, R.; Petracci, E.; Mele, D. Value of baseline left lateral wall postsystolic displacement assessed by M-mode to predict reverse remodeling by cardiac resynchronization therapy. Am. J. Cardiol. 2007, 100, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-M.; Fung, J.W.-H.; Zhang, Q.; Chan, C.-K.; Chan, Y.-S.; Lin, H.; Kum, L.C.; Kong, S.-L.; Zhang, Y.; Sanderson, J.E.; et al. Tissue Doppler imaging is superior to strain rate imaging and postsystolic shortening on the prediction of reverse remodeling in both ischemic and nonischemic heart failure after cardiac resynchronization therapy. Circulation 2004, 110, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Helm, R.H.; Leclercq, C.; Faris, O.P.; Ozturk, C.; McVeigh, E.; Lardo, A.C.; Kass, D.A. Cardiac Dyssynchrony Analysis Using Circumferential versus Longitudinal Strain: Implications for Assessing Cardiac Resynchronization. Circulation 2005, 111, 2760. [Google Scholar] [CrossRef] [Green Version]
- Westenberg, J.J.; Lamb, H.J.; van der Geest, R.J.; Bleeker, G.B.; Holman, E.R.; Schalij, M.J.; de Roos, A.; van der Wall, E.E.; Reiber, J.H.; Bax, J.J. Assessment of left ventricular dyssynchrony in patients with conduction delay and idiopathic dilated cardiomyopathy: Head-to-head comparison between tissue doppler imaging and velocity-encoded magnetic resonance imaging. J. Am. Coll. Cardiol. 2006, 47, 2042–2048. [Google Scholar] [CrossRef] [PubMed]
- Szulik, M.; Tillekaerts, M.; Vangeel, V.; Ganame, J.; Willems, R.; Lenarczyk, R.; Rademakers, F.; Kalarus, Z.; Kukulski, T.; Voigt, J.-U. Assessment of apical rocking: A new, integrative approach for selection of candidates for cardiac resynchronization therapy. Eur. J. Echocardiogr. 2010, 11, 863–869. [Google Scholar] [CrossRef] [Green Version]
- Gurel, E.; Tigen, K.; Karaahmet, T.; Dundar, C.; Guler, A.; Basaran, Y. Apical transverse motion is associated with speckle-tracking radial dyssynchrony in patients with non-ischemic dilated cardiomyopathy. Anatol. J. Cardiol. 2015, 15, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Tani, T.; Sumida, T.; Tanabe, K.; Ehara, N.; Yamaguchi, K.; Kawai, J.; Yagi, T.; Morioka, S.; Fujiwara, H.; Okada, Y.; et al. Left ventricular systolic dyssynchrony index by three-dimensional echocardiography in patients with decreased left ventricular function: Comparison with tissue Doppler echocardiography. Echocardiography 2012, 29, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Marsan, N.A.; Bleeker, G.B.; Ypenburg, C.; Ghio, S.; Van De Veire, N.R.; Holman, E.R.; Van Der Wall, E.E.; Tavazzi, L.; Schalij, M.J.; Bax, J.J. Real-time three-dimensional echocardiography permits quantification of left ventricular mechanical dyssynchrony and predicts acute response to cardiac resynchronization therapy. J. Cardiovasc. Electrophysiol. 2008, 19, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Auger, D.; Bertini, M.; Marsan, N.A.; Hoke, U.; Ewe, S.H.; Thijssen, J.; Witkowski, T.; Yiu, K.-H.; Ng, A.; Van Der Wall, E.E.; et al. Prediction of response to cardiac resynchronization therapy combining two different three-dimensional analyses of left ventricular dyssynchrony. Am. J. Cardiol. 2011, 108, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Suffoletto, M.S.; Dohi, K.; Cannesson, M.; Saba, S.; Gorcsan, J. Novel speckle-tracking radial strain from routine black-and-white echocardiographic images to quantify dyssynchrony and predict response to cardiac resynchronization therapy. Circulation 2006, 113, 960–968. [Google Scholar] [CrossRef] [Green Version]
