Differential Expression of Glucose Transporter Proteins GLUT-1, GLUT-3, GLUT-8 and GLUT-12 in the Placenta of Macrosomic, Small-for-Gestational-Age and Growth-Restricted Foetuses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Immunohistochemical Staining and Quantitative Morphometric Analysis of Glucose Transporters Expression in Placental Tissue Sections
2.3. Statistical Analysis
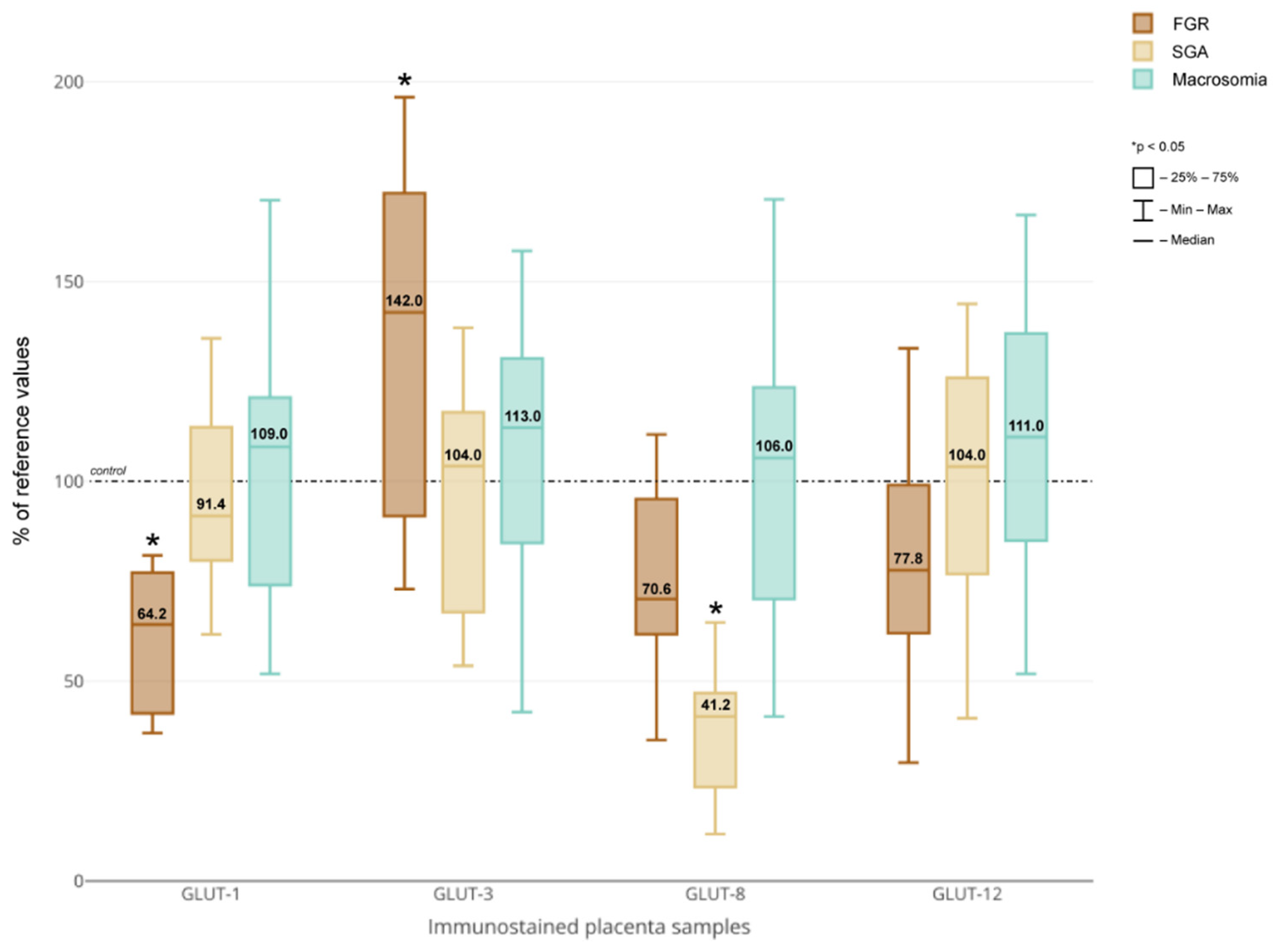
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Romo, A.; Carceller, R.; Tobajas, J. Intrauterine growth retardation (IUGR): Epidemiology and etiology. Pediatric Endocrinol. Rev. 2009, 6 (Suppl. 3), 332–336. [Google Scholar]
- Salihu, H.M.; Dongarwar, D.; King, L.M.; Yusuf, K.K.; Ibrahimi, S.; Salinas-Miranda, A.A. Trends in the incidence of fetal macrosomia and its phenotypes in the United States, 1971–2017. Arch. Gynecol. Obstet. 2020, 301, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Wang, J.; Duan, Y.; Yang, Z. Prevalence of low birth weight and macrosomia estimates based on heaping adjustment method in China. Sci. Rep. 2021, 11, 15016. [Google Scholar] [CrossRef] [PubMed]
- Nardozza, L.M.; Caetano, A.C.; Zamarian, A.C.; Mazzola, J.B.; Silva, C.P.; Marçal, V.M.; Lobo, T.F.; Peixoto, A.B.; Araujo Júnior, E. Fetal growth restriction: Current knowledge. Arch. Gynecol. Obstet. 2017, 295, 1061–1077. [Google Scholar] [CrossRef] [PubMed]
- Boulet, S.L.; Alexander, G.R.; Salihu, H.M.; Pass, M. Macrosomic births in the United States: Determinants, outcomes, and proposed grades of risk. Am. J. Obs. Gynecol. 2003, 188, 1372–1378. [Google Scholar] [CrossRef] [PubMed]
- Jansson, T.; Myatt, L.; Powell, T.L. The role of trophoblast nutrient and ion transporters in the development of pregnancy complications and adult disease. Curr. Vasc. Pharmacol. 2009, 7, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Dimasuay, K.G.; Boeuf, P.; Powell, T.L.; Jansson, T. Placental Responses to Changes in the Maternal Environment Determine Fetal Growth. Front. Physiol. 2016, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Chassen, S.; Jansson, T. Complex, coordinated and highly regulated changes in placental signaling and nutrient transport capacity in IUGR. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165373. [Google Scholar] [CrossRef]
