Risk Assessment of the Increased Occurrence of Congenital Cardiac and Non-Cardiac Defects in Fetuses with a Normal Karyotype after Assisted Fertilization in Comparison to Natural Fertilization Based on Ultrasound Diagnostics
Abstract
1. Introduction
2. Experimental Section
2.1. Patient Characteristics
2.2. Prenatal Diagnosis Performed in the First Trimester of Pregnancy
2.3. Statistical Analysis
3. Results
3.1. Analysis of the Ultrasound Examination of the Fetus with and without Detected Heart Defects among Women Who Became Pregnant without the Use of Assisted Reproduction Methods
3.2. Analysis of the Ultrasound Examination of the Fetus with or without a Detected Congenital Heart Defect amongst Patients Who Became Pregnant after the Induction of Ovulation Taking into Account the Method of Fertilization
3.3. Analysis of the Ultrasound Examination of the Fetus with or without a Detected Congenital Heart Defect amongst Patients Who Became Pregnant as a Result of In-Vitro Fertilization
3.4. Analysis of the Ultrasound Examination of the Fetus with a Diagnosed Non-Heart Developmental Defect or after Its Exclusion among Women Who Became Pregnant without Using Assisted Reproduction Techniques
3.5. Analysis of the Ultrasound Examination of the Fetus with or without a Detected Congenital Non-Heart Defect among Patients Who Became Pregnant as a Result of Extracorporeal In-Vitro Fertilization
3.6. The Relationship between the Risk of Congenital Heart Defects Occurring in Fetuses Depending on the Used Ovulation Method before Fertilization
3.7. The Relationship between the Risk of Congenital Non-Heart Defects Occurring in Fetuses Depending on the Used Ovulation Method before Fertilization
3.8. The Risk of Congenital Heart Defects Occurring in a Fetus Depending on the Fertilization Technique
3.9. The Relationship between the Risk of Congenital Non-Heart Defects Occurring in a Fetus Depending on the Fertilization Technique
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ilioi, E.C.; Golombok, S. Psychological adjustment in adolescents conceived by assisted reproduction techniques: A systematic review. Hum. Reprod. Update 2014, 21, 84–96. [Google Scholar] [CrossRef]
- Łukaszuk, K.; Kozioł, K.; Jakiel, G.; Jakimiuk, A.; Jędrzejczak, P.; Kuczyński, W.; Kurzawa, R.; Pawelczyk, L.; Radwan, M.; Spaczyński, R.; et al. Diagnostyka i leczenie niepłodności-rekomendacje Polskiego Towarzystwa Medycyny Rozrodu i Embriologii (PTMRiE) oraz Polskiego Towarzystwa Ginekologów i Położników (PTGP). Ginekologia i Perinatologia Praktyczna 2018, 3, 112–140. [Google Scholar]
- Wielgoś, M.; Bomba-Opoń, D.; Bręborowicz, G.H.; Czajkowski, K.; Dębski, R.; Leszczyńska-Gorzelak, B.; Oszukowski, P.; Radowicki, S.; Zimmer, M. Rekomendacje Polskiego Towarzystwa Ginekologów i Położników dotyczące cięcia cesarskiego. Ginekologia i Perinatologia Praktyczna 2018, 3, 159–174. [Google Scholar]
- Sinha, P.; Pandey, K.; Srivastava, A. Factors determining successful intrauterine insemination. Int. J. Reprod. Contracept. Obstet. Gynecol. 2017, 6, 3887–3891. [Google Scholar] [CrossRef]