- Tatsumi, K.; Tanaka, H.; Tsuji, T.; Kaneko, A.; Ryo, K.; Yamawaki, K.; Omar, A.M.; Fukuda, Y.; Norisada, K.; Matsumoto, K.; et al. Strain dyssynchrony index determined by three-dimensional speckle area tracking can predict response to cardiac resynchronization therapy. Cardiovasc. Ultrasound 2011, 9, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celikyurt, U.; Acar, B.; Hidayet, S.; Karauzum, I.; Karauzum, K.; Vural, A.; Agacdiken, A. Systolic aortic root motion predicts response to cardiac resynchronization therapy. Pacing Clin. Electrophysiol. 2019, 42, 1471–1476. [Google Scholar] [CrossRef]
- Gallard, A.; Hubert, A.; Smiseth, O.; Voigt, J.-U.; Le Rolle, V.; Leclercq, C.; Bidaut, A.; Galli, E.; Donal, E.; Hernandez, A.I. Prediction of response to cardiac resynchronization therapy using a multi-feature learning method. Int. J. Cardiovasc. Imaging 2020, 37, 989–998. [Google Scholar] [CrossRef]
- Gallard, A.; Bidaut, A.; Hubert, A.; Sade, E.; Marechaux, S.; Sitges, M.; Separovic-Hanzevacki, J.; Rolle, V.L.; Galli, E.; Hernandez, A.; et al. Characterization of Responder Profiles for Cardiac Resynchronization Therapy through Unsupervised Clustering of Clinical and Strain Data. J. Am. Soc. Echocardiogr. 2021, 34, 483–493. [Google Scholar] [CrossRef]
- Lancellotti, P.; Tribouilloy, C.; Hagendorff, A.; Popescu, B.A.; Edvardsen, T.; Pierard, L.A.; Badano, L.; Zamorano, J.L. Recommendations for the echocardiographic assessment of native valvular regurgitation: An executive summary from the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 611–644. [Google Scholar] [CrossRef] [Green Version]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef]
- Ben Zekry, S.; Nagueh, S.F.; Little, S.H.; Quinones, M.A.; McCulloch, M.L.; Karanbir, S.; Herrera, E.L.; Lawrie, G.M.; Zoghbi, W.A. Comparative accuracy of two-and three-dimensional transthoracic and transesophageal echocardiography in identifying mitral valve pathology in patients undergoing mitral valve repair: Initial observations. J. Am. Soc. Echocardiogr. 2011, 24, 1079–1085. [Google Scholar] [CrossRef]
- Chen, C.G.; Thomas, J.D.; Anconina, J.; Harrigan, P.; Mueller, L.; Picard, M.H.; Levine, R.A.; Weyman, A.E. Impact of impinging wall jet on color Doppler quantification of mitral regurgitation. Circulation 1991, 84, 712–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Topilsky, Y.; Michelena, H.; Bichara, V.; Maalouf, J.; Mahoney, D.W.; Enriquez-Sarano, M. Mitral valve prolapse with mid-late systolic mitral regurgitation: Pitfalls of evaluation and clinical outcome compared with holosystolic regurgitation. Circulation 2012, 125, 1643–1651. [Google Scholar] [CrossRef] [Green Version]
- Matsumura, Y.; Fukuda, S.; Tran, H.; Greenberg, N.L.; Agler, D.A.; Wada, N.; Toyono, M.; Thomas, J.D.; Shiota, T. Geometry of the proximal isovelocity surface area in mitral regurgitation by 3-dimensional color Doppler echocardiography: Difference between functional mitral regurgitation and prolapse regurgitation. Am. Heart J. 2008, 155, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Tribouilloy, C.; Shen, W.F.; Rey, J.L.; Adam, M.C.; Lesbre, J.P. Mitral to aortic velocity-time integral ratio. A non-geometric pulsed-Doppler regurgitant index in isolated pure mitral regurgitation. Eur. Heart J. 1994, 15, 1335–1339. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Dini, F.L.; Faggiano, P.; Agricola, E.; Cicoira, M.; Frattini, S.; Simioniuc, A.; Gullace, M.; Ghio, S.; Enriquez-Sarano, M.; et al. Independent prognostic value of functional mitral regurgitation in patients with heart failure. A quantitative analysis of 1256 patients with ischaemic and non-ischaemic dilated cardiomyopathy. Heart 2011, 97, 1675–1680. [Google Scholar] [CrossRef]