- Jansson, T.; Cetin, I.; Powell, T.L.; Desoye, G.; Radaelli, T.; Ericsson, A.; Sibley, C.P. Placental transport and metabolism in fetal overgrowth -- a workshop report. Placenta 2006, 27 (Suppl. A), S109–S113. [Google Scholar] [CrossRef] [Green Version]
- Illsley, N.P.; Baumann, M.U. Human placental glucose transport in fetoplacental growth and metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165359. [Google Scholar] [CrossRef]
- Mueckler, M.; Thorens, B. The SLC2 (GLUT) family of membrane transporters. Mol. Asp. Med. 2013, 34, 121–138. [Google Scholar] [CrossRef] [Green Version]
- Stanirowski, P.J.; Lipa, M.; Bomba-Opoń, D.; Wielgoś, M. Expression of placental glucose transporter proteins in pregnancies complicated by fetal growth disorders. Adv. Protein Chem. Struct. Biol. 2021, 123, 95–131. [Google Scholar]
- Sakata, M.; Kurachi, H.; Imai, T.; Tadokoro, C.; Yamaguchi, M.; Yoshimoto, Y.; Oka, Y.; Miyake, A. Increase in human placental glucose transporter-1 during pregnancy. Eur. J. Endocrinol. 1995, 132, 206–212. [Google Scholar] [CrossRef]
- Brown, K.; Heller Ds Zamudio, S.; Illsley, N. Glucose transporter 3 (GLUT3) protein expression in human placenta across gestation. Placenta 2011, 32, 1041–1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janzen, C.; Lei, M.Y.; Cho, J.; Sullivan, P.; Shin, B.C.; Devaskar, S.U. Placental glucose transporter 3 (GLUT3) is up-regulated in human pregnancies complicated by late-onset intrauterine growth restriction. Placenta 2013, 34, 1072–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, H.N.; Crombleholme, T.; Habli, M. Adenoviral-Mediated Placental Gene Transfer of IGF-1 Corrects Placental Insufficiency via Enhanced Placental Glucose Transport Mechanisms. PLoS ONE 2013, 8, e74632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janzen, C.; Lei, M.Y.Y.; Jeong, I.S.D.; Ganguly, A.; Sullivan, P.; Paharkova, V.; Capodanno, G.; Nakamura, H.; Perry, A.; Shin, B.C.; et al. Humanin (HN) and glucose transporter 8 (GLUT8) in pregnancies