- Crawford, G.E.; Ledger, W.L. In vitro fertilisation/intracytoplasmic sperm injection beyond 2020. BJOG Int. J. Obstet. Gynaecol. 2018, 126, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Sicchieri, F.; Silva, A.B.; e Silva, A.C.J.S.R.; Navarro, P.A.A.S.; Ferriani, R.A.; dos Reis, R.M. Prognostic factors in intrauterine insemination cycles. JBRA Assist. Reprod. 2018, 22, 2–7. [Google Scholar] [CrossRef]
- Liu, J.; Li, T.C.; Wang, J.; Wang, W.; Hou, Z.; Liu, J. The impact of ovarian stimulation on the outcome of intrauterine insemination treatment: An analysis of 8893 cycles. BJOG Int. J. Obstet. Gynaecol. 2016, 123, 70–75. [Google Scholar] [CrossRef]
- Honda, T.; Tsutsumi, M.; Komoda, F.; Tatsumi, K. Acceptable pregnancy rate of unstimulated intrauterine insemination: A retrospective analysis of 17,830 cycles. Reprod. Med. Biol. 2014, 14, 27–32. [Google Scholar] [CrossRef]
- Katz, D.J.; Teloken, P.; Ohad, S. Male infertility—The other side of the equation. Aust. Fam. Physician 2017, 46, 641–646. [Google Scholar]
- Dhillon, R.K.; McLernon, D.J.; Smith, P.P.; Fishel, S.; Dowell, K.; Deeks, J.J.; Bhattacharya, S.; Coomarasamy, A. Predicting the chance of live birth for women undergoing IVF: A novel pretreatment counselling tool. Hum. Reprod. 2016, 31, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, D.M.; Barbosa, M.A.P.; Ferriani, R.A.; Navarro, P.A.; Raine-Fenning, N.; Nastri, C.O.; Martins, W.P. Regular (ICSI) versus ultra-high magnification (IMSI) sperm selection for assisted reproduction. Cochrane Database Syst. Rev. 2020, 2, 1465–1858. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Xun, Y.; Hu, S.; Lai, Q.; Jin, L. GnRH antagonist versus follicular-phase single-dose GnRH agonist protocol in patients of normal ovarian responses during controlled ovarian stimulation. Gynecol. Endocrinol. 2019, 35, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Alper, M.M.; Fauser, B.C. Ovarian stimulation protocols for IVF: Is more better than less? Reprod. Biomed. Online 2017, 34, 345–353. [Google Scholar] [CrossRef]
- Roztocka, A. Vaginal bleeding during the first 20 weeks of pregnancy and its impact on adverse perinatal outcome. Arch. Perinat. Med. 2016, 22, 7–16. [Google Scholar]
- Carloson, L.M.; Vora, N.L. Prenatal Diagnosis Screening and Diagnostic Tools. Clin. N. Am. 2017, 44, 245–256. [Google Scholar]
- Farhud, D.; Pourkalhor, H. Prenatal diagnostic methods. Med. Lab. Diagn. 2019, 11, 25–35. [Google Scholar]
- Kapersky, E.; Wierzba, J.; Limon, J.; Latos-Bieleńska, A.; Czauderna, P. Epidemiologia wrodzonych wad rozwojowych zarejestrowanych w województwie pomorskim w latach 2003–2005. Ann. Acad. Med. Gedanensis 2008, 38, 25–35. [Google Scholar]
- Pietryga, M.; Borowski, D.; Brązert, J.; Cnota, W.; Czekierdowski, A.; Czuba, B.; Dubiel, M.; Iciek, R.; Kaczmarek, P.; Oszukowski, P.; et al. Rekomendacje sekcji ultrasonografii polskiego towarzystwa ginekologicznego w zakresie przesiewowej diagnostyki ultrasonograficznej w ciąży o przebiegu prawidłowym w 2015r. Ginekol. Pol. 2015, 83, 309–315. [Google Scholar]