- Zeng, X.; Levine, R.A.; Hua, L.; Morris, E.L.; Kang, Y.; Flaherty, M.; Morgan, N.V.; Hung, J. Diagnostic value of vena contracta area in the quantification of mitral regurgitation severity by color Doppler 3D echocardiography. Circ. Cardiovasc. Imaging 2011, 4, 506–513. [Google Scholar] [CrossRef] [Green Version]
- Golba, K.; Mokrzycki, K.; Drozdz, J.; Cherniavsky, A.; Wrobel, K.; Roberts, B.J.; Haddad, H.; Maurer, G.; Yii, M.; Asch, F.M.; et al. Mechanisms of functional mitral regurgitation in ischemic cardiomyopathy determined by transesophageal echocardiography (from the Surgical Treatment for Ischemic Heart Failure Trial). Am. J. Cardiol. 2013, 112, 1812–1818. [Google Scholar] [CrossRef] [Green Version]
- Hübscher, A.; Schwerg, M.; Hoffmann, S.; Baldenhofer, G.; Heupel, C.; Jasaityte, R.; Kruck, S.; Stangl, K.; Dreger, H.; Knebel, F. Automated quantification of mitral valve tenting volume in functional mitral regurgitation by three-dimensional echocardiography. Echocardiography 2020, 37, 1043–1048. [Google Scholar] [CrossRef]
- Sköldborg, V.; Madsen, P.L.; Dalsgaard, M.; Abdulla, J. Quantification of mitral valve regurgitation by 2D and 3D echocardiography compared with cardiac magnetic resonance a systematic review and meta-analysis. Int. J. Cardiovasc. Imaging 2020, 36, 279–289. [Google Scholar] [CrossRef]
- Hahn, R.T.; Thomas, J.D.; Khalique, O.K.; Cavalcante, J.L.; Praz, F.; Zoghbi, W.A. Imaging Assessment of Tricuspid Regurgitation Severity. JACC. Cardiovasc. Imaging 2019, 12, 469–490. [Google Scholar] [CrossRef]
- Surkova, E.; Muraru, D.; Iliceto, S.; Badano, L.P. The use of multimodality cardiovascular imaging to assess right ventricular size and function. Int. J. Cardiol. 2016, 214, 54–69. [Google Scholar] [CrossRef]
- Velayudhan, D.E.; Brown, T.M.; Nanda, N.C.; Patel, V.; Miller, A.P.; Mehmood, F.; Rajdev, S.; Fang, L.; Frans, E.E.; Vengala, S.; et al. Quantification of tricuspid regurgitation by live three-dimensional transthoracic echocardiographic measurements of vena contracta area. Echocardiography 2006, 23, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Fatima, H.; Mahmood, F.; Sehgal, S.; Belani, K.; Sharkey, A.; Chaudhary, O.; Baribeau, Y.; Matyal, R.; Khabbaz, K.R. Artificial Intelligence for Dynamic Echocardiographic Tricuspid Valve Analysis: A New Tool in Echocardiography. J. Cardiothorac. Vasc. Anesth. 2020, 34, 2703–2706. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, F.; Karthik, S.; Subramaniam, B.; Panzica, P.J.; Mitchell, J.; Lerner, A.B.; Jervis, K.; Maslow, A.D. Intraoperative application of geometric three-dimensional mitral valve assessment package: A feasibility study. J. Cardiothorac. Vasc. Anesth. 2008, 22, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Ferrans, V.J. Pathologic anatomy of the dilated cardiomyopathies. Am. J. Cardiol. 1989, 64, C9–C11. [Google Scholar] [CrossRef]
- Falk, R.H.; Foster, E.; Coats, M.H. Ventricular thrombi and thromboembolism in dilated cardiomyopathy: A prospective follow-up study. Am. Heart J. 1992, 123, 136–142. [Google Scholar] [CrossRef]
- Wilensky, R.L.; Jung, S.C. Thromboembolism in patients with decreased left ventricular function: Incidence, risk, and treatment. J. Cardiovasc. Risk 1995, 2, 91–96. [Google Scholar] [CrossRef]
- Di Odoardo, L.A.F.; Stefanini, G.G.; Vicenzi, M. Uncertainties about left ventricular thrombus after STEMI. Nat. Rev. Cardiology 2021, 18, 381–382. [Google Scholar] [CrossRef]
- Porter, T.R.; Mulvagh, S.L.; Abdelmoneim, S.S.; Becher, H.; Belcik, J.T.; Bierig, M.; Choy, J.; Gaibazzi, N.; Gillam, L.D.; Janardhanan, R.; et al. Clinical Applications of Ultrasonic Enhancing Agents in Echocardiography: 2018 American Society of Echocardiography Guidelines Update. J. Am. Soc. Echocardiogr. 2018, 31, 241–274. [Google Scholar] [CrossRef]
