Evaluation of the Use of Antibiofilmogram Technology in the Clinical Evolution of Foot Ulcers Infected by Staphylococcus aureus in Persons Living with Diabetes: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Bacteriological Study
2.3. Antibiofilmogram
2.4. Statistical Analysis
3. Results
3.1. Studied Population
3.2. Presence of S. aureus during the Follow-Up of the Patients
3.3. Antibiofilmogram and Evolution of the DFU
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lipsky, B.A.; Senneville, E.; Abbas, Z.G.; Aragón-Sánchez, J.; Diggle, M.; Embil, J.M.; Kono, S.; Lavery, L.A.; Malone, M.; van Asten, S.A.; et al. International Working Group on the Diabetic Foot (IWGDF). Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes/Metab. Res. Rev. 2020, 36, e3280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diabetic Foot: Facts and Figures. Available online: https://diabeticfootonline.com/diabetic-foot-facts-and-figures/ (accessed on 10 October 2021).
- Liu, C.; Ponsero, A.J.; Armstrong, D.G.; Lipsky, B.A.; Hurwitz, B.L. The dynamic wound microbiome. BMC Med. 2020, 18, 358. [Google Scholar] [CrossRef] [PubMed]
- Radzieta, M.; Sadeghpour-Heravi, F.; Peters, T.J.; Hu, H.; Vickery, K.; Jeffries, T.; Dickson, H.G.; Schwarzer, S.; Jensen, S.O.; Malone, M. A multiomics approach to identify host-microbe alterations associated with infection severity in diabetic foot infections: A pilot study. NPJ Biofilms Microbiomes 2021, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Gardner, S.E.; Hillis, S.L.; Heilmann, K.; Segre, J.A.; Grice, E.A. The neuropathic diabetic foot ulcer microbiome is associated with clinical factors. Diabetes 2013, 62, 923–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunyach-Remy, C.; Ngba Essebe, C.; Sotto, A.; Lavigne, J.P. Staphylococcus aureus toxins and diabetic foot ulcers: Role in pathogenesis and interest in diagnosis. Toxins 2016, 8, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pouget, C.; Dunyach-Remy, C.; Pantel, A.; Schuldiner, S.; Sotto, A.; Lavigne, J.P. Biofilms in diabetic foot ulcers: Significance and clinical relevance. Microorganisms 2020, 8, 1580. [Google Scholar] [CrossRef]
- Percival, S.L.; McCarty, S.M.; Lipsky, B. Biofilms and Wounds: An Overview of the Evidence. Adv. Wound Care 2015, 4, 373–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malone, M.; Bjarnsholt, T.; McBain, A.J.; James, G.A.; Stoodley, P.; Leaper, D.; Tachi, M.; Schultz, G.; Swanson, T.; Wolcott, R.D. The prevalence of biofilms in chronic wounds: A systematic review and meta-analysis of published data. J. Wound Care 2017, 26, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Ferrer, M.D.; Rodriguez, J.C.; Álvarez, L.; Artacho, A.; Royo, G.; Mira, A. Effect of antibiotics on biofilm inhibition and induction measured by real-time cell analysis. J. Appl. Microbiol. 2017, 122, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Tasse, J.; Croisier, D.; Badel-Berchoux, S.; Chavanet, P.; Bernardi, T.; Provot, C.; Laurent, F. Preliminary results of a new antibiotic susceptibility test against biofilm installation in device-associated infections: The Antibiofilmogram®. Pathog. Dis. 2016, 74, ftw057. [Google Scholar] [CrossRef] [Green Version]
- Edmonds, M.; Lázaro-Martínez, J.L.; Alfayate-García, J.M.; Martini, J.; Petit, J.M.; Rayman, G.; Lobmann, R.; Uccioli, L.; Sauvadet, A.; Bohbot, S.; et al. Sucrose octasulfate dressing versus control dressing in patients with neuroischaemic diabetic foot ulcers (Explorer): An international, multicentre, double-blind, randomized, controlled trial. Lancet Diabetes Endocrinol. 2018, 6, 186–196. [Google Scholar] [CrossRef]
- Commission Nationale D’évaluation des Dispositifs Médicaux et des Technologies de Santé. Available online: https://www.has-sante.fr/plugins/ModuleXitiKLEE/types/FileDocument/doXiti.jsp?id=c_2867129 (accessed on 8 October 2021).