- Borowski, D.; Pietryga, M.; Basta, P.; Cnota, W.; Czuba, B.; Dubiel, M. Rekomendacje sekcji ultrasonografii polskiego towarzystwa ginekologów i położników w zakresie przesiewowej diagnostyki ultrasonograficznej w ciąży o przebiegu prawidłowym—2020 rok. Ginekologia i Perinatologia Praktyczna 2020, 5, 63–75. [Google Scholar]
- Giorgione, V.; Parazzini, F.; Fesslova, V.; Cipriani, S.; Candiani, M.; Inversetti, A.; Sigismondi, C.; Tibero, P.; Cavoretto, P. Congenital heart defects in IVF/ICSI pregnancy: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2018, 51, 33–42. [Google Scholar] [CrossRef]
- Mussa, A.; Molinatto, C.; Cerrato, F.; Palumbo, O.; Carella, M.; Baldassarre, G.; Carli, D.; Peris, C.; Riccio, A.; Ferrero, G.B. Assisted reproductive techniques and risk of Beckwith-Wiedemann syndrome. Pediatrics 2017, 140, e20164311. [Google Scholar] [CrossRef] [PubMed]
- Berntsen, S.; Söderström-Anttila, V.; Wennerholm, U.B.; Laivuori, H.; Lof, A.; Oldereid, N.B.; Romundstad, L.B.; Bergh, C.; Pinborg, A. The health of children conceived by ART: ‘the chicken or the egg? ’ Hum. Reprod. Update 2019, 25, 137–158. [Google Scholar] [CrossRef] [PubMed]
- Lui, G.K.; Rogers, I.S.; Ding, V.Y.; Hedlin, H.K.; Macmillen, K.; Maron, D.J.; Sillman, C.; Romfh, A.; Dade, T.C.; Haeffele, C.; et al. Risk estimates for atherosclerotic cardiovascular disease in adults with congenital heart disease. Am. J. Cardiol. 2017, 119, 112–118. [Google Scholar] [CrossRef]
- Jay, P.Y.; Akhirome, E.; Magnan, R.A.; Zhang, M.R.; Kang, L.; Qin, Y.; Ugwu, N.; Regmi, S.D.; Nogee, J.M.; Cheverud, J.M. Transgenerational cardiology: One way to a baby’s heart is through the mother. Mol. Cell. Endocrinol. 2016, 435, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Van Velzen, C.L.; Clur, S.A.; Rijlaarsdam, M.E.B.; Bax, C.J.; Pajkrt, E.; Heymans, M.W.; Bekker, M.N.; Hruda, J.; de Groot, C.J.M.; Blom, N.A.; et al. Prenatal detection of congenital heart disease-results of a national screening programme. BJOG Int. J. Obstet. Gynaecol. 2015, 123, 400–407. [Google Scholar] [CrossRef]
- Gil, M.M.; Galeva, S.; Jani, J.C.; Konstantinidou, L.; Akolekar, R.; Plana, M.N.; Nicolaides, K.H. Screening for trisomies by cfDNA testing of maternal blood in twin pregnancy: Update of The Fetal Medicine Foundation results and meta-analysis. Ultrasound Obstet. Gynecol. 2019, 53, 734–742. [Google Scholar]
- Ramaekers, P.; Mannaerts, D.; Jacquemyn, Y. Re: Prenatal detection of congenital heart diseases-results of a national screening programme. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 1420–1421. [Google Scholar] [CrossRef]
- Van Velzen, C.L.; Ket, J.C.F.; van de Ven, P.M.; Blom, N.A.; Haak, M.C. Systematic review and meta-analysis of the performance of second-trimester screening for prenatal detection of congenital heart defects. Int. J. Gynaecol. Obstet. 2018, 140, 137–145. [Google Scholar] [CrossRef]
- Meller, C.H.; Grinenco, S.; Aiello, H.; Córdoba, A.; Sáenz-Tejeira, M.M.; Marantz, P.; Otaño, L. Congenital heart disease, prenatal diagnosis and management. Arch. Argent. Pediatr. 2020, 118, e149–e161. [Google Scholar]