- Roifman, I.; Connelly, K.A.; Wright, G.A.; Wijeysundera, H.C. Echocardiography vs Cardiac Magnetic Resonance Imagingfor the Diagnosis of Left Ventricular Thrombus: A Systematic Review. Can. J. Cardiol. 2015, 31, 785–791. [Google Scholar] [CrossRef]
- Anwar, A.M.; Nosir, Y.F.M.; Ajam, A.; Chamsi-Pasha, H. Central role of real-time three-dimensional echocardiography in the assessment of intracardiac thrombi. Int. J. Cardiovasc. Imaging 2010, 26, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, A.; Mantovani, F.; Bursi, F.; Faggiano, A.; Boriani, G.; Faggiano, P. Optimal Use of Echocardiography in Management of Thrombosis after Anterior Myocardial Infarction. Echocardiography 2020, 37, 1287–1295. [Google Scholar] [CrossRef]
- Niemann, M.; Gaudron, P.D.; Bijnens, B.; Störk, S.; Beer, M.; Hillenbrand, H.; Cikes, M.; Herrmann, S.; Hu, K.; Ertl, G.; et al. Differentiation between fresh and old left ventricular thrombi by deformation imaging. Circ. Cardiovasc. Imaging 2012, 5, 667–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strzelecki, M.; Materka, A.; Drozdz, J.; Krzeminska-Pakula, M.; Kasprzak, J.D. Classification and segmentation of intracardiac masses in cardiac tumor echocardiograms. Comput. Med. Imaging Graph. 2006, 30, 95–107. [Google Scholar] [CrossRef]
- Bose, P.; Shankar, O.; Singh, B.; Bhola, R.; Singh, R. Histological changes of myocardium in dilated cardiomyopathy. J. Anat. Soc. India 2017, 66, 109–111. [Google Scholar] [CrossRef]
- Moreo, A.; Ambrosio, G.; Chiara, B.D.; Pu, M.; Tran, T.; Mauri, F.; Raman, S.V. Influence of Myocardial Fibrosis on Left Ventricular Diastolic Function. Circ. Cardiovasc. Imaging 2009, 2, 437–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arbustini, E.; Disertori, M.; Narula, J. Primary Prevention of Sudden Arrhythmic Death in Dilated Cardiomyopathy: Current Guidelines and Risk Stratification. JACC Heart Fail. 2017, 5, 39–43. [Google Scholar] [CrossRef]
- Hoyt, R.H.; Collins, S.M.; Skorton, D.J.; Ericksen, E.E.; Conyers, D. Assessment of fibrosis in infarcted human hearts by analysis of ultrasonic backscatter. Circulation 1985, 71, 740–744. [Google Scholar] [CrossRef] [Green Version]
- Naito, J.; Masuyama, T.; Mano, T.; Kondo, H.; Yamamoto, K.; Nagano, R.; Doi, Y.; Hori, M.; Kamada, T. Ultrasonic myocardial tissue characterization in patients with dilated cardiomyopathy: Value in noninvasive assessment of myocardial fibrosis. Am. Heart J. 1996, 131, 115–121. [Google Scholar] [CrossRef]
- Mizuno, R.; Fujimoto, S.; Saito, Y.; Nakamura, S. Non-invasive quantitation of myocardial fibrosis using combined tissue harmonic imaging and integrated backscatter analysis in dilated cardiomyopathy. Cardiology 2007, 108, 11–17. [Google Scholar] [CrossRef]
- Gaibazzi, N.; Tuttolomondo, D.; Guaricci, A.I.; di Giannuario, G. Pulse-Cancellation Echocardiography for Clinical Evaluation of Myocardial Scar Burden. Curr. Cardiol. Rep. 2021, 23, 1–7. [Google Scholar] [CrossRef]
- Montant, P.; Chenot, F.; Goffinet, C.; Poncelet, A.; Vancraeynest, D.; Pasquet, A.; Gerber, B.L.; Vanoverschelde, J.L.J. Detection and quantification of myocardial scars by contrast-enhanced 3D echocardiography. Circ. Cardiovasc. Imaging 2010, 3, 415–423. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhang, Y.; Zhang, L.; Tian, F.; Wang, B.; Xie, Y.; Sun, W.; Sun, Z.; Yang, Y.; Lv, Q.; et al. Assessment of Myocardial Fibrosis Using Two-Dimensional and Three-Dimensional Speckle Tracking Echocardiography in Dilated Cardiomyopathy With Advanced Heart Failure. J. Card. Fail. 2021, 27, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Gaibazzi, N.; Bianconcini, M.; Marziliano, N.; Parrini, I.; Conte, M.R.; Siniscalchi, C.; Faden, G.; Faggiano, P.; Pigazzani, F.; Grassi, F.; et al. Scar Detection by Pulse-Cancellation Echocardiography: Validation by CMR in Patients with Recent STEMI. JACC Cardiovasc. Imaging 2016, 9, 1239–1251. [Google Scholar] [CrossRef] [PubMed]
- Faggiano, A.; Santangelo, G.; Carugo, S.; Pressman, G.; Picano, E.; Faggiano, P. Cardiovascular Calcification as a Marker of Increased Cardiovascular Risk and a Surrogate for Subclinical Atherosclerosis: Role of Echocardiography. J. Clin. Med. 2021, 10, 1668. [Google Scholar] [CrossRef] [PubMed]
- Gaibazzi, N.; Suma, S.; Lorenzoni, V.; Sartorio, D.; Pressman, G.; Siniscalchi, C.; Garibaldi, S. Myocardial Scar by Pulse-Cancellation Echocardiography Is Independently Associated with Appropriate Defibrillator Intervention for Primary Prevention after Myocardial Infarction. J. Am. Soc. Echocardiogr. 2020, 33, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Kagiyama, N.; Shrestha, S.; Cho, J.S.; Khalil, M.; Singh, Y.; Challa, A.; Casaclang-Verzosa, G.; Sengupta, P.P. A low-cost texture-based pipeline for predicting myocardial tissue remodeling and fibrosis using cardiac ultrasound: Texture-based myocardial tissue characterization using cardiac ultrasound. EBioMedicine 2020, 54, 102726. [Google Scholar] [CrossRef]
| Assessing Left Ventricular Dimensions, Geometry and Systolic Function | |||||
|---|---|---|---|---|---|
| Standard Echocardiographic Techniques | Emerging Echocardiographic Techniques | ||||
| Technique Name and Related Parameters | Limitations | New Recent Findings | Technique Name and Related Parameters | Potential Benefits | Current Key Studies in DCM |
| 2D Transthoracic Echocardiography | Conditioned by foreshortening of apex Based on geometrical assumptions; Risk of endocardial dropout; Conditioned by shape distortions. | Corrects for shape distortions; Less geometrical assumptions compared with linear dimensions [8]. | Non-invasive LV pressure-strain loop and GWI | Powerful and independent predictor of outcome; Deepens the relationships between LV remodeling and increased after-load; Better predicts LV fibrosis; Useful to assess therapeutic response. | Prospective Study [36] |
| 3D Transthoracic Echocardiography | Lower temporal resolution; Lacking data on normal values; Image-quality dependent. | No geometrical assumption Unaffected by foreshortening More accurate and reproducible compared to other imaging modalities Predictive of CRT response [32]. | Reverse remodeling index | It is an independent predictor of all-cause mortality and heart transplantation | Prospective Study [44] |
| Post-systolic shortening and Early systolic lengthening | It can predict adverse cardiac outcomes. | Retrospective Study [48] | |||
| GLS | Lacking set of normal values; High endor-dependent. | Angle independent High prognostic value Predict major arrhythmic events independently from EF [22]. | MVC tissue longitudinal elongation | It can predict LV remodeling sphericity leading to new diagnostic tools. | Retrospective Study [57] |
| Artificial intelligence | Reduce observer variability, providing more consistent and reproducible data; Allow big data analysis, predicting future data; Time and cost saving. | Retrospective Study [63] | |||
| Diastolic Function Assessment | |||||
|---|---|---|---|---|---|
| Standard Echocardiographic Techniques | Emerging Echocardiographic Techniques | ||||
| Technique Name and Related Parameters | Limitations | New Recent Findings | Technique Name and Related Parameters | Potential Benefits | Current Key Studies in DCM |