- Enright, M.C.; Day, N.P.; Davies, C.E.; Peacock, S.J.; Spratt, B.G. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 2000, 38, 1008–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staphylococcus aureus MLST Databases. Available online: http://www.mlst.net/ (accessed on 31 August 2021).
- Versey, Z.; da Cruz Nizer, W.S.; Russell, E.; Zigic, S.; DeZeeuw, K.G.; Marek, J.E.; Overhage, J.; Cassol, E. Biofilm-Innate Immune Interface: Contribution to Chronic Wound Formation. Front. Immunol. 2021, 12, 648554. [Google Scholar] [CrossRef] [PubMed]
- Harika, K.; Shenoy, V.P.; Narasimhaswamy, N.; Chawla, K. Detection of Biofilm Production, and Its Impact on Antibiotic Resistance Profile of Bacterial Isolates from Chronic Wound Infections. J. Glob. Infect. Dis. 2020, 12, 129–134. [Google Scholar]
- Dowd, S.E.; Wolcott, R.D.; Sun, Y.; McKeehan, T.; Smith, E.; Rhoads, D. Polymicrobial nature of chronic diabetic foot ulcer biofilm infections determined using bacterial tag encoded FLX amplicon pyrosequencing (bTEFAP). PLoS ONE 2008, 3, e3326. [Google Scholar] [CrossRef]
- Zheng, Z.; Stewart, P.S. Penetration of rifampin through Staphylococcus epidermidis biofilms. Antimicrob. Agents Chemother. 2002, 46, 900–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.; Ray, P.; Das, A.; Sharma, M. Penetration of antibiotics through Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Antimicrob. Chemother. 2010, 65, 1955–1958. [Google Scholar] [CrossRef] [Green Version]
- Olivares, E.; Badel-Berchoux, S.; Provot, C.; Prévost, G.; Bernardi, T.; Jehl, F. Clinical impact of antibiotics for the treatment of Pseudomonas aeruginosa biofilm infections. Front. Microbiol. 2020, 10, 2894. [Google Scholar] [CrossRef] [PubMed]
