Current Knowledge and Future Challenges in Takotsubo Syndrome: Part 1—Pathophysiology and Diagnosis
Abstract
1. Introduction
2. Clinical Manifestation and Outcome
3. Epidemiology
4. Diagnosis
4.1. ECG Patterns
4.2. Biomarkers
4.3. Angiography and Ventriculography
4.4. Echocardiography
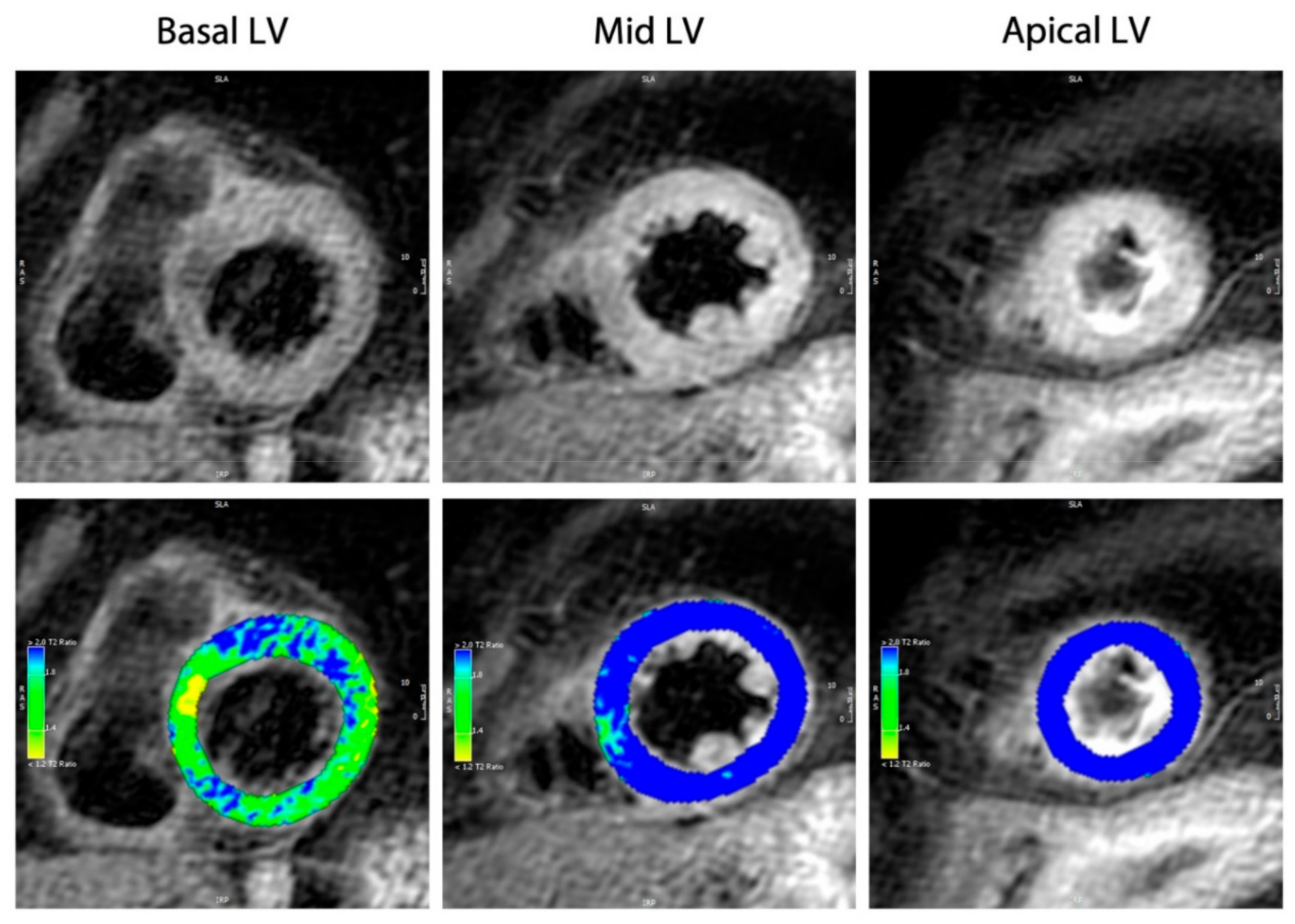
4.5. Cardiac Magnetic Resonance Imaging
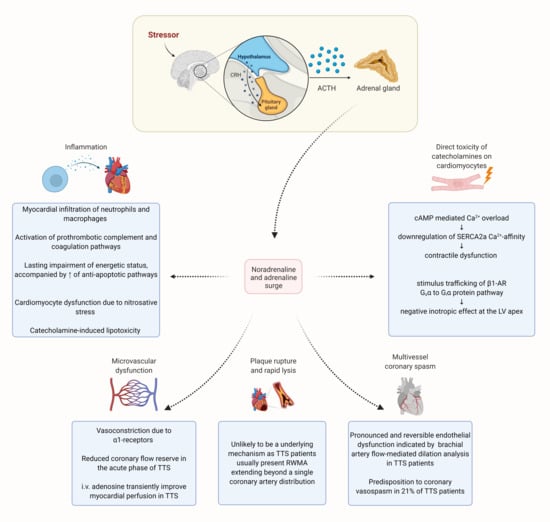
5. Pathophysiology
5.1. Sympathetic Hyperactivity
5.1.1. Multivessel Coronary Spasm
5.1.2. Plaque Rupture
5.1.3. Microvascular Dysfunction
5.1.4. Direct Toxicity of Catecholamines on Cardiomyocytes
5.1.5. Genetic Predisposition
5.2. Hormonal Factors
5.3. The Role of Inflammation in TTS
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sato, H.; Tateishi, H.; Uchida, T.; Dote, K.; Ishihara, M.; Kodama, K.; Haze, K.; Hori, M. Clinical Aspect of Myocardial Injury: From Ischemia to Heart Failure; Kagaku Hyoronsha: Tokyo, Japan, 1990; pp. 55–64. [Google Scholar]
- Eitel, I.; Behrendt, F.; Schindler, K.; Kivelitz, D.; Gutberlet, M.; Schuler, G.; Thiele, H. Differential diagnosis of suspected apical ballooning syndrome using contrast-enhanced magnetic resonance imaging. Eur. Heart J. 2008, 29, 2651–2659. [Google Scholar] [CrossRef] [PubMed]
- Ghadri, J.R.; Cammann, V.L.; Napp, L.C.; Jurisic, S.; Diekmann, J.; Bataiosu, D.R.; Seifert, B.; Jaguszewski, M.; Sarcon, A.; Neumann, C.A.; et al. Differences in the Clinical Profile and Outcomes of Typical and Atypical Takotsubo Syndrome. JAMA Cardiol. 2016, 1, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Templin, C.; Ghadri, J.R.; Diekmann, J.; Napp, L.C.; Bataiosu, D.R.; Jaguszewski, M.; Cammann, V.L.; Sarcon, A.; Geyer, V.; Neumann, C.A.; et al. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N. Engl. J. Med. 2015, 373, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Akashi, Y.J.; Nef, H.M.; Lyon, A.R. Epidemiology and pathophysiology of Takotsubo syndrome. Nat. Rev. Cardiol. 2015, 12, 387–397. [Google Scholar] [CrossRef]
- Aizawa, K.; Suzuki, T. Takotsubo Cardiomyopathy. Heart Fail. Clin. 2013, 9, 243–247. [Google Scholar] [CrossRef]
- Stähli, B.E.; Ruschitzka, F.; Enseleit, F. Isolated right ventricular ballooning syndrome: A new variant of transient cardiomyopathy. Eur. Heart J. 2011, 32, 1821. [Google Scholar] [CrossRef]
- Shams, Y.; Tornvall, P.; Tornerud, M.; Henareh, L. Capecitabine caused cardiogenic shock through induction of global Takotsubo syndrome. Cardiovasc. Revascularization Med. 2013, 14, 57–61. [Google Scholar] [CrossRef]
- Eitel, I.; von Knobelsdorff-Brenkenhoff, F.; Bernhardt, P.; Carbone, I.; Muellerleile, K.; Aldrovandi, A.; Francone, M.; Desch, S.; Gutberlet, M.; Strohm, O.; et al. Clinical Characteristics and Cardiovascular Magnetic Resonance Findings in Stress (Takotsubo) Cardiomyopathy. JAMA 2011, 306, 277–286. [Google Scholar] [CrossRef]
- Ghadri, J.-R.; Wittstein, I.S.; Prasad, A.; Sharkey, S.; Dote, K.; Akashi, Y.J.; Cammann, V.L.; Crea, F.; Galiuto, L.; Desmet, W.; et al. International Expert Consensus Document on Takotsubo Syndrome (Part I): Clinical Characteristics, Diagnostic Criteria, and Pathophysiology. Eur. Heart J. 2018, 39, 2032–2046. [Google Scholar] [CrossRef]
- Scally, C.; Ahearn, T.; Rudd, A.; Neil, C.J.; Srivanasan, J.; Jagpal, B.; Horowitz, J.; Frenneaux, M.; Dawson, D.K. Right Ventricular Involvement and Recovery After Acute Stress-Induced (Tako-tsubo) Cardiomyopathy. Am. J. Cardiol. 2016, 117, 775–780. [Google Scholar] [CrossRef]