- Chen, L.; Zhu, L.; Cai, C.; Yan, G.; Sun, H. Clinical and neonatal outcomes of intrauterine insemination with frozen donor sperm. Syst. Biol. Reprod. Med. 2018, 64, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Iwashima, S.; Ishikawa, T.; Itoh, H. Reproductive technologies and the risk of congenital heart defects. Hum. Fertil. 2015, 20, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Hautala, J.; Gissler, M.; Ritvanen, A.; Tekay, A.; Pitkänen-Argillander, O.; Stefanovic, V.; Sarkola, T.; Helle, E.; Pihkala, J.; Pätilä, T.; et al. The implementation of a nationwide anomaly screening programme improves prenatal detection of major cardiac defects: An 11-year national population-based cohort study. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.W.; Miao, Q.; Taljaard, M.; Lougheed, J.; Gaudet, L.; Davies, M.; Lanes, A.; Leader, A.; Corsi, D.J.; Sprague, A.E.; et al. Associations of assisted reproductive technology and twin pregnancy with risk of congenital heart defects. JAMA Pediatr. 2020, 174, 446–454. [Google Scholar] [CrossRef]
- Sene, A.A.; Ghorbani, S.; Ashrafi, M. Comparison of the pregnancy outcomes and the incidence of fetal congenital abnormalities in infertile women with letrozole and clomiphene citrate. J. Obstet. Gynaecol. Res. 2018, 44, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, T.; Jwa, S.C.; Kuwahara, A.; Irahara, M.; Kubota, T.; Saito, H. No increased risk of major congenital anomalies or adverse pregnancy or neonatal outcomes following letrozole use in assisted reproductive technology. Hum. Reprod. 2016, 32, 125–132. [Google Scholar] [CrossRef][Green Version]
- Jelliffe-Pawlowski, L.L.; Norton, M.E.; Shaw, G.M.; Baer, R.J.; Flessel, M.C.; Goldman, S.; Currier, R. Risk of critical congenital heart defects by nuchal translucency norms. Am. J. Obstet. Gynecol. 2015, 212, 518.e1–518.e10. [Google Scholar] [CrossRef]
- Borelli, M.; Baer, R.J.; Chambers, C.D.; Smith, T.C.; Jelliffe-Pawloski, L. Critical congenital heart defects and abnormal levels of routinely collected first- and second-trimester biomarkers. Am. J. Obstet. Gynecol. 2016, 173, 368–374. [Google Scholar] [CrossRef]
- Alanen, J.; Leskinen, M.; Sairanen, M.; Korpimaki, T.; Kouru, H.; Gissler, M.; Ryynanen, M.; Nevalainen, J. Fetal nuchal translucency in severe congenital heart defects: Experiences in Northern Finland. J. Matern. Fetal Neonatal Med. 2017, 32, 1454–1460. [Google Scholar] [CrossRef]
- De Mooij, Y.M.; van den Akker, N.M.S.; Bekker, M.N.; Bartelings, M.M.; van Vugt, J.M.G.; Gittenberger-de Groot, A.C. Aberrant lymphatic development in euploid fetuses with increased nuchal translucency including Noonan syndrome. Prenat. Diagn. 2011, 31, 159–166. [Google Scholar] [CrossRef]
- Etchegaray, A.; Juarez-Peñalva, S.; Petracchi, F.; Igarzabal, L. Prenatal genetic considerations in congenital ventriculomegaly and hydrocephalus. Child’s Nerv. Syst. 2020, 36, 1645–1660. [Google Scholar] [CrossRef]