complicated by intrauterine growth restriction. PLoS ONE 2018, 13, e0193583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gude, N.M.; Stevenson, J.L.; Rogers, S.; Best, J.D.; Kalionis, B.; Huisman, M.A.; Erwich, J.J.; Timmer, A.; King, R.G. GLUT12 expression in human placenta in first trimester and term. Placenta 2003, 24, 566–570. [Google Scholar] [CrossRef]
- Stanirowski, P.J.; Szukiewicz, D.; Majewska, A.; Wątroba, M.; Pyzlak, M.; Bomba-Opoń, D.; Wielgoś, M. Placental expression of glucose transporters GLUT-1, GLUT-3, GLUT-8 and GLUT-12 in pregnancies complicated by gestational and type 1 diabetes mellitus. J. Diabetes Investig. 2021. [Google Scholar] [CrossRef]
- Jansson, T.; Wennergren, M.; Illsley, N.P. Glucose transporter expression and distribution in the human placenta throughout gestation and in intrauterine growth retardation. J. Clin. Endocrinol. Metab. 1993, 77, 1554–1562. [Google Scholar]
- Jansson, T.; Ylvén, K.; Wennergren, M.; Powell, T.L. Glucose transport and system A activity in syncytiotrophoblast microvillous and basal plasma membranes in intrauterine growth restriction. Placenta 2002, 23, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Kainulainen, H.; Jarvinen, T.; Heinonen, P.K. Placental glucose transporters in fetal intrauterine growth retardation and macrosomia. Gynecol. Obstet. Investig. 1997, 44, 89–92. [Google Scholar] [CrossRef]
- Gordijn, S.J.; Beune, I.M.; Thilaganathan, B.; Papageorghiou, A.; Baschat, A.A.; Baker, P.N.; Silver, R.M.; Wynia, K.; Ganzevoort, W. Consensus definition of fetal growth restriction: A Delphi procedure. Ultrasound Obs. Gynecol. 2016, 48, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Beune, I.M.; Bloomfield, F.H.; Ganzevoort, W.; Embleton, N.D.; Rozance, P.J.; van Wassenaer-Leemhuis, A.G.; Wynia, K.; Gordijn, S.J. Consensus Based Definition of Growth Restriction in the Newborn. J. Pediatr. 2018, 196, 71–76.e1. [Google Scholar] [CrossRef]
- Villar, J.; Cheikh Ismail, L.; Victora, C.G.; Ohuma, E.O.; Bertino, E.; Altman, D.G.; Lambert, A.; Papageorghiou, A.T.; Carvalho, M.; Jaffer, Y.A.; et al. International standards for newborn weight, length, and head circumference by gestational age and sex: The Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 2014, 384, 857–868. [Google Scholar] [CrossRef]