- Orazi, G.; Jean-Pierre, F.; O’Toole, G.A. Pseudomonas aeruginosa PA14 Enhances the Efficacy of Norfloxacin against Staphylococcus aureus Newman Biofilms. J. Bacteriol. 2020, 202, e00159-20. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, J.P.; Hosny, M.; Dunyach-Remy, C.; Boutet-Dubois, A.; Schuldiner, S.; Cellier, N.; Yahiaoui-Martinez, A.; Molle, V.; La Scola, B.; Marchandin, H.; et al. Long-term intrahost evolution of Staphylococcus aureus among diabetic patients with foot infections. Front. Microbiol. 2021, 12, 741406. [Google Scholar] [CrossRef] [PubMed]
- Kahl, B.C.; Duebbers, A.; Lubritz, G.; Haeberle, J.; Koch, H.G.; Ritzerfeld, B.; Reilly, M.; Harms, E.; Proctor, R.A.; Hermann, M.; et al. Population dynamics of persistent Staphylococcus aureus isolated from the airways of cystic fibrosis patients during a 6-year prospective study. J. Clin. Microbiol. 2003, 41, 4424–4427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunyach-Remy, C.; Courtais-Coulon, C.; DeMattei, C.; Jourdan, N.; Schuldiner, S.; Sultan, A.; Carrière, C.; Alonso, S.; Sotto, A.; Lavigne, J.P. Link between nasal carriage of Staphylococcus aureus and infected diabetic foot ulcers. Diabetes Metab. 2017, 43, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Senneville, E.; Brière, M.; Neut, C.; Messad, N.; Lina, G.; Richard, J.L.; Sotto, A.; Lavigne, J.P. French Study Group on the Diabetic Foot. First report of the predominance of clonal complex 398 Staphylococcus aureus strains in osteomyelitis complicating diabetic foot ulcers: A national French study. Clin. Microbiol. Infect. 