- Pelliccia, F.; Parodi, G.; Greco, C.; Antoniucci, D.; Brenner, R.; Bossone, E.; Cacciotti, L.; Capucci, A.; Citro, R.; Delmas, C.; et al. Comorbidities frequency in Takotsubo syndrome: An international collaborative systematic review including 1109 patients. Am. J. Med. 2015, 128, 654.e11–654.e19. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, S.W.; Lesser, J.R.; Maron, M.S.; Maron, B.J. Why not just call it tako-tsubo cardiomyopathy: A discussion of nomenclature. J. Am. Coll. Cardiol. 2011, 57, 1496–1497. [Google Scholar] [CrossRef] [PubMed]
- Ghadri, J.R.; Sarcon, A.; Diekmann, J.; Bataiosu, D.R.; Cammann, V.L.; Jurisic, S.; Napp, L.C.; Jaguszewski, M.; Scherff, F.; Brugger, P.; et al. Happy heart syndrome: Role of positive emotional stress in takotsubo syndrome. Eur. Heart J. 2016, 37, 2823–2829. [Google Scholar] [CrossRef] [PubMed]
- Ghadri, J.R.; Bataisou, R.D.; Diekmann, J.; Lüscher, T.F.; Templin, C. First case of atypical takotsubo cardiomyopathy in a bilateral lung-transplanted patient due to acute respiratory failure. Eur. Heart J. Acute Cardiovasc. Care 2015, 4, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Cheah, C.F.; Kofler, M.; Schiefecker, A.J.; Beer, R.; Klug, G.; Pfausler, B.; Helbok, R. Takotsubo Cardiomyopathy in Traumatic Brain Injury. Neurocrit. Care 2017, 26, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Minhas, A.S.; Scheel, P.; Garibaldi, B.; Liu, G.; Horton, M.; Jennings, M.; Jones, S.R.; Michos, E.D.; Hays, A.G. Takotsubo Syndrome in the Setting of COVID-19. JACC Case Rep. 2020, 2, 1321–1325. [Google Scholar] [CrossRef]
- Mathew, B.; Villarreal, D. Two unusual cases of Takotsubo cardiomyopathy presenting with sudden cardiac death. Am. J. Med. Sci. 2009, 337, 473–475. [Google Scholar] [CrossRef]
- Oindi, F.M.; Sequeira, E.; Sequeira, H.R.; Mutiso, S.K. Takotsubo cardiomyopathy in pregnancy: A case report and literature review. BMC Pregnancy Childbirth 2019, 19, 89. [Google Scholar] [CrossRef]
- Shams, Y. Takotsubo Syndrome Triggered by Acute Coronary Syndrome in a Cohort of 20 Patients: An often Missed Diagnosis. Int. J. Cardiol. Res. 2015, 2, 28–33. [Google Scholar] [CrossRef]
- Templin, C.; Napp, L.C.; Ghadri, J.R. Takotsubo Syndrome: Underdiagnosed, Underestimated, but Understood? J. Am. Coll. Cardiol. 2016, 67, 1937–1940. [Google Scholar] [CrossRef]
- Yaguchi, M.; Yaguchi, H.; Takahashi, N. A case of asymptomatic takotsubo cardiomyopathy with intraventricular thrombus associated with epileptic seizure. Brain Nerve 2011, 63, 897–900. [Google Scholar] [PubMed]
- Kyi, H.H.; Aljariri Alhesan, N.; Upadhaya, S.; Al Hadidi, S. Seizure Associated Takotsubo Syndrome: A Rare Combination. Case Rep. Cardiol. 2017, 2017, 8458054. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.M.; Kim, J.G.; Kim, J.B.; Cho, K.H.; Yu, S.; Oh, K.; Kim, Y.H.; Choi, J.Y.; Seo, W.K. Takotsubo-Like Myocardial Dysfunction in Ischemic Stroke: A Hospital-Based Registry and Systematic Literature Review. Stroke 2016, 47, 2729–2736. [Google Scholar] [CrossRef] [PubMed]
- Song, B.G.; Yang, H.S.; Hwang, H.K.; Kang, G.H.; Park, Y.H.; Chun, W.J.; Oh, J.H. The impact of stressor patterns on clinical Features in patients with tako-tsubo cardiomyopathy: Experiences of two tertiary cardiovascular centers. Clin. Cardiol. 2012, 35, E6-13. [Google Scholar] [CrossRef] [PubMed]
- Stiermaier, T.; Moeller, C.; Oehler, K.; Desch, S.; Graf, T.; Eitel, C.; Vonthein, R.; Schuler, G.; Thiele, H.; Eitel, I. Long-term excess mortality in takotsubo cardiomyopathy: Predictors, causes and clinical consequences. Eur. J. Heart Fail. 2016, 18, 650–656. [Google Scholar] [CrossRef]
- Shams, Y. Clinical Features and Outcome of Pheochromocytoma-Induced Takotsubo Syndrome: Analysis of 80 Published Cases. Am. J. Cardiol. 2016, 117, 1836–1844. [Google Scholar] [CrossRef]
- Shoukat, S.; Awad, A.; Nam, D.K.; Hoskins, M.H.; Samuels, O.; Higginson, J.; Clements, S.D., Jr. Cardiomyopathy with inverted tako-tsubo pattern in the setting of subarachnoid hemorrhage: A series of four cases. Neurocrit. Care 2013, 18, 257–260. [Google Scholar] [CrossRef]
- Elesber, A.; Lerman, A.; Bybee, K.A.; Murphy, J.G.; Barsness, G.; Singh, M.; Rihal, C.S.; Prasad, A. Myocardial perfusion in apical ballooning syndrome correlate of myocardial injury. Am. Heart J. 2006, 152, 469.e9–469.e13. [Google Scholar] [CrossRef]
- Stiermaier, T.; Santoro, F.; Graf, T.; Guastafierro, F.; Tarantino, N.; De Gennaro, L.; Caldarola, P.; Di Biase, M.; Thiele, H.; Brunetti, N.D.; et al. Prognostic value of N-Terminal Pro-B-Type Natriuretic Peptide in Takotsubo syndrome. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2018, 107, 597–606. [Google Scholar] [CrossRef]
- Dias, A.; Núñez Gil, I.J.; Santoro, F.; Madias, J.E.; Pelliccia, F.; Brunetti, N.D.; Salmoirago-Blotcher, E.; Sharkey, S.W.; Eitel, I.; Akashi, Y.J.; et al. Takotsubo syndrome: State-of-the-art review by an expert panel—Part 1. Cardiovasc. Revascularization Med. 2019, 20, 70–79. [Google Scholar] [CrossRef]
- Stiermaier, T.; Santoro, F.; El-Battrawy, I.; Möller, C.; Graf, T.; Novo, G.; Santangelo, A.; Mariano, E.; Romeo, F.; Caldarola, P.; et al. Prevalence and Prognostic Impact of Diabetes in Takotsubo Syndrome: Insights From the International, Multicenter GEIST Registry. Diabetes Care 2018, 41, 1084. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, A.; Núñez-Gil Iván, J.; Conty, D.A.; Vedia, O.; Almendro-Delia, M.; Duran Cambra, A.; Martin-Garcia Agustin, C.; Barrionuevo-Sánchez, M.; Martínez-Sellés, M.; Raposeiras-Roubín, S.; et al. Short- and Long-Term Prognosis of Patients With Takotsubo Syndrome Based on Different Triggers: Importance of the Physical Nature. J. Am. Heart Assoc. 2019, 8, e013701. [Google Scholar] [CrossRef]
- Santoro, F.; Nunez Gil, I.J.; Stiermaier, T.; El-Battrawy, I.; Guerra, F.; Novo, G.; Guastafierro, F.; Tarantino, N.; Novo, S.; Mariano, E.; et al. Assessment of the German and Italian Stress Cardiomyopathy Score for Risk Stratification for In-hospital Complications in Patients With Takotsubo Syndrome. JAMA Cardiol. 2019, 4, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Arcari, L.; Musumeci, M.B.; Stiermaier, T.; El-Battrawy, I.; Möller, C.; Guerra, F.; Novo, G.; Mariano, E.; Limite, L.R.; Cacciotti, L.; et al. Incidence, determinants and prognostic relevance of dyspnea at admission in patients with Takotsubo syndrome: Results from the international multicenter GEIST registry. Sci. Rep. 2020, 10, 13603. [Google Scholar] [CrossRef] [PubMed]
- Kurowski, V.; Kaiser, A.; von Hof, K.; Killermann, D.P.; Mayer, B.; Hartmann, F.; Schunkert, H.; Radke, P.W. Apical and midventricular transient left ventricular dysfunction syndrome (tako-tsubo cardiomyopathy): Frequency, mechanisms, and prognosis. Chest 2007, 132, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Akashi, Y.J.; Goldstein, D.S.; Barbaro, G.; Ueyama, T. Takotsubo cardiomyopathy: A new form of acute, reversible heart failure. Circulation 2008, 118, 2754–2762. [Google Scholar] [CrossRef]
- Deshmukh, A.; Kumar, G.; Pant, S.; Rihal, C.; Murugiah, K.; Mehta, J.L. Prevalence of Takotsubo cardiomyopathy in the United States. Am. Heart J. 2012, 164, 66–71.e61. [Google Scholar] [CrossRef]
- Shams, Y.; Tornvall, P. Epidemiology, pathogenesis, and management of takotsubo syndrome. Clin Auton. Res. 2018, 28, 53–65. [Google Scholar] [CrossRef]
- Minhas, A.S.; Hughey, A.B.; Kolias, T.J. Nationwide Trends in Reported Incidence of Takotsubo Cardiomyopathy from 2006 to 2012. Am. J. Cardiol. 2015, 116, 1128–1131. [Google Scholar] [CrossRef]
- Schneider, B.; Athanasiadis, A.; Stöllberger, C.; Pistner, W.; Schwab, J.; Gottwald, U.; Schoeller, R.; Gerecke, B.; Hoffmann, E.; Wegner, C.; et al. Gender differences in the manifestation of tako-tsubo cardiomyopathy. Int. J. Cardiol. 2013, 166, 584–588. [Google Scholar] [CrossRef]