| PW Doppler(E/A ratio) | U-shaped relation with LV diastolic function; Preload dependent. | Easy to obtain and interpret in most cases; Strong predictor of mortality in DCM, independently from EF and age. | Ventricular 2D-Speckle Tracking (Ds, DSr) | Better predictor of LV filling pressure; SRe: predictor of response to therapy in DCM. | Prospective Study [77] |
| Tissue Doppler Imaging (E/E’) | Highly angle dependent; Presence of a grey zone. | Preload independent; Correlation with heart catheterization Tau; Applicable in several diseases [70]. | Atrial 2D- Speckle Tracking | Easy to perform; possibility of off-line processing; Practical for serial follow-up; Angle independent; PALS associated with functional capacity during exercise in DCM. | Prospective Study [88] |
| 2D echocardiography (LAVi) | Elevated volume index in several other conditions: AF, atrial flutter, mitral valve diseases, high-output states (e.g., anemia). | Efficiently reflects cumulative effects of LV filling pressure [68]. | Artificial Intelligence and Machine Learning | Improves diagnostic accuracy, reducing indeterminate classification Enhances prognostication Opens to novel parameters. | Retrospective Study [88] |
| Right Ventricular Disfunction Evaluation | |||||
|---|---|---|---|---|---|
| Standard Echocardiographic Techniques | Emerging Echocardiographic Techniques | ||||
| Technique Name and Related Parameters | Limitations | New Recent Findings | Technique Name and Related Parameters | Potential Benefits | Current Key Studies in DCM |
| Fractional Area Change (FAC) | Challenging in case of suboptimal image quality of RV free wall; Only acceptable inter-observer reproducibility; Neglects the contribution of RV outflow tract. | RV-FAC provide better prognostic information than TAPSE or S’ in DCM and has been shown to strongly correlate with CMR [97]. | RV S/D ratio at CW Doppler | Easy to perform; Prognostic value in advanced HF with DCM. | Prospective Study [99] |
| TAPSE | Angle-dependent; Influenced by regional wall-motion abnormalities. | It is an accurate marker of RV dysfunction in pediatric patients with DCM [107]. | 2D RV Speckle tracking | Angle and load-independent; Good correlation with RVEF at CMR; Better mortality predictor than other echocardiographic or CMR-parameters. | Prospective Study [105] Retrospective Study [106] |
| Tissue Doppler Imaging (S’) | Angle-dependent; Not representative of RV global function after thoracotomy, pulmonary thromboendarterectomy or heart transplantation. | S’ combined with increased plasma BNP additively predict adverse cardiac outcomes in DCM [98]. | 3D echocardiography | Includes RV outflow tract; correlates well with EF by CMR; Independent prognostic value in DCM; Practical for serial follow-up. | Prospective Study [110] |
| Left Ventricular Dyssynchrony Assessment | |||||
|---|---|---|---|---|---|
| Standard Echocardiographic Techniques | Emerging Echocardiographic Techniques | ||||
| Technique Name and Related Parameters | Limitations | New Recent Findings | Technique Name and Related Parameters | Potential Benefits | Current Key Studies in DCM |
| Standard 2D and M-Mode echocardiographic detection of LVD | Lack of correlation with CMR; Modest specificity and sensitivity; Unable to analyse the various components of the cardiac contraction movement; Conditioned by translation and tethering effects. | Time differences between early septal and delayed displacement of posterolateral wall on M-mode images improve the predictive ability for CRT responses [117]. | Apical Trasverse Motion by Tissue Doppler Imaging | Proved superior over conventional techniques to define LVD; Precise assessment of radial dyssynchrony. | Cross-Sectional Study [122] |