- Jouannic, J.-M.; Thieulin, A.-C.; Bonnet, D.; Houyel, L.; Lelong, N.; Goffinet, F.; Khoshnood, B. Measurement of nuchal translucency for prenatal screening of congenital heart defects: A population-based evaluation. Prenat. Diagn. 2011, 31, 1264–1269. [Google Scholar] [CrossRef]
- Curado, J.; Bhide, A. The use of ultrasound in the antenatal diagnosis of structural abnormalities. Obstet. Gynaecol. Reprod. Med. 2018, 28, 301–307. [Google Scholar] [CrossRef]
- Donofrio, M.T.; Moon-Grady, A.J.; Hornberger, L.K.; Copel, J.A.; Sklansky, M.S.; Abuhamad, A.; Cuneo, B.F.; Huhta, J.C.; Jonas, R.A.; Krishnan, A.; et al. Diagnosis and treatment of fetal cardiac disease: A scientific statement from the American Heart Association. Circulation 2014, 129, 2183–2242. [Google Scholar] [CrossRef] [PubMed]
- Burger, N.B.; Bekker, M.N.; de Groot, C.J.M.; Christoffels, V.M.; Hakk, M.C. Why increased nuchal translucency is associated with congenital heart disease: A systematic review on genetic mechanisms. Prenat. Diagn. 2015, 35, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Moaddab, A.; Tonni, G.; Grisolia, G.; Bonasoni, M.P.; Júnior, E.A.; Rolo, L.C.; Prefumo, F.; de la Fuente, S.; Sepulveda, W.; Ayres, N.; et al. Predicting outcome in 259 fetuses with agenesis of ductus venosus-a multicenter experience and systematic review of the literature. J. Matern. Fetal Neonatal Med. 2016, 29, 3606–3614. [Google Scholar] [CrossRef]
- Seravalli, V.; Miller, J.L.; Block-Abraham, D.; Baschat, A.A. Ductus venosus Doppler in the assessment of fetal cardiovascular health: An updated practical approach. Acta Obstet. Gynecol. Scand. 2016, 95, 635–644. [Google Scholar] [CrossRef]
- Baran, Ş.Y.; Kalaycı, H.; Durdağ, G.D.; Yetkinel, S.; Arslan, A.; Kılıçdağ, E.B. Does abnormal ductus venosus pulsatility index at the first-trimester effect on adverse pregnancy outcomes? J. Gyncol. Obstet. Hum. Reprod. 2020, 49, 101851. [Google Scholar] [CrossRef]
- Kalayci, H.; Baran, Ş.Y.; Durdağ, G.D.; Yetkinel, S.; Alemdaroğlu, S.; Özdoğan, S. Reference values of the ductus venosus pulsatility index for pregnant women between 11 and 13 + 6 weeks of gestation. J. Matern. Fetal Neonatal Med. 2020, 33, 1134–1139. [Google Scholar] [CrossRef]
- Orlic, N.K.; Egic, A.; Damnjanovic-Pazin, B.; Lukic, R.; Joksic, I.; Mikovic, Z. Screening performance of congenital heart defects in first trimester using simple cardiac scan, nuchal translucency, abnormal ductus venosus blood flow and tricuspid regurgitation. Congenit. Heart Dis. 2019, 14, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Socolov, D.; Socolov, R.; Gorduza, V.E.; Butureanu, T.; Stanculescu, R.; Carauleanu, A.; Pavaleanu, I. Increased nuchal translucency in fetuses with a normal karyotype-diagnosis and management: An observational study. Medicine 2017, 96, e7521. [Google Scholar] [CrossRef]
- Edwards, L.; Hui, L. First and second trimester screening for fetal structural anomalies. Semin. Fetal Neonatal Med. 2018, 23, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Baer, R.J.; Norton, M.E.; Shaw, G.M.; Flessel, M.C.; Goldman, S.; Currier, R.; Jelliffe-Pawlowski, L.L. Risk of selected structural abnormalities in infants after increased nuchal translucency measurement. Am. J. Obstet. Gynecol. 2014, 211, 675.e1–675.e19. [Google Scholar] [CrossRef] [PubMed]