2014, 20, O274–O277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shettigar, K.; Murali, T.S. Virulence factors and clonal diversity of Staphylococcus aureus in colonization and wound infection with emphasis on diabetic foot infection. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 2235–2246. [Google Scholar] [CrossRef] [PubMed]
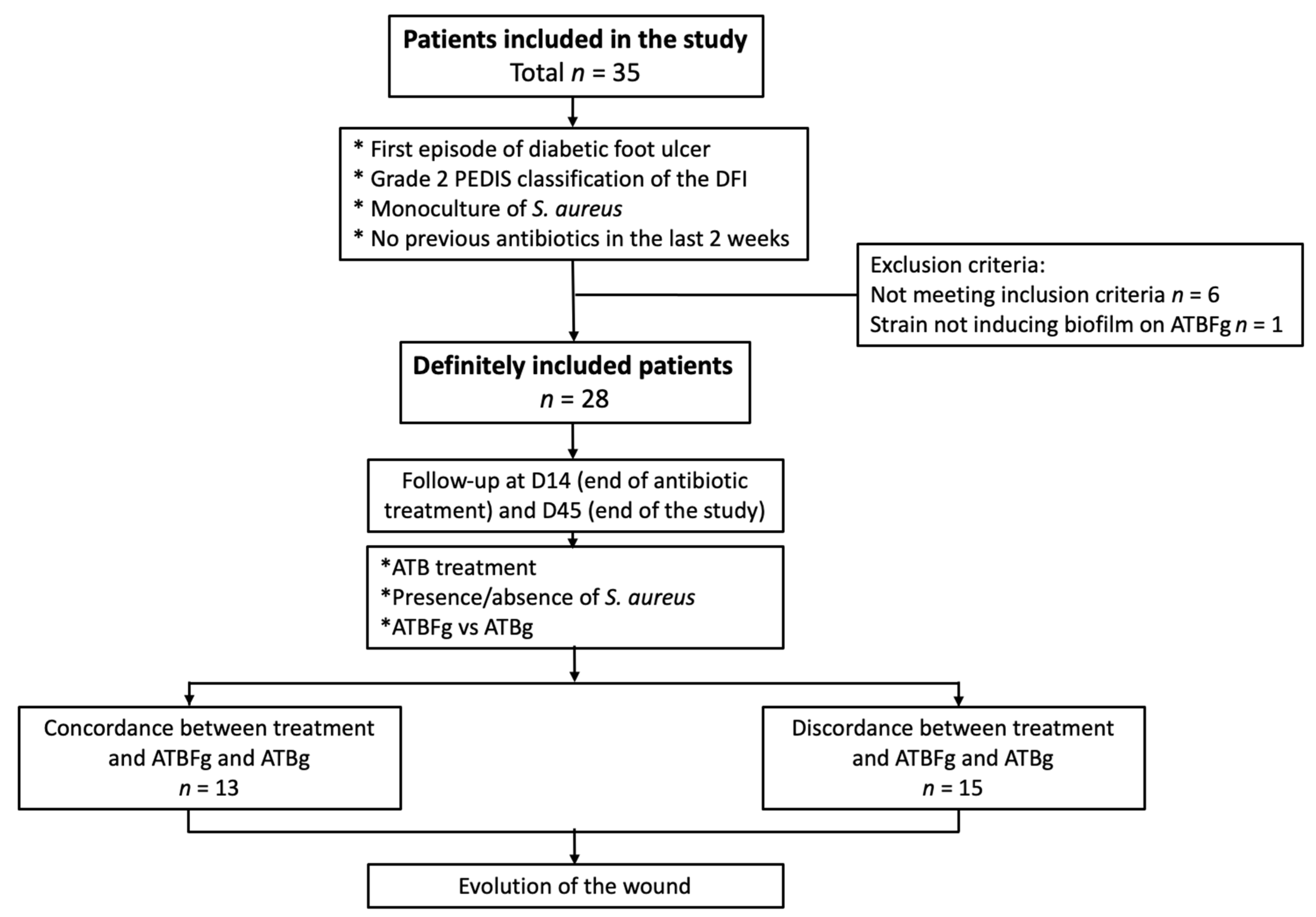
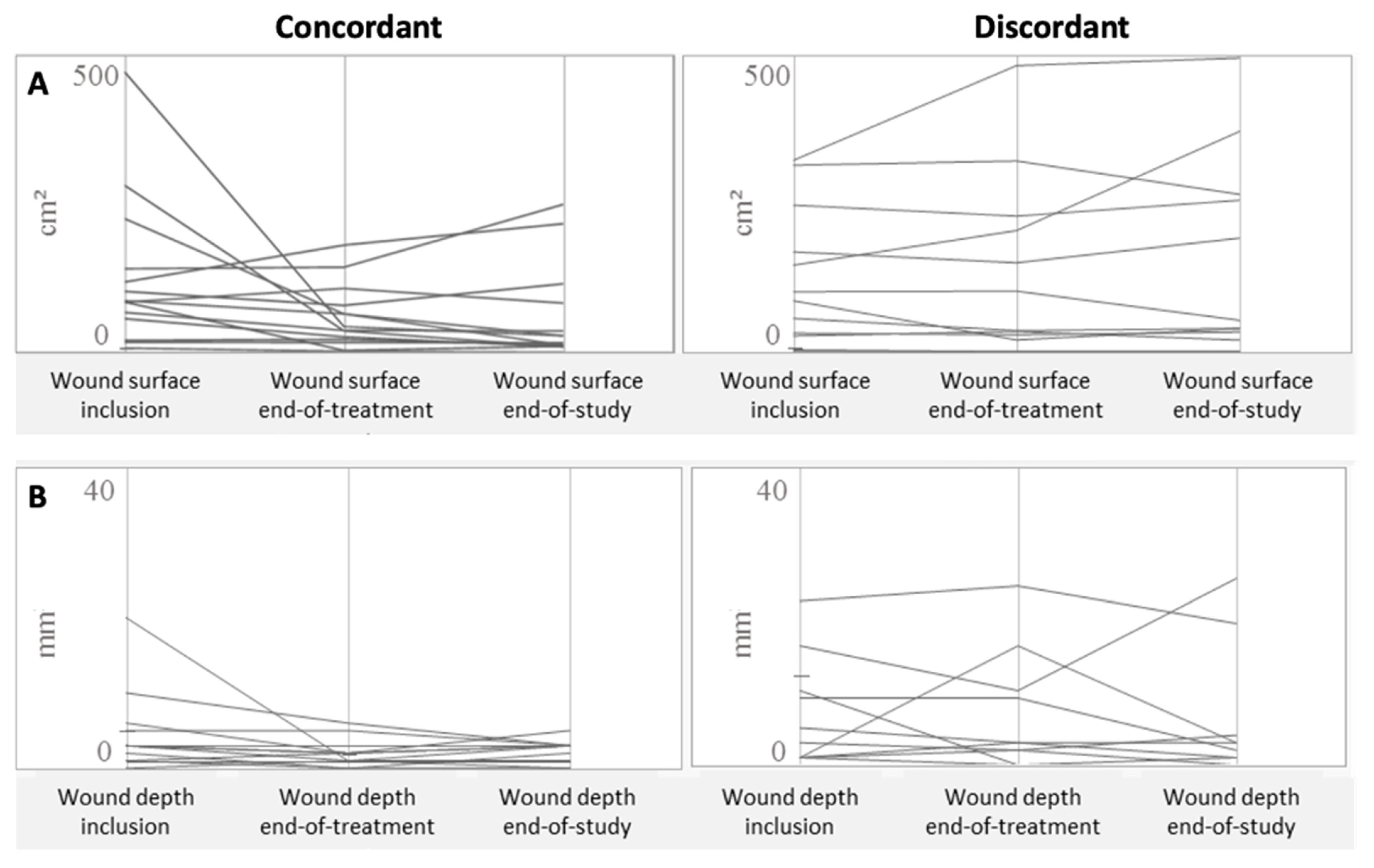
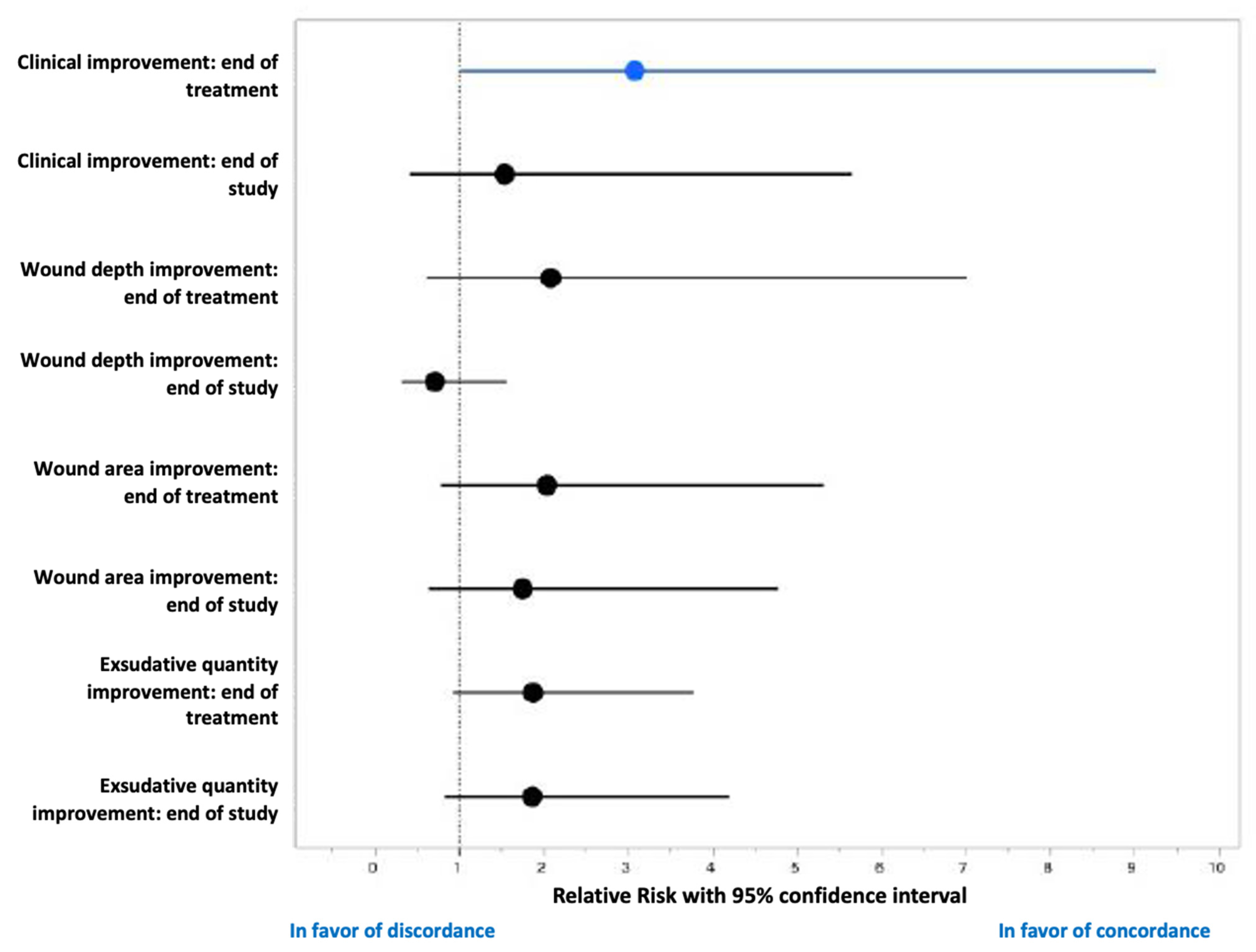
| Characteristics | Concordant Group (n = 15) | Disconcordant Group (n = 13) | Total (n = 28) | p-Value Concordant vs. Disconcordant |
|---|---|---|---|---|
| Age (years, SD a) | 60.1 (±13.1) | 62.4 (±10.9) | 61.2 (±11.9) | 0.6273 |
| Male/Female (n,%) | 11 (73.3)/4 (26.7) | 11 (84.6)/2(15.4) | 22 (78.6)/6 (21.4) | 0.4865 |
| BMI b (kg/m2, SD) | 29.93 (±5.18) | 33.67 (±7.94) | 31.66 (±6.75) | 0.1462 |
| Comorbidities | ||||
| Charlson index (median, IQ c) | 2 (3) | 4 (1.5) | 3 (2) | 0.4591 |
| McCabe Score | 1 | 1 | 1 | >0.99 |
| Arteriopathy (n,%) | 14 (93.3) | 12 (92.3) | 26 (92.3) | >0.99 |
| Neuropathy (n,%) | 13 (86.7) | 12 (92.3) | 25 (89.3) | >0.99 |
| Diabetes duration median (years, IQ) | 15 (±10) | 16 (±10) | 15.5 (±10.7) | 0.473 |
| HbA1c mean (%, SD) | 8.60 (±2.18) | 7.93 (±1.4) | 8.29 (±1.86) | 0.3536 |
| Type 1/Type 2 diabetes mellitus (n,%/n,%) | 1 (6.7)/14 (93.3) | 1 (7.7)/12 (92.3) | 2 (7.1)/26 (92.9) | 0.9201 |
| Characteristics of the wounds | ||||
| Initial wound depth median (mm, IQ) | 3 (±4.25) | 7 (±14) | 3 (±9) | 0.2621 |
| Initial wound surface area median (mm2, IQ) | 117.8 (±168.8) | 120.1 (±265.75) | 119 (±197.65) | 0.9632 |
| Exsudative wound (n,%) | 2 (13.3) | 4 (30.8) | 6 (24) | 0.3720 |