- Sharkey, S.W.; Windenburg, D.C.; Lesser, J.R.; Maron, M.S.; Hauser, R.G.; Lesser, J.N.; Haas, T.S.; Hodges, J.S.; Maron, B.J. Natural History and Expansive Clinical Profile of Stress (Tako-Tsubo) Cardiomyopathy. J. Am. Coll. Cardiol. 2010, 55, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Topal, Y.; Topal, H.; Dogan, C.; Tiryaki, S.B.; Biteker, M. Takotsubo (stress) cardiomyopathy in childhood. Eur. J. Pediatr. 2020, 179, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Urbinati, A.; Pellicori, P.; Guerra, F.; Capucci, A.; Clark, A.L. Takotsubo syndrome in the paediatric population: A case report and a systematic review. J. Cardiovasc. Med. 2017, 18, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.; Franco, E.; Koshkelashvili, N.; Pressman, G.S.; Hebert, K.; Figueredo, V.M. Racial and ethnic differences in Takotsubo cardiomyopathy presentation and outcomes. Int. J. Cardiol. 2015, 194, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Franco, E.; Dias, A.; Koshkelashvili, N.; Pressman, G.S.; Hebert, K.; Figueredo, V.M. Distinctive Electrocardiographic Features in African Americans Diagnosed with Takotsubo Cardiomyopathy. Ann. Noninvasive Electrocardiol. 2016, 21, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Carson, K.; Usmani, Z.; Sawhney, G.; Shah, R.; Horowitz, J. Systematic review and meta-analysis of incidence and correlates of recurrence of takotsubo cardiomyopathy. Int. J. Cardiol. 2014, 174, 696–701. [Google Scholar] [CrossRef]
- Patel, S.M.; Chokka, R.G.; Prasad, K.; Prasad, A. Distinctive Clinical Characteristics According to Age and Gender in Apical Ballooning Syndrome (Takotsubo/Stress Cardiomyopathy): An Analysis Focusing on Men and Young Women. J. Card. Fail. 2013, 19, 306–310. [Google Scholar] [CrossRef]
- El-Battrawy, I.; Santoro, F.; Stiermaier, T.; Möller, C.; Guastafierro, F.; Novo, G.; Novo, S.; Mariano, E.; Romeo, F.; Romeo, F.; et al. Incidence and Clinical Impact of Recurrent Takotsubo Syndrome: Results From the GEIST Registry. J. Am. Heart Assoc. 2019, 8, e010753. [Google Scholar] [CrossRef]
- Prasad, A.; Lerman, A.; Rihal, C.S. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): A mimic of acute myocardial infarction. Am. Heart J. 2008, 155, 408–417. [Google Scholar] [CrossRef]
- Lyon, A.R.; Bossone, E.; Schneider, B.; Sechtem, U.; Citro, R.; Underwood, S.R.; Sheppard, M.N.; Figtree, G.A.; Parodi, G.; Akashi, Y.J.; et al. Current state of knowledge on Takotsubo syndrome: A Position Statement from the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2016, 18, 8–27. [Google Scholar] [CrossRef]
- Namgung, J. Electrocardiographic Findings in Takotsubo Cardiomyopathy: ECG Evolution and Its Difference from the ECG of Acute Coronary Syndrome. Clin. Med. Insights Cardiol. 2014, 8, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Kurisu, S.; Inoue, I.; Kawagoe, T.; Ishihara, M.; Shimatani, Y.; Nakamura, S.; Yoshida, M.; Mitsuba, N.; Hata, T.; Sato, H. Time course of electrocardiographic changes in patients with tako-tsubo syndrome: Comparison with acute myocardial infarction with minimal enzymatic release. Circ. J. Off. J. Jpn. Circ. Soc. 2004, 68, 77–81. [Google Scholar] [CrossRef]
- Frangieh Antonio, H.; Obeid, S.; Ghadri, J.R.; Imori, Y.; D’Ascenzo, F.; Kovac, M.; Ruschitzka, F.; Lüscher Thomas, F.; Duru, F.; Templin, C.; et al. ECG Criteria to Differentiate Between Takotsubo (Stress) Cardiomyopathy and Myocardial Infarction. J. Am. Heart Assoc. 2016, 5, e003418. [Google Scholar] [CrossRef] [PubMed]
- Behr, E.R.; Mahida, S. Takotsubo cardiomyopathy and the long-QT syndrome: An insult to repolarization reserve. EP Eur. 2009, 11, 697–700. [Google Scholar] [CrossRef] [PubMed]