- Stanirowski, P.J.; Szukiewicz, D.; Pyzlak, M.; Abdalla, N.; Sawicki, W.; Cendrowski, K. Impact of pre-gestational and gestational diabetes mellitus on the expression of glucose transporters GLUT-1, GLUT-4 and GLUT-9 in human term placenta. Endocrine 2017, 55, 799–808. [Google Scholar] [CrossRef]
- Zamudio, S.; Baumann, M.U.; Illsley, N.P. Effects of chronic hypoxia in vivo on the expression of human placental glucose transporters. Placenta 2006, 27, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Zamudio, S.; Torricos, T.; Fik, E.; Oyala, M.; Echalar, L.; Pullockaran, J.; Tutino, E.; Martin, B.; Belliappa, S.; Balanza, E.; et al. Hypoglycemia and the origin of hypoxia-induced reduction in human fetal growth. PLoS ONE 2010, 5, e8551. [Google Scholar] [CrossRef] [Green Version]
- Chandrasiri, U.P.; Chua, C.L.; Umbers, A.J.; Chaluluka, E.; Glazier, J.D.; Rogerson, S.J.; Boeuf, P. Insight into the pathogenesis of fetal growth restriction in placental malaria: Decreased placental glucose transporter isoform 1 expression. J. Infect. Dis. 2014, 209, 1663–1667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lüscher, B.P.; Marini, C.; Joerger-Messerli, M.S.; Huang, X.; Hediger, M.A.; Albrecht, C.; Baumann, M.U.; Surbek, D.V. Placental glucose transporter (GLUT)-1 is down-regulated in preeclampsia. Placenta 2017, 55, 94–99. [Google Scholar] [CrossRef]
- Chang, Y.L.; Chao, A.S.; Chang, S.D.; Cheng, P.J. Placental glucose transporter 1 and 3 gene expression in Monochorionic twin pregnancies with selective fetal growth restriction. BMC Pregnancy Childbirth 2021, 21, 260. [Google Scholar] [CrossRef] [PubMed]
- Challis, D.E.; Pfarrer, C.D.; Ritchie, J.W.; Koren, G.; Adamson, S.L. Glucose metabolism is elevated and vascular resistance and maternofetal transfer is normal in perfused placental cotyledons from severely growth-restricted fetuses. Pediatr. Res. 2000, 47, 309–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, S.; Joost, H.G.; Schürmann, A. GLUT8, the enigmatic intracellular hexose transporter. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E614–E618. [Google Scholar] [CrossRef]