| Monotherapy/Bitherapy (n,%) | 9 (60.0)/6 (40.0) | 2 (15.4)/11 (84.6) | 11 (39.3)/17 (60.7) | 0.0238 |
| Treatment duration (day, SD) | 13 ± 5 | 14 ± 3.5 | 13 ± 3.8 | 0.0393 |
| β-lactams (n,%) | 10 (66.7) | 9 (69.2) | 19 (67.9) | 0.7051 |
| Macrolides and related (n,%) | 7 (46.7) | 3 (23.1) | 10 (35.7) | 0.1145 |
| Cotrimoxazole (n,%) | 0 (0) | 5 (38.5) | 5 (17.9) | 0.0131 |
| Glycopeptides (n,%) | 0 (0) | 2 (15.4) | 2 (7.1) | 0.2063 |
| Fluoroquinolones (n,%) | 0 (0) | 6 (46.2) | 6 (21.4) | 0.0046 |
| Rifampicin (n,%) | 0 (0) | 5 (38.5) | 5 (17.9) | 0.0131 |
| Classification Group | Patients | Antibiotics Prescription a | Result of Antibiogram b | Results of Antibiofilmogram |
|---|---|---|---|---|
| Concordant | C01P004 | CLN | S | S |
| C01P009 | CLN | S | S | |
| C01P012 | AMC | S | S | |
| C03P001 | AMC | S | S | |
| C03P003 | CLN | S | S | |
| C03P004 | CLN | S | S | |
| C03P006 | CLN | S | S | |
| C03P008 | AMC and CLN | R/S | R/S | |
| C03P009 | CLN | S | S | |
| C03P010 | AMC and CLN | R/S | R/S | |
| C04P002 | AMC | R | R | |
| C04P003 | AMC | S | S | |
| C04P004 | AMC | S | S | |
| C04P005 | AMC | S | S | |
| C06P001 | AMC | S | S | |
| Discordant | C01P001 | OFX + RIF | S/S | R/S |
| C01P002 | AMC + OFX | S/S | S/R | |
| C01P005 | AMC + OFX | S/S | S/R | |
| C01P008 | AMC + SXT | S/S | R/R | |
| C01P010 | OFX + CLN | S/S | R/S | |
| C01P011 | SXT + OFX | S/S | R/R | |
| C01P013 | SXT + RIF | S/S | R/S | |
| C03P002 | OFX + SXT | S/S | R/R | |
| C03P007 | CLN + VAN | S/S | S/R | |
| C04P001 | SXT | S | R | |
| C04P007 | AMC | S | R | |
| C04P008 | AMC + RIF | S/S | R/S | |
| C06P003 | AMC | S | R |