- Bilińska, M.; Osmola, K. Cleft lip and palate -risk factors, prenatal diagnosis and health consequences. Ginekol. Pol. 2015, 86, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.L.; Cox, T.C.; Uribe, L.M.M.; Zhu, Y.; Richter, C.T.; Nidey, N.; Standley, J.M.; Deng, M.; Blue, E.; Chong, J.X.; et al. Mutations in the epithelial cadherin-p120-catenin complex cause mendelian non-syndromic cleft clip with or without cleft palate. Am. J. Hum. Genet. 2018, 102, 1143–1157. [Google Scholar] [CrossRef]
- Mossey, P.A.; Little, J.; Steegers-Theunissen, R.; Molloy, A.; Peterlin, B.; Shaw, W.C.; Johnson, C.; FitzPatrick, D.R.; Franceschelli, P.; Rubini, M. Genetic interactions in nonsyndromic orofacial clefts in europe-EUROSCAN study. Cleft Palate Craniofac. J. 2017, 54, 623–630. [Google Scholar] [CrossRef]
- De Queiroz Herkrath, A.P.C.; Herkrath, F.J.; Rebelo, M.A.B.; Vettore, M.V. Parental age as a risk factor for non-syndromic oral clefts: A meta-analysis. J. Dent. 2012, 40, 3–14. [Google Scholar] [CrossRef]
- Norwitz, E.R.; Brynn, L. Noninvasive prenatal testing: The future is now. Rev. Obstet Gynecol. 2013, 6, 48–62. [Google Scholar] [CrossRef]
- Zaami, S.; Orrico, A.; Signore, F.; Cavaliere, A.F.; Mazzi, M.; Marinelli, E. Ethical, Legal and Social Issues (ELSI) Associated with Non-Invasive Prenatal Testing: Reflections on the Evolution of Prenatal Diagnosis and Procreative Choices. Genes 2021, 12, 204. [Google Scholar] [CrossRef]
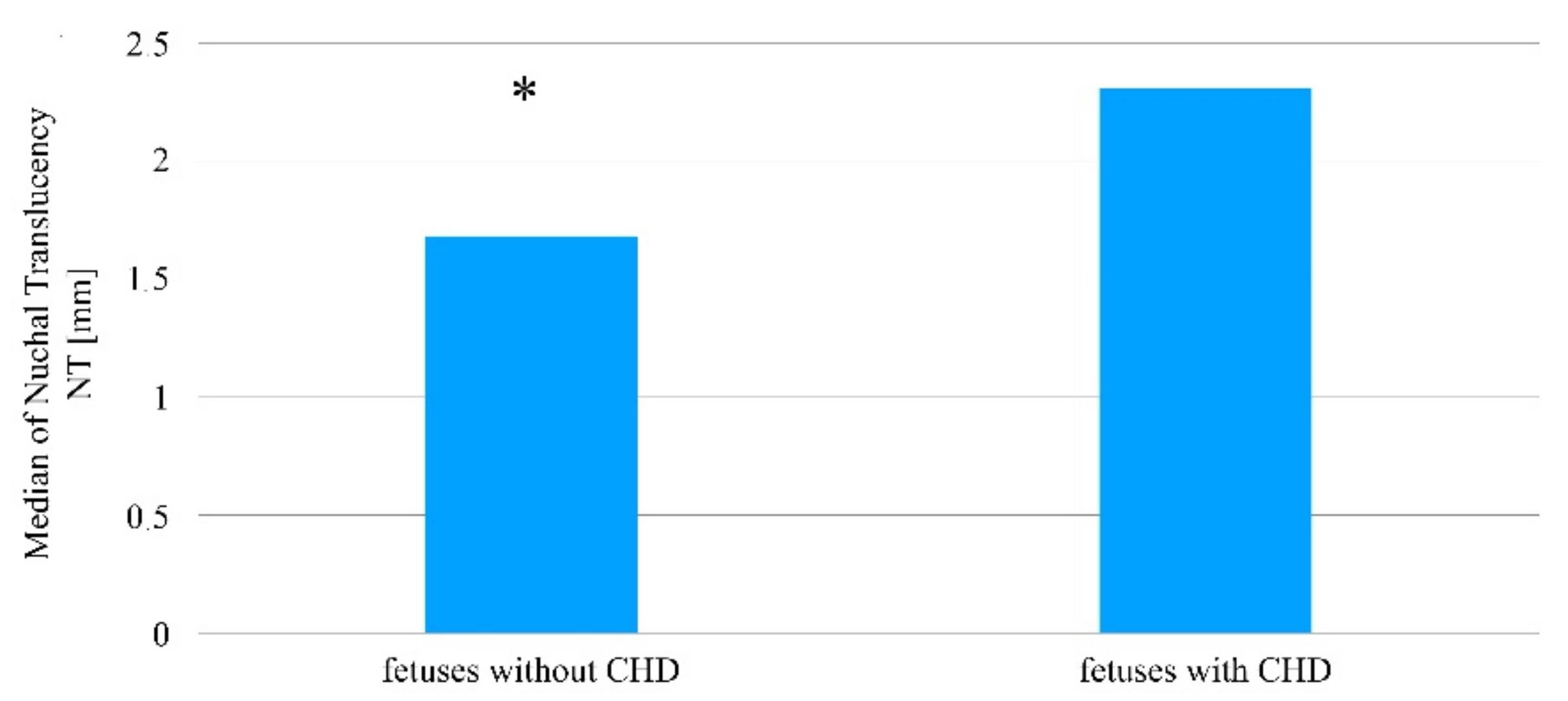






| Parameter | Fetus Group | Q1 | Me | Q3 | p < 0.05 (Mann–Whitney U Test) |
|---|---|---|---|---|---|
| CRL (mm) | No heart defect (1255 cases; 96.98%) | 57.70 | 63.00 | 68.80 | p > 0.05 NS |
| Heart defect (11 cases; 0.85%) | 62.25 | 66.85 | 69.23 | ||
| NT (mm) | No heart defect (1255 cases; 96.98%) | 1.40 | 1.70 | 1.98 | p = 0.0015 |
| Heart defect (11 cases; 0.85%) | 1.65 | 2.30 | 2.68 | ||
| FHR (beats/min) | No heart defect (1255 cases; 96.98%) | 155.00 | 159.00 | 162.00 | p > 0.05 NS |
| Heart defect (11 cases; 0.85%%) | 157.00 | 159.00 | 162.50 | ||
| DV-PI (m/s) | No heart defect (1255 cases; 96.98%) | 0.93 | 1.06 | 1.20 | p = 0.0068 |
| Heart defect (11 cases; 0.85%) | 0.99 | 1.25 | 1.38 |
| Parameter | Fetus Group | Q1 | Me | Q3 | p < 0.05 (Mann–Whitney U Test) |
|---|---|---|---|---|---|
| CRL (mm) | Heart defect (3 cases; 1.69%) | 57.80 | 63.20 | 69.00 | p > 0.05 NS |
| No heart defect (173 cases; 97.19%) | 62.50 | 66.80 | 69.15 | ||
| NT (mm) | Heart defect (3 cases; 1.69%) | 1.50 | 1.70 | 2.00 | p = 0.0007 |
| No heart defect (173 cases; 97.19%) | 1.95 | 2.30 | 3.00 | ||
| FHR (beats/min) | Heart defect (3 cases; 1.69%) | 155.00 | 159.00 | 162.00 | p > 0.05 NS |
| No heart defect (173 cases; 97.19%) | 157.00 | 158.00 | 162.00 | ||
| DV-PI (m/s) | Heart defect (3 cases; 1.69%) | 0.92 | 1.05 | 1.20 | p = 0.0285 |
| No heart defect (173 cases; 97.19%) | 0.97 | 1.24 | 1.30 |