- Adastra, K.L.; Frolova, A.I.; Chi, M.M.; Cusumano, D.; Bade, M.; Carayannopoulos, M.O.; Moley, K.H. Slc2a8 deficiency in mice results in reproductive and growth impairments. Biol. Reprod. 2012, 87, 49. [Google Scholar] [CrossRef] [PubMed]
- Scheepers, A.; Doege, H.; Joost, H.G.; Schürmann, A. Mouse GLUT8: Genomic organization and regulation of expression in 3T3-L1 adipocytes by glucose. Biochem. Biophys. Res. Commun. 2001, 288, 969–974. [Google Scholar] [CrossRef]
- Shao, Y.; Wellman, T.L.; Lounsbury, K.M.; Zhao, F.Q. Differential regulation of GLUT1 and GLUT8 expression by hypoxia in mammary epithelial cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R237–R247. [Google Scholar] [CrossRef] [Green Version]
- Stanirowski, P.J.; Szukiewicz, D.; Pyzlak, M.; Abdalla, N.; Sawicki, W.; Cendrowski, K. Analysis of correlations between the placental expression of glucose transporters GLUT-1, GLUT-4 and GLUT-9 and selected maternal and fetal parameters in pregnancies complicated by diabetes mellitus. J. Matern. Fetal. Neonatal Med. 2019, 32, 650–659. [Google Scholar] [CrossRef]
- Acosta, O.; Ramirez, V.I.; Lager, S.; Gaccioli, F.; Dudley, D.J.; Powell, T.L.; Jansson, T. Increased glucose and placental GLUT-1 in large infants of obese nondiabetic mothers. Am. J. Obs. Gynecol. 2015, 212, 227.e1–227.e7. [Google Scholar] [CrossRef]
- Toyoda, Y.; Takada, T.; Miyata, H.; Matsuo, H.; Kassai, H.; Nakao, K.; Nakatochi, M.; Kawamura, Y.; Shimizu, S.; Shinomiya, N.; et al. Identification of GLUT12/SLC2A12 as a urate transporter that regulates the blood urate level in hyperuricemia model mice. Proc. Natl. Acad. Sci. USA 2020, 117, 18175–18177. [Google Scholar] [CrossRef]
- Bainbridge, S.A.; Roberts, J.M. Uric acid as a pathogenic factor in preeclampsia. Placenta 2008, 29 (Suppl. A), S67–S72. [Google Scholar] [CrossRef] [Green Version]
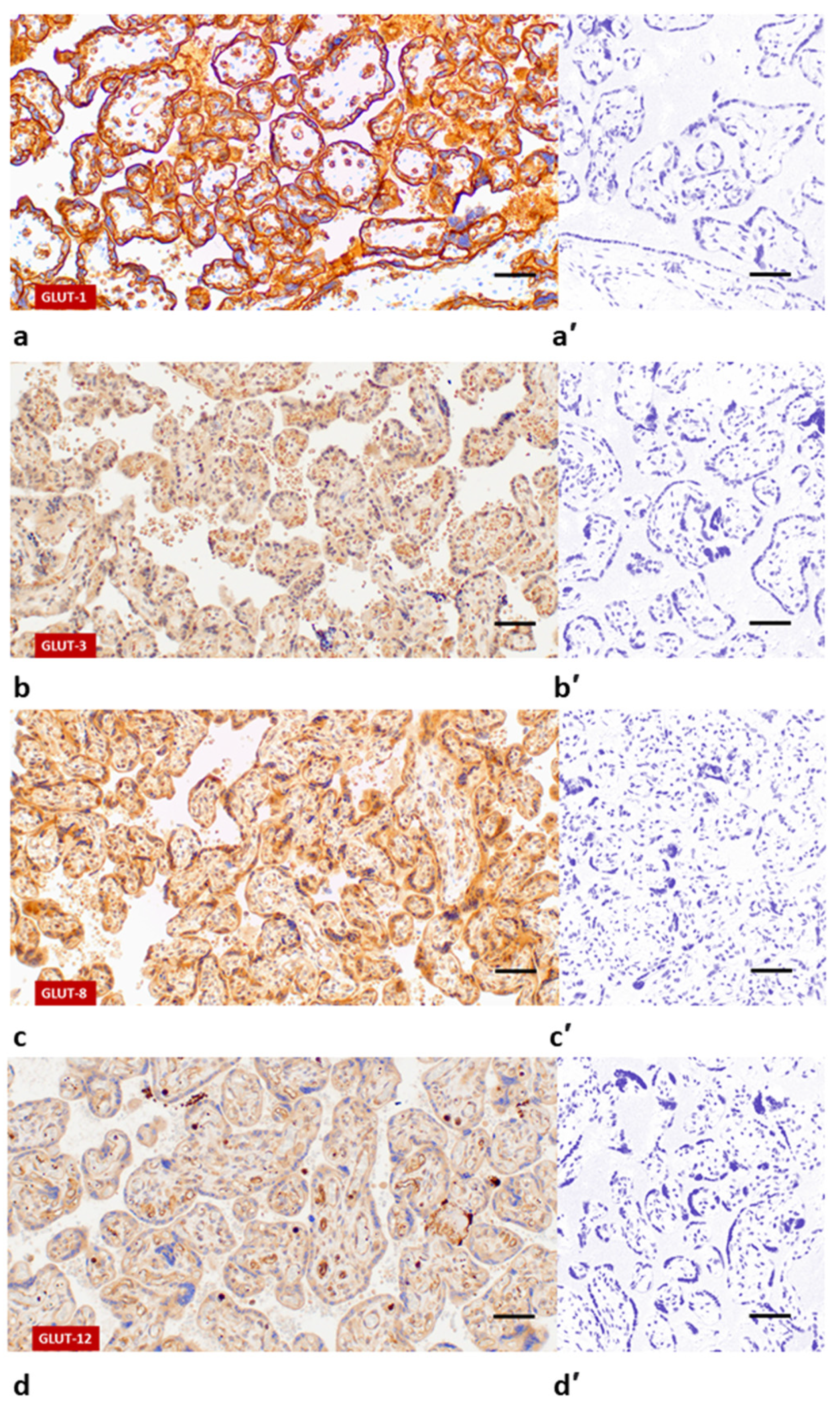

| No. | Group | Gestational Age (Weeks) | Sex | FBW (g) | Percentile a | Length (cm) | Percentile a | HC (cm) | Percentile a | Prenatal FGR Diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | SGA | 38 | F | 2500 | 10 | 47.9 | 10–50 | 33 | 10–50 | No |