| Characteristics | Concordant Group (n = 15) | Discordant Group (n = 13) | Total (n = 28) | p-Value Concordant vs. Discordant |
|---|---|---|---|---|
| End of treatment (Day 14) | ||||
| Wound depth median (mm, IQ a) | 2 ± 1.25 | 3 ± 14 | 2 ± 4.5 | 0.0516 |
| Wound surface area (mm2, IQ) | 43.55 ± 72.12 | 75.8 ± 220.15 | 43.55 ± 135.8 | 0.4556 |
| Exsudative wound (n, %) | 0 (0) | 4 (30.8) | 4 (14.3) | 0.0282 |
| Inflammatory signs (n, %) | 8 (53.3) | 11 (84.6) | 19 (67.9) | 0.0823 |
| Number of species (mean, SD b) | 1.79 ± 1.05 | 1.58 ± 0.51 | 1.69 ± 0.84 | 0.55 |
| Gram-Negative Bacilli (n, %) | 5 (33.3) | 6 (46.2) | 11 (39.3) | 0.6922 |
| Gram-Positive Cocci (n, %) | 8 (53.3) | 7 (53.8) | 15 (53.6) | >0.999 |
| Anaerobes (n, %) | 0 (0) | 0 (0) | 0 (0) | >0.999 |
| Clinical improvement (n, %) | 12 (80.0) | 5 (38.5) | 17 (60.7) | 0.0245 |
| Wound healing (n, %) | 8 (53.3) | 4 (30.8) | 12 (42.9) | 0.2219 |
| End of follow-up (Day 45) | ||||
| Wound depth median (mm, IQ) | 3 ± 2 | 3 ± 18 | 3 ± 2 | 0.5482 |
| Wound surface area (mm2, IQ) | 22 ± 70.2 | 42.4 ± 185.3 | 29.85 ± 157.62 | 0.0595 |
| Exsudative wound (n, %) | 2 (13.3) | 3 (20.0) | 5 (17.9) | 0.3217 |
| Inflammatory signs (n, %) | 7 (46.7) | 9 (69.2) | 16 (57.1) | 0.4404 |
| Number of species (mean, SD) | 1.87 ± 1.13 | 1.69 ± 0.75 | 1.79 ± 0.96 | 0.6395 |
| Gram-Negative Bacilli (n, %) | 5 (33.3) | 3 (20.0) | 8 (28.6) | 0.686 |
| Gram-Positive Cocci (n, %) | 10 (66.7) | 7 (53.8) | 17 (60.7) | 0.7 |
| Anaerobes (n, %) | 0 (0) | 3 (20.0) | 3 (10.7) | 0.0873 |
| Clinical improvement (n, %) | 12 (80) | 8 (61.5) | 21 (75.0) | 0.5295 |