- Stöllberger, C.; Finsterer, J.; Schneider, B. Transient left ventricular dysfunction (tako-tsubo phenomenon): Findings and potential pathophysiological mechanisms. Can. J. Cardiol. 2006, 22, 1063–1068. [Google Scholar] [CrossRef]
- Ogura, R.; Hiasa, Y.; Takahashi, T.; Yamaguchi, K.; Fujiwara, K.; Ohara, Y.; Nada, T.; Ogata, T.; Kusunoki, K.; Yuba, K.; et al. Specific findings of the standard 12-lead ECG in patients with ‘Takotsubo’ cardiomyopathy: Comparison with the findings of acute anterior myocardial infarction. Circ. J. Off. J. Jpn. Circ. Soc. 2003, 67, 687–690. [Google Scholar] [CrossRef]
- Santoro, F.; Brunetti, N.D.; Tarantino, N.; Romero, J.; Guastafierro, F.; Ferraretti, A.; Di Martino, L.F.M.; Ieva, R.; Pellegrino, P.L.; Di Biase, M.; et al. Dynamic changes of QTc interval and prognostic significance in takotsubo (stress) cardiomyopathy. Clin. Cardiol. 2017, 40, 1116–1122. [Google Scholar] [CrossRef]
- Santoro, F.; Stiermaier, T.; Tarantino, N.; Guastafierro, F.; Graf, T.; Möller, C.; Di Martino, L.F.M.; Thiele, H.; Di Biase, M.; Eitel, I.; et al. Impact of persistent ST elevation on outcome in patients with Takotsubo syndrome. Results from the GErman Italian STress Cardiomyopathy (GEIST) registry. Int. J. Cardiol. 2018, 255, 140–144. [Google Scholar] [CrossRef]
- Pilgrim, T.M.; Wyss, T.R. Takotsubo cardiomyopathy or transient left ventricular apical ballooning syndrome: A systematic review. Int. J. Cardiol. 2008, 124, 283–292. [Google Scholar] [CrossRef]
- Pirlet, C.; Pierard, L.; Legrand, V.; Gach, O. Ratio of high-sensitivity troponin to creatine kinase-MB in takotsubo syndrome. Int. J. Cardiol. 2017, 243, 300–305. [Google Scholar] [CrossRef]
- Fröhlich, G.M.; Schoch, B.; Schmid, F.; Keller, P.; Sudano, I.; Lüscher, T.F.; Noll, G.; Ruschitzka, F.; Enseleit, F. Takotsubo cardiomyopathy has a unique cardiac biomarker profile: NT-proBNP/myoglobin and NT-proBNP/troponin T ratios for the differential diagnosis of acute coronary syndromes and stress induced cardiomyopathy. Int. J. Cardiol. 2012, 154, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Dagrenat, C.; Von Hunolstein, J.J.; Matsushita, K.; Thebaud, L.; Greciano, S.; Tuzin, N.; Meyer, N.; Trinh, A.; Jesel, L.; Ohlmann, P.; et al. Value of Cardiac Biomarkers in the Early Diagnosis of Takotsubo Syndrome. J. Clin. Med. 2020, 9, 2985. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Neil, C.J.; Sverdlov, A.L.; Mahadavan, G.; Chirkov, Y.Y.; Kucia, A.M.; Stansborough, J.; Beltrame, J.F.; Selvanayagam, J.B.; Zeitz, C.J.; et al. N-terminal pro-brain natriuretic protein levels in takotsubo cardiomyopathy. Am. J. Cardiol. 2011, 108, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, P.; Zaidi, R.; Sardar, M.R. Takotsubo cardiomyopathy: Pathophysiology and role of cardiac biomarkers in differential diagnosis. World J. Cardiol. 2017, 9, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Acher, R.; Chauvet, J.; Rouille, Y. Dynamic Processing of Neuropeptides. J. Mol. Neurosci. 2002, 18, 223–228. [Google Scholar] [CrossRef]
- Boeckel, J.-N.; Oppermann, J.; Anadol, R.; Fichtlscherer, S.; Zeiher, A.M.; Keller, T. Analyzing the Release of Copeptin from the Heart in Acute Myocardial Infarction Using a Transcoronary Gradient Model. Sci. Rep. 2016, 6, 20812. [Google Scholar] [CrossRef]
- Reichlin, T.; Hochholzer, W.; Stelzig, C.; Laule, K.; Freidank, H.; Morgenthaler, N.G.; Bergmann, A.; Potocki, M.; Noveanu, M.; Breidthardt, T.; et al. Incremental Value of Copeptin for Rapid Rule Out of Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2009, 54, 60–68. [Google Scholar] [CrossRef]