| 2 | SGA | 40 | F | 2580 | 3–10 | 51.1 | 50–90 | 33.9 | 10–50 | No |
| 3 | SGA | 39 | F | 2650 | 10 | 51.8 | >97 | 32.5 | 10–50 | No |
| 4 | SGA | 38 | F | 2500 | 10 | 54.3 | >97 | 32 | 10–50 | No |
| 5 | SGA | 39 | F | 2525 | 3–10 | 51.5 | 90–97 | 32.5 | 10–50 | No |
| 6 | SGA | 38 | M | 2505 | 3–10 | 49.8 | 50–90 | 32.8 | 10–50 | No |
| 7 | SGA | 39 | M | 2720 | 3–10 | 50.8 | 50–90 | 34.5 | 50–90 | No |
| 8 | SGA | 40 | M | 2800 | 3–10 | 56 | >97 | 32.6 | 3–10 | No |
| 9 | SGA | 40 | M | 2840 | 3–10 | 51.5 | 50–90 | 33.5 | 10–50 | No |
| 10 | SGA | 39 | M | 2570 | 3–10 | 52.3 | 90–97 | 31.9 | 3–10 | No |
| 11 | SGA | 40 | M | 2880 | 10 | 54 | >97 | 32.5 | 3–10 | No |
| 12 | FGR | 38 | F | 2430 | 3–10 | 49.8 | 50–90 | 31.4 | 3–10 | Yes (abnormal Doppler) |
| 13 | FGR | 38 | M | 2405 | 3–10 | 50.2 | 50–90 | 31 | <3 | Yes (abnormal Doppler) |
| 14 | FGR | 37 | M | 2120 | <3 | 48.5 | 50–90 | 31.3 | 3–10 | Yes (abnormal Doppler) |
| 15 | FGR | 39 | F | 2410 | <3 | 48.6 | 10–50 | 31.9 | 3–10 | No |
| 16 | FGR | 37 | F | 1985 | <3 | 46.6 | 10–50 | 30.8 | 3–10 | Yes (abnormal Doppler) |
| 17 | FGR | 38 | F | 2100 | <3 | 49.9 | 50–90 | 31.5 | 3–10 | No |
| 18 | FGR | 37 | F | 2040 | <3 | 47.1 | 10–50 | 30.2 | <3 | Yes (abnormal Doppler) |
| 19 | FGR | 36 | M | 1625 | <3 | 46.8 | 10–50 | 30.5 | 3–10 | Yes (abnormal Doppler) |
| 20 | FGR | 36 | F | 1610 | <3 | 41.2 | <3 | 30.2 | 3–10 | No |
| 21 | FGR | 37 | F | 2015 | <3 | 49.2 | 50–90 | 31.1 | 3–10 | No |
| 22 | FGR | 37 | F | 2320 | 3–10 | 52.5 | >97 | 30.9 | 3–10 | Yes (abnormal Doppler) |
| 23 | FGR | 37 | F | 2240 | 3–10 | 51.9 | >97 | 30.2 | <3 | Yes (abnormal Doppler) |
| 24 | FGR | 36 | M | 1820 | <3 | 47.3 | 50–90 | 30.1 | <3 | Yes (abnormal Doppler) |
| Placental Specimens | N | Central (A) Peripheral (B) | GLUT-1 (Sections × Visual Fields) | GLUT-3 (Sections × Visual Fields) | GLUT-8 (Sections × Visual Fields) | GLUT-12 (Sections × Visual Fields) | |
|---|---|---|---|---|---|---|---|
| Group | |||||||
| FGR | 13 | A: 13 | 39 × 3 | 39 × 3 | 39 × 3 | 39 × 3 | |