| Wound healing (n, %) | 11 (73.3) | 8 (61.5) | 19 (67.9) | 0.4953 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sotto, A.; Laurent, F.; Schuldiner, S.; Vouillarmet, J.; Corvec, S.; Bemer, P.; Boutoille, D.; Dunyach-Rémy, C.; Lavigne, J.-P. Evaluation of the Use of Antibiofilmogram Technology in the Clinical Evolution of Foot Ulcers Infected by Staphylococcus aureus in Persons Living with Diabetes: A Pilot Study. J. Clin. Med. 2021, 10, 5928. https://doi.org/10.3390/jcm10245928
Sotto A, Laurent F, Schuldiner S, Vouillarmet J, Corvec S, Bemer P, Boutoille D, Dunyach-Rémy C, Lavigne J-P. Evaluation of the Use of Antibiofilmogram Technology in the Clinical Evolution of Foot Ulcers Infected by Staphylococcus aureus in Persons Living with Diabetes: A Pilot Study. Journal of Clinical Medicine. 2021; 10(24):5928. https://doi.org/10.3390/jcm10245928
Chicago/Turabian StyleSotto, Albert, Frédéric Laurent, Sophie Schuldiner, Julien Vouillarmet, Stéphane Corvec, Pascale Bemer, David Boutoille, Catherine Dunyach-Rémy, and Jean-Philippe Lavigne. 2021. "Evaluation of the Use of Antibiofilmogram Technology in the Clinical Evolution of Foot Ulcers Infected by Staphylococcus aureus in Persons Living with Diabetes: A Pilot Study" Journal of Clinical Medicine 10, no. 24: 5928. https://doi.org/10.3390/jcm10245928
APA StyleSotto, A., Laurent, F., Schuldiner, S., Vouillarmet, J., Corvec, S., Bemer, P., Boutoille, D., Dunyach-Rémy, C., & Lavigne, J.-P. (2021). Evaluation of the Use of Antibiofilmogram Technology in the Clinical Evolution of Foot Ulcers Infected by Staphylococcus aureus in Persons Living with Diabetes: A Pilot Study. Journal of Clinical Medicine, 10(24), 5928. https://doi.org/10.3390/jcm10245928