- Burgdorf, C.; Schubert, A.; Schunkert, H.; Kurowski, V.; Radke, P.W. Release patterns of copeptin and troponin in Tako-Tsubo cardiomyopathy. Peptides 2012, 34, 389–394. [Google Scholar] [CrossRef]
- Budnik, M.; Białek, S.; Peller, M.; Kiszkurno, A.; Kochanowski, J.; Kucharz, J.; Sitkiewicz, D.; Opolski, G. Serum copeptin and copeptin/NT-proBNP ratio—New tools to differentiate takotsubo syndrome from acute myocardial infarction. Folia Med. Crac. 2020, 60, 5–14. [Google Scholar] [CrossRef]
- Yang, H.S.; Kim, H.J.; Shim, H.J.; Kim, S.J.; Hur, M.; Di Somma, S.; Network, G. Soluble ST2 and troponin I combination: Useful biomarker for predicting development of stress cardiomyopathy in patients admitted to the medical intensive care unit. Heart Lung J. Crit. Care 2015, 44, 282–288. [Google Scholar] [CrossRef]
- Højagergaard, M.A.; Hassager, C.; Christensen, T.E.; Bang, L.E.; Gøtze, J.P.; Ostrowski, S.R.; Holmvang, L.; Frydland, M. Biomarkers in patients with Takotsubo cardiomyopathy compared to patients with acute anterior ST-elevation myocardial infarction. Biomark. Biochem. Indic. Expo. Responseand Susceptibility Chem. 2020, 25, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, S.R.; Pedersen, S.H.; Jensen, J.S.; Mogelvang, R.; Johansson, P.I. Acute myocardial infarction is associated with endothelial glycocalyx and cell damage and a parallel increase in circulating catecholamines. Crit. Care 2013, 17, R32. [Google Scholar] [CrossRef] [PubMed]
- Pirzer, R.; Elmas, E.; Haghi, D.; Lippert, C.; Kralev, S.; Lang, S.; Borggrefe, M.; Kälsch, T. Platelet and monocyte activity markers and mediators of inflammation in Takotsubo cardiomyopathy. Heart Vessel. 2012, 27, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Santoro, F.; Costantino, M.D.; Guastafierro, F.; Triggiani, G.; Ferraretti, A.; Tarantino, N.; Saguner, A.; Di Biase, M.; Brunetti, N.D. Inflammatory patterns in Takotsubo cardiomyopathy and acute coronary syndrome: A propensity score matched analysis. Atherosclerosis 2018, 274, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Stiermaier, T.; Adams, V.; Just, M.; Blazek, S.; Desch, S.; Schuler, G.; Thiele, H.; Eitel, I. Growth differentiation factor-15 in Takotsubo cardiomyopathy: Diagnostic and prognostic value. Int. J. Cardiol. 2014, 173, 424–429. [Google Scholar] [CrossRef]
- Tarantino, N.; Santoro, F.; Di Biase, L.; Di Terlizzi, V.; Vitale, E.; Barone, R.; Della Rocca, D.G.; De Leon De La Cruz, N.S.; Di Biase, M.; Brunetti, N.D. Chromogranin-A serum levels in patients with takotsubo syndrome and ST elevation acute myocardial infarction. Int. J. Cardiol. 2020, 320, 12–17. [Google Scholar] [CrossRef]
- Jaguszewski, M.; Osipova, J.; Ghadri, J.R.; Napp, L.C.; Widera, C.; Franke, J.; Fijalkowski, M.; Nowak, R.; Fijalkowska, M.; Volkmann, I.; et al. A signature of circulating microRNAs differentiates takotsubo cardiomyopathy from acute myocardial infarction. Eur. Heart J. 2014, 35, 999–1006. [Google Scholar] [CrossRef]
- Baudry, A.; Mouillet-Richard, S.; Schneider, B.; Launay, J.M.; Kellermann, O. MiR-16 Targets the Serotonin Transporter: A New Facet for Adaptive Responses to Antidepressants. Science 2010, 329, 1537–1541. [Google Scholar] [CrossRef]
- Dwivedi, Y. Evidence demonstrating role of microRNAs in the etiopathology of major depression. J. Chem. Neuroanat. 2011, 42, 142–156. [Google Scholar] [CrossRef]
- Ghadri, J.-R.; Wittstein, I.S.; Prasad, A.; Sharkey, S.; Dote, K.; Akashi, Y.J.; Cammann, V.L.; Crea, F.; Galiuto, L.; Desmet, W.; et al. International Expert Consensus Document on Takotsubo Syndrome (Part II): Diagnostic Workup, Outcome, and Management. Eur. Heart J. 2018, 39, 2047–2062. [Google Scholar] [CrossRef]