| Parameter | Way of Fertilization (41 Cases; 100%) | Q1 | Me | Q3 | p < 0.05 (Mann–Whitney U Test) |
|---|---|---|---|---|---|
| CRL (mm) | AIH (28 cases; 68.29%) | 52.90 | 58.50 | 64.03 | p > 0.05 NS |
| AID (13 cases; 31.72%) | 58.90 | 60.60 | 65.00 | ||
| NT (mm) | AIH (28 cases; 68.29%) | 1.30 | 1.60 | 1.93 | p > 0.05 NS |
| AID (13 cases; 31.72%) | 1.50 | 1.70 | 2.00 | ||
| FHR (beats/min) | AIH (28 cases; 68.29%) | 158.75 | 161.00 | 164.00 | p > 0.05 NS |
| AID (13 cases; 31.72%) | 155.00 | 161.00 | 170.00 | ||
| DV-PI (m/s) | AIH (28 cases; 68.29%) | 1.00 | 1.08 | 1.15 | p > 0.05 NS |
| AID (13 cases; 31.72%) | 1.00 | 1.11 | 1.21 |
| Parameter | Way of Fertilization (105 Cases; 100%) | Q1 | Me | Q3 | p < 0.05 (Mann–Whitney U Test) |
|---|---|---|---|---|---|
| CRL (mm) | ICSI (53 cases; 50.48%) | 55.80 | 61.10 | 66.90 | p > 0.05 NS |
| IMSI (52 cases; 49.52%) | 60.85 | 66.20 | 73.60 | ||
| NT (mm) | ICSI (53 cases; 50.48%) | 1.30 | 1.60 | 1.90 | p > 0.05 NS |
| IMSI (52 cases; 49.52%) | 1.45 | 1.70 | 1.90 | ||
| FHR (beats/min) | ICSI (53 cases; 50.48%) | 156.00 | 161.00 | 164.25 | p > 0.05 NS |
| IMSI (52 cases; 49.52%) | 157.00 | 161.00 | 163.50 | ||
| DV-PI (m/s) | ICSI (53 cases; 50.48%) | 0.93 | 1.00 | 1.13 | p > 0.05 NS |
| IMSI (52 cases; 49.52%) | 1.00 | 1.16 | 1.26 |
| Parameter | Fetus Group | Q1 | Me | Q3 | p < 0.05 (Mann–Whitney U Test) |
|---|---|---|---|---|---|
| CRL (mm) | No congenital non-heart defect (1255 cases; 96.98%) | 57.80 | 63.00 | 68.80 | p > 0.05 NS |
| congenital non-heart defect (32 cases; 2.47%) | 56.47 | 62.80 | 70.00 | ||
| NT (mm) | No congenital non-heart defect (1255 cases; 96.98%) | 1.40 | 1.70 | 1.92 | p = 0.0207 |
| congenital non-heart defect (32 cases; 2.47%) | 1.50 | 1.90 | 2.93 | ||
| FHR (beats/min) | No congenital non-heart defect (1255 cases; 96.98%) | 155.00 | 159.00 | 162.00 | p > 0.05 NS |
| congenital non-heart defect (32 cases; 2.47%) | 152.75 | 159.50 | 166.00 | ||
| DV-PI (m/s) | No congenital non-heart defect (1255 cases; 96.98%) | 0.93 | 1.06 | 1.20 | p > 0.05 NS |
| congenital non-heart defect (32 cases; 2.47%) | 0.98 | 1.10 | 1.17 |
| Parameter | Fetus Group (137 Cases; 100%) | Q1 | Me | Q3 | p < 0.05 (Mann–Whitney U Test) |
|---|---|---|---|---|---|
| CRL (mm) | No congenital non-heart defect (135 cases; 98.54%) | 57.90 | 63.30 | 69.00 | p > 0.05 NS |
| congenital non-heart defect (2 cases; 1.46%) | 56.15 | 61.35 | 69.32 | ||
| NT (mm) | No congenital non-heart defect (135 cases; 98.54%) | 1.50 | 1.70 | 2.00 | p = 0.0262 |
| congenital non-heart defect (2 cases; 1.46%) | 1.50 | 1.90 | 3.08 | ||
| FHR (beats/minute) | No congenital non-heart defect (135 cases; 98.54%) | 155.00 | 159.00 | 162.00 | p > 0.05 NS |
| congenital non-heart defect (2 cases; 1.46%) | 155.00 | 160.00 | 166.25 | ||
| DV-PI (m/s) | No congenital non-heart defect (135 cases; 98.54%) | 0.92 | 1.05 | 1.20 | p > 0.05 NS |
| congenital non-heart defect (2 cases; 1.46%) | 0.99 | 1.10 | 1.16 |
| Parameter | Way of Fertilization | Q1 | Me | Q3 | p < 0.05 (Mann–Whitney U Test) |
|---|---|---|---|---|---|