| 3D Echocardiography | Good ability in identifying mechanical delays in myocardial walls; Possibility of obtaining data on longitudinal, radial and circumferential timing of the myocardial segments. | Prospective Study [123] | |||
| Tissue Doppler Imaging | Extensively angle-dependent; Prone to noise and artifacts; Inability to distinguish between active and passive movements. | Good correlation with velocity-encoded cardiac CMR; Good predictive value for LVRR in patients undergoing CRT [118]. | 3D GLS Speckle Tracking | Reflect true regional mechanics Allows the coupling of 3D area strain with both 3D longitudinal and circumferential strain; More sensitive to changes in myocardial function. | Prospective Study [127] |
| Systolic Aortic Root Motion | Easily obtained by M-Mode echocardiography; Good ability to predict non-response to CRT. | Retrospective Study [128] | |||
| 2D GLS Speckle Tracking | Angle-dependent; Dependent on frame rate, image resolution and noise; Risk of oversimplifying the complexity of LVD. | High sensitivity in identifying long-term responses to CRT [126]. | AI technology | Combines LVD echocardiographic, electrocardiographic and clinical parameters to improve the predicting value of imaging approaches for the response to CRT. | Retrospective Study [130] |
| Secondary Mitral and Tricuspid Regurgitation Evaluation | |||||
|---|---|---|---|---|---|
| Standard Echocardiographic Techniques | Emerging Echocardiographic Techniques | ||||
| Technique Name and Related Parameters | Limitations | New Recent Findings | Technique Name and Related Parameters | Potential Benefits | Current Key Studies in DCM |
| Standard 2D Transthoracic and Trans-oesophageal Echocardiography with Color Doppler | Highly influenced by settings, hemodynamic conditions, dynamic changes in the orifice area and mechanism of mitral and tricuspid regurgitation; Need for cumbersome manual measurements in which small errors result in significant inaccuracies; Risk of overestimation of valvular defects; Corrected quantification of the severity in less than two-thirds of cases. | For proximal flow convergence: Rapid qualitative assessment; Absence of PISA is usually a sign of mild regurgitation; For VC; Surrogate for regurgitant orifice size; Independent of flow rate; Can be applied to eccentric jets; Less dependent on technical factors; >For jet area; Easy to measure [132]. | Real-Time 3D Transthoracic and Trans-oesophageal Echocardiography with color Doppler | Faithful reconstruction of the valve anatomy; More accurate measurements of quantitative and semiquantitative parameters; Good reproducibility with CMR findings Availability of automated approaches more accurate and reproducible than spectral Doppler velocity profiles and 2D areas; Higher accuracy to identify severe regurgitation than CMR. | Metanalysis: [142] |
| AI technology | Integration of 2D and 3D echocardiographic parameters with automated quantification of disease severity; Reduced time to analyze cardiac structures and good reproducibility with minimal user intervention. | Review [146] | |||
| Identifying Left Ventricular Thrombus | |||||
|---|---|---|---|---|---|
| Standard Echocardiographic Techniques | Emerging Echocardiographic Techniques | ||||
| Technique Name and Related Parameters | Limitations | New Recent Findings | Technique Name and Related Parameters | Potential Benefits | Current Key Studies in DCM |