| B: 13 | 39 × 3 | 39 × 3 | 39 × 3 | 39 × 3 | |||
| SGA | 11 | A: 11 | 33 × 3 | 33 × 3 | 33 × 3 | 33 × 3 | |
| B: 11 | 33 × 3 | 33 × 3 | 33 × 3 | 33 × 3 | |||
| Macrosomia | 26 | A: 26 | 78 × 3 | 78 × 3 | 78 × 3 | 78 × 3 | |
| B: 26 | 78 × 3 | 78 × 3 | 78 × 3 | 78 × 3 | |||
| Control | 20 | A: 20 | 60 × 3 | 60 × 3 | 60 × 3 | 60 × 3 | |
| B: 20 | 60 × 3 | 60 × 3 | 60 × 3 | 60 × 3 | |||
| Total: | 70 | A: 70 | 210 × 3 | 210 × 3 | 210 × 3 | 210 × 3 | |
| B: 70 | 210 × 3 | 210 × 3 | 210 × 3 | 210 × 3 | |||
| A + B = 140 | A + B = 1680 × 3 = 5040 images | ||||||
| FGR (n = 13) | SGA (n = 11) | Foetal Macrosomia a (n = 26) | Control (n = 20) | p Value | |
|---|---|---|---|---|---|
| Age (years) | 30 [30–36] | 30 [25.5–34] | 30 [28.2–33] | 31.5 [28.5–34.2] | 0.65 |
| Gestational age (weeks) | 37 [37–37] | 39 [38.5–40] | 40 [39–40] | 39 [38–39] | <0.001 * |
| Gravidity | 1 [1-2] | 2 [1–3] | 2 [1–2] | 2 [1–2] | 0.64 |
| Parity | 1 [1–2] | 1 [1–2.5] | 2 [1–2] | 2 [1–2] | 0.52 |
| Pre-pregnancy weight (kg) | 64 [55–73] | 55 [54.5–60.5] | 74 [62.5–80.7] | 59.5 [55–66] | <0.05 † |
| Gestational weight gain (kg) | 10 [7–17] | 11 [10–12] | 15.5 [12–17.8] | 13 [10.7–15] | <0.05 ‡ |
| Height (m) | 1.63 [1.6–1.65] | 1.62 [1.59–1.66] | 1.67 [1.65–1.72] | 1.64 [1.6–1.7] | <0.05 ‡ |
| Pre-pregnancy BMI (kg/m2) | 23.8 [21.6–25] | 21 [20.2–22.6] | 25.3 [22.3–28.3] | 21.4 [20.4–24] | <0.05 † |
| Fasting plasma glucose (mg/dl) b | 78 [77–79] | 77 [74–80.7] | 83 [78.2–85.7] | 78 [74.2–83.7] | 0.13 |
| 1 h plasma glucose (mg/dl) b | 139 [133–147] | 112.5 [104.5–125.2] | 123.5 [107.2–150.7] | 122.5 [93.7–139.5] | 0.11 |
| 2 h plasma glucose (mg/dl) b | 105 [93–109] | 100.5 [68.7–118] | 97.5 [80.2–119] | 99.5 [86.5–115.2] | 0.68 |
| Foetal sex Male Female | 4 (30.8%) 9 (69.2%) | 6 (54.5%) 5 (45.5%) | 16 (61.5%) 10 (38.5%) | 8 (40%) 12 (60%) | 0.25 |
| Foetal birth weight (g) | 2100 [1985–2330] | 2580 [2515–2760] | 4207.5 [4102.5–4371.3] | 3240 [3078.8–3495] | <0.001 ‡,§ |
| Placental weight (g) | 321 [306–428] | 379 [349.5–457] | 657 [590.2–711.7] | 593 [496.2–631.2] | <0.001 ‡ <0.05 ¶ |
| Estimate | 95% CI | p Value | |
|---|---|---|---|
| GLUT-1 | |||