- Pan, X.-Y.; Zhang, Z.-W. MFGE8, ALB, APOB, APOE, SAA1, A2M, and C3 as Novel Biomarkers for Stress Cardiomyopathy. Cardiovasc. Ther. 2020, 2020, 1615826. [Google Scholar] [CrossRef] [PubMed]
- Citro, R.; Okura, H.; Ghadri, J.R.; Izumi, C.; Meimoun, P.; Izumo, M.; Dawson, D.; Kaji, S.; Eitel, I.; Kagiyama, N.; et al. Multimodality imaging in takotsubo syndrome: A joint consensus document of the European Association of Cardiovascular Imaging (EACVI) and the Japanese Society of Echocardiography (JSE). J. Echocardiogr. 2020, 18, 199–224. [Google Scholar] [CrossRef] [PubMed]
- Napp, L.C.; Cammann, V.L.; Jaguszewski, M.; Szawan, K.A.; Wischnewsky, M.; Gili, S.; Knorr, M.; Heiner, S.; Citro, R.; Bossone, E.; et al. Coexistence and outcome of coronary artery disease in Takotsubo syndrome. Eur. Heart J. 2020, 41, 3255–3268. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.M.; Lennon, R.J.; Prasad, A. Regional wall motion abnormality in apical ballooning syndrome (Takotsubo/stress cardiomyopathy): Importance of biplane left ventriculography for differentiating from spontaneously aborted anterior myocardial infarction. Int. J. Cardiovasc. Imaging 2012, 28, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Napp, L.C.; Ghadri, J.; Bauersachs, J.; Templin, C. Acute coronary syndrome or Takotsubo cardiomyopathy: The suspect may not always be the culprit. Int. J. Cardiol. 2015, 187, 116–119. [Google Scholar] [CrossRef]
- Desmet, W.; Bennett, J.; Ferdinande, B.; De Cock, D.; Adriaenssens, T.; Coosemans, M.; Sinnaeve, P.; Kayaert, P.; Dubois, C. The apical nipple sign: A useful tool for discriminating between anterior infarction and transient left ventricular ballooning syndrome. Eur. Heart J. Acute Cardiovasc. Care 2013, 3, 264–267. [Google Scholar] [CrossRef]
- Chou, A.Y.; Sedlak, T.; Aymong, E.; Sheth, T.; Starovoytov, A.; Humphries, K.H.; Mancini, G.B.J.; Saw, J. Spontaneous Coronary Artery Dissection Misdiagnosed as Takotsubo Cardiomyopathy: A Case Series. Can. J. Cardiol. 2015, 31, 1073.e1075–1073.e1078. [Google Scholar] [CrossRef]
- Shams, Y. Spontaneous coronary artery dissection and takotsubo syndrome: An often overlooked association; review. Cardiovasc. Revascularization Med. 2018, 19. [Google Scholar] [CrossRef]
- Madias, J.E. On a Plausible Association of Spontaneous Coronary Artery Dissection and Takotsubo Syndrome. Can. J. Cardiol. 2015, 31, 1410.e1411. [Google Scholar] [CrossRef]
- Eitel, I.; Stiermaier, T.; Graf, T.; Möller, C.; Rommel, K.-P.; Eitel, C.; Schuler, G.; Thiele, H.; Desch, S. Optical Coherence Tomography to Evaluate Plaque Burden and Morphology in Patients With Takotsubo Syndrome. J. Am. Heart Assoc. 2016, 5, e004474. [Google Scholar] [CrossRef]
- De Backer, O.; Debonnaire, P.; Gevaert, S.; Missault, L.; Gheeraert, P.; Muyldermans, L. Prevalence, associated factors and management implications of left ventricular outflow tract obstruction in takotsubo cardiomyopathy: A two-year, two-center experience. BMC Cardiovasc. Disord. 2014, 14, 147. [Google Scholar] [CrossRef] [PubMed]
- Citro, R.; Piscione, F.; Parodi, G.; Salerno-Uriarte, J.; Bossone, E. Role of Echocardiography in Takotsubo Cardiomyopathy. Heart Fail. Clin. 2013, 9, 157–166. [Google Scholar] [CrossRef]
- Citro, R.; Bossone, E.; Parodi, G.; Rigo, F.; Nardi, F.; Provenza, G.; Zito, C.; Novo, G.; Vitale, G.; Prota, C.; et al. Independent Impact of RV Involvement on In-Hospital Outcome of Patients With Takotsubo Syndrome. JACC Cardiovasc. Imaging 2016, 9, 894–895. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, M.; Rihal, C.S.; Lerman, A.; Prasad, A. Acute Heart Failure in Apical Ballooning Syndrome (TakoTsubo/Stress Cardiomyopathy). J. Am. Coll. Cardiol. 2011, 57, 1400–1401. [Google Scholar] [CrossRef] [PubMed]