| Standard 2D Transthoracic Echocardiography | Misses up to two-thirds of thrombi → very low sensitivity. | The majority (56%) of LVT in DCM are diagnosed by Standard Transthoracic Echocardiography [155]. | Real-Time 3D Echocardiography | Identification of the attachment point of the thrombus to the cardiac wall; Delineation of the changes in thrombi structure (e.g., calcification, degeneration); More accurate assessment of thrombus mobility; Accurate calculation of thrombus volume. | Cross-Sectional [154] |
| Contrast-Enhanced 2D Echocardiography | Need for ultrasound contrast agents; Time consuming; Not able to evaluate the changes in thrombi structure. | Contrast-Enhanced echocardiography o nearly doubled sensitivity and yielded improved accuracy versus non-contrast echo [152] | Strain-Rate by Tissue Doppler Imaging | Allows to differentiate between fresh (range: 5–27 days) and old (4–26 months) thrombi; Allows to obtain an approximate calculation of the thrombus stiffness. | Missing specific data in non-ischemic DCM → studies are needed |
| AI technology | Already applied to recognize intracardiac masses (e.g., left atrium thrombosis, cardiac tumors and vegetation) → it is realistic to expect AI to be a near-future application for detect LVT | Missing specific data in non-ischemic DCM → studies are needed | |||
| Assessing Myocardial Scars and Fibrosis | |||||
|---|---|---|---|---|---|
| Standard Echocardiographic Techniques | Emerging Echocardiographic Techniques | ||||
| Technique Name and Related Parameters | Limitations | New Recent Findings | Technique Name and Related Parameters | Potential Benefits | Current Key Studies in DCM |
| THI-Calibrated Myocardial Integrated Backscatter 2D Echocardiography | Lack of correlation with CMR; Lack of correlation with histopathology findings; Failed though to demonstrate feasibility in the real-life clinical practice. | It is the only echocardiography technique proven to evaluate quantitatively myocardial fibrosis specifically in DCM patients [163]. | Contrast-Enhanced 3D Echocardiography | Strong correlation with CMR; Available and cheaper than CMR; Rapid learning curve. | Missing specific data in non-ischemic DCM → studies are needed |
| 3D GLS Speckle Tracking | Strong correlation with histopathology findings; Good intra/inter-observer reproducibility; Practical for serial follow-up. | Cross-Sectional Study [166] | |||
| Pulse Cancellation Ultrasound [eSCAR] | Standard 2D phase array probe with contrast opacification preset (power-modulation/pulse inversion harmonic imaging; transmit 1.6 MHz/receive 3.2 MHz) without the need for contrast administration; Strong correlation with CMR Prognostic value for ICD appropriate discharge; Bedside, fast and easy, perfect for screening. | Missing specific data in non-ischemic DCM → studies are needed | |||
| Radiomics-Based Texture Analysis | Simple and cheap application of AI to standard echocardiography software; Strong correlation with CMR Vendor-independent; Good interobserver agreement. | Cross-Sectional Study [170] | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faggiano, A.; Avallone, C.; Gentile, D.; Provenzale, G.; Toriello, F.; Merlo, M.; Sinagra, G.; Carugo, S. Echocardiographic Advances in Dilated Cardiomyopathy. J. Clin. Med. 2021, 10, 5518. https://doi.org/10.3390/jcm10235518
Faggiano A, Avallone C, Gentile D, Provenzale G, Toriello F, Merlo M, Sinagra G, Carugo S. Echocardiographic Advances in Dilated Cardiomyopathy. Journal of Clinical Medicine. 2021; 10(23):5518. https://doi.org/10.3390/jcm10235518
Chicago/Turabian StyleFaggiano, Andrea, Carlo Avallone, Domitilla Gentile, Giovanni Provenzale, Filippo Toriello, Marco Merlo, Gianfranco Sinagra, and Stefano Carugo. 2021. "Echocardiographic Advances in Dilated Cardiomyopathy" Journal of Clinical Medicine 10, no. 23: 5518. https://doi.org/10.3390/jcm10235518