| Gestational weight gain (kg) | −0.001 | −0.004−0.002 | 0.54 |
| FBW (kg) | 0.007 | 0.003−0.01 | <0.001 |
| Pre-pregnancy BMI (kg/m2) | −0.001 | −0.004−0.001 | 0.32 |
| Foetal sex—male | −0.003 | −0.009−0.003 | 0.37 |
| GLUT-3 | |||
| Pre-pregnancy BMI (kg/m2) | 0.002 | 0−0.005 | <0.05 |
| Maternal height (m) | 0.031 | −0.007−0.069 | 0.11 |
| FBW (kg) | −0.003 | −0.005−0 | 0.06 |
| GLUT-8 | |||
| FBW (kg) | 0.003 | 0.001−0.004 | <0.01 |
| Foetal sex—male | −0.002 | −0.005−0.001 | 0.11 |
| GLUT-12 | |||
| Gestational weight gain (kg) | −0.002 | −0.004−0.001 | 0.17 |
| FBW (kg) | 0.004 | 0.002−0.007 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanirowski, P.J.; Szukiewicz, D.; Majewska, A.; Wątroba, M.; Pyzlak, M.; Bomba-Opoń, D.; Wielgoś, M. Differential Expression of Glucose Transporter Proteins GLUT-1, GLUT-3, GLUT-8 and GLUT-12 in the Placenta of Macrosomic, Small-for-Gestational-Age and Growth-Restricted Foetuses. J. Clin. Med. 2021, 10, 5833. https://doi.org/10.3390/jcm10245833
Stanirowski PJ, Szukiewicz D, Majewska A, Wątroba M, Pyzlak M, Bomba-Opoń D, Wielgoś M. Differential Expression of Glucose Transporter Proteins GLUT-1, GLUT-3, GLUT-8 and GLUT-12 in the Placenta of Macrosomic, Small-for-Gestational-Age and Growth-Restricted Foetuses. Journal of Clinical Medicine. 2021; 10(24):5833. https://doi.org/10.3390/jcm10245833
Chicago/Turabian StyleStanirowski, Paweł Jan, Dariusz Szukiewicz, Agata Majewska, Mateusz Wątroba, Michał Pyzlak, Dorota Bomba-Opoń, and Mirosław Wielgoś. 2021. "Differential Expression of Glucose Transporter Proteins GLUT-1, GLUT-3, GLUT-8 and GLUT-12 in the Placenta of Macrosomic, Small-for-Gestational-Age and Growth-Restricted Foetuses" Journal of Clinical Medicine 10, no. 24: 5833. https://doi.org/10.3390/jcm10245833
APA StyleStanirowski, P. J., Szukiewicz, D., Majewska, A., Wątroba, M., Pyzlak, M., Bomba-Opoń, D., & Wielgoś, M. (2021). Differential Expression of Glucose Transporter Proteins GLUT-1, GLUT-3, GLUT-8 and GLUT-12 in the Placenta of Macrosomic, Small-for-Gestational-Age and Growth-Restricted Foetuses. Journal of Clinical Medicine, 10(24), 5833. https://doi.org/10.3390/jcm10245833