- Scally, C.; Rudd, A.; Mezincescu, A.; Wilson, H.; Srivanasan, J.; Horgan, G.; Broadhurst, P.; Newby, D.E.; Henning, A.; Dawson, D.K. Persistent Long-Term Structural, Functional, and Metabolic Changes After Stress-Induced (Takotsubo) Cardiomyopathy. Circulation 2018, 137, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, K.; Ahearn, T.; Srinivasan, J.; Neil, C.J.; Scally, C.; Rudd, A.; Jagpal, B.; Frenneaux, M.P.; Pislaru, C.; Horowitz, J.D.; et al. Alterations in Cardiac Deformation, Timing of Contraction and Relaxation, and Early Myocardial Fibrosis Accompany the Apparent Recovery of Acute Stress-Induced (Takotsubo) Cardiomyopathy: An End to the Concept of Transience. J. Am. Soc. Echocardiogr. 2017, 30, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.-J.; Sohn, I.S. A case of biventricular involvement of Takotsubo cardiomyopathy: 3D echocardiographic imaging. J. Echocardiogr. 2014, 12, 48–49. [Google Scholar] [CrossRef]
- Santoro, F.; Stiermaier, T.; Tarantino, N.; De Gennaro, L.; Moeller, C.; Guastafierro, F.; Marchetti, M.F.; Montisci, R.; Carapelle, E.; Graf, T.; et al. Left Ventricular Thrombi in Takotsubo Syndrome: Incidence, Predictors, and Management: Results From the GEIST (German Italian Stress Cardiomyopathy) Registry. J. Am. Heart Assoc. 2017, 6, e006990. [Google Scholar] [CrossRef]
- Stiermaier, T.; Graf, T.; Möller, C.; Eitel, C.; Ledwoch, J.; Desch, S.; Gutberlet, M.; Schuler, G.; Thiele, H.; Eitel, I. Transient left atrial dysfunction is a feature of Takotsubo syndrome. J. Cardiovasc. Magn. Reson. 2017, 19, 15. [Google Scholar] [CrossRef]
- Schuster, A.; Hor Kan, N.; Kowallick Johannes, T.; Beerbaum, P.; Kutty, S. Cardiovascular Magnetic Resonance Myocardial Feature Tracking. Circ. Cardiovasc. Imaging 2016, 9, e004077. [Google Scholar] [CrossRef]
- Backhaus, S.J.; Stiermaier, T.; Lange, T.; Chiribiri, A.; Lamata, P.; Uhlig, J.; Kowallick, J.T.; Raaz, U.; Villa, A.; Lotz, J.; et al. Temporal changes within mechanical dyssynchrony and rotational mechanics in Takotsubo syndrome: A cardiovascular magnetic resonance imaging study. Int. J. Cardiol. 2018, 273, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Plácido, R.; Cunha Lopes, B.; Almeida, A.G.; Rochitte, C.E. The role of cardiovascular magnetic resonance in takotsubo syndrome. J. Cardiovasc. Magn. Reson. 2016, 18, 68. [Google Scholar] [CrossRef] [PubMed]
- Rolf, A.; Nef, H.M.; Mollmann, H.; Troidl, C.; Voss, S.; Conradi, G.; Rixe, J.; Steiger, H.; Beiring, K.; Hamm, C.W.; et al. Immunohistological basis of the late gadolinium enhancement phenomenon in tako-tsubo cardiomyopathy. Eur. Heart J. 2009, 30, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Naruse, Y.; Sato, A.; Kasahara, K.; Makino, K.; Sano, M.; Takeuchi, Y.; Nagasaka, S.; Wakabayashi, Y.; Katoh, H.; Satoh, H.; et al. The clinical impact of late gadolinium enhancement in Takotsubo cardiomyopathy: Serial analysis of cardiovascular magnetic resonance images. J. Cardiovasc. Magn. Reson. 2011, 13, 67. [Google Scholar] [CrossRef]
- Eitel, I.; Friedrich, M.G. T2-weighted cardiovascular magnetic resonance in acute cardiac disease. J. Cardiovasc. Magn. Reson. 2011, 13, 13. [Google Scholar] [CrossRef]
- Wittstein, I.S.; Thiemann, D.R.; Lima, J.A.; Baughman, K.L.; Schulman, S.P.; Gerstenblith, G.; Wu, K.C.; Rade, J.J.; Bivalacqua, T.J.; Champion, H.C. Neurohumoral features of myocardial stunning due to sudden emotional stress. N. Engl. J. Med. 2005, 352, 539–548. [Google Scholar] [CrossRef]
- Madias, J.E. Blood norepinephrine/epinephrine/dopamine measurements in 108 patients with takotsubo syndrome from the world literature: Pathophysiological implications. Acta Cardiol. 2020, 1–9. [Google Scholar] [CrossRef]
- Shams, Y. Plasma Epinephrine Level and its Causal Link to Takotsubo Syndrome Revisited