| CRL (mm) | AIH (28 cases; 68.29%) | 52.90 | 52.90 | 52.90 | p > 0.05 NS |
| AID (13 cases; 31.72%) | 58.50 | 58.50 | 58.50 | ||
| NT (mm) | AIH (28 cases; 68.29%) | 1.30 | 1.60 | 1.93 | p > 0.05 NS |
| AID (13 cases; 31.72%) | 1.50 | 1.70 | 2.00 | ||
| FHR (beats/min) | AIH (28 cases; 68.29%) | 158.75 | 161.00 | 164.00 | p > 0.05 NS |
| AID (13 cases; 31.72%) | 155.00 | 161.00 | 170.00 | ||
| DV-PI (m/s) | AIH (28 cases; 68.29%) | 1.00 | 1.08 | 1.15 | p > 0.05 NS |
| AID (13 cases; 31.72%) | 1.00 | 1.11 | 1.21 |
| Parameter | Intracytoplasmic Sperm Injection Technique | Q1 | Me | Q3 | p < 0.05 (Mann–Whitney U Test) | |
|---|---|---|---|---|---|---|
| CRL (mm) | ICSI (53 cases; 50.48%) | No defect (96.23%) | 55.80 | 60.80 | 66.25 | p > 0.05 NS |
| Defect (2 cases; 3.77%) | 66.90 | 67.00 | 67.10 | |||
| IMSI (52 cases; 49.52%) No defect | 61.13 | 67.45 | 74.25 | |||
| NT (mm) | ICSI (53 cases; 50.48%) | No defect (96.23%) | 1.30 | 1.60 | 1.80 | p = 0.0445 |
| Defect (2 cases; 3.77%) | 2.20 | 2.40 | 2.60 | |||
| IMSI (52 cases; 49.52%) No defect | 1.48 | 1.70 | 1.90 | |||
| FHR (beats/min) | ICSI (53 cases; 50.48%) | No defect (96.23%) | 156.00 | 161.00 | 164.50 | p > 0.05 NS |
| Defect (2 cases; 3.77%) | 163.25 | 163.50 | 163.75 | |||
| IMSI (52 cases; 49.52%) No defect | 157.00 | 161.00 | 164.00 | |||
| DV-PI (m/s) | ICSI (53 cases; 50.48%) | No defect (96.23%) | 0.93 | 1.00 | 1.14 | p > 0.05 NS |
| Defect (2 cases; 3.77%) | 0.82 | 0.82 | 0.83 | |||
| IMSI (52 cases; 49.52%) No defect | 1.00 | 1.16 | 1.27 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serafin, D.; Grabarek, B.O.; Boroń, D.; Madej, A.; Czuba, B. Risk Assessment of the Increased Occurrence of Congenital Cardiac and Non-Cardiac Defects in Fetuses with a Normal Karyotype after Assisted Fertilization in Comparison to Natural Fertilization Based on Ultrasound Diagnostics. J. Clin. Med. 2021, 10, 5630. https://doi.org/10.3390/jcm10235630
Serafin D, Grabarek BO, Boroń D, Madej A, Czuba B. Risk Assessment of the Increased Occurrence of Congenital Cardiac and Non-Cardiac Defects in Fetuses with a Normal Karyotype after Assisted Fertilization in Comparison to Natural Fertilization Based on Ultrasound Diagnostics. Journal of Clinical Medicine. 2021; 10(23):5630. https://doi.org/10.3390/jcm10235630
Chicago/Turabian StyleSerafin, Dawid, Beniamin Oskar Grabarek, Dariusz Boroń, Andrzej Madej, and Bartosz Czuba. 2021. "Risk Assessment of the Increased Occurrence of Congenital Cardiac and Non-Cardiac Defects in Fetuses with a Normal Karyotype after Assisted Fertilization in Comparison to Natural Fertilization Based on Ultrasound Diagnostics" Journal of Clinical Medicine 10, no. 23: 5630. https://doi.org/10.3390/jcm10235630
APA StyleSerafin, D., Grabarek, B. O., Boroń, D., Madej, A., & Czuba, B. (2021). Risk Assessment of the Increased Occurrence of Congenital Cardiac and Non-Cardiac Defects in Fetuses with a Normal Karyotype after Assisted Fertilization in Comparison to Natural Fertilization Based on Ultrasound Diagnostics. Journal of Clinical Medicine, 10(23), 5630. https://doi.org/10.3390/jcm10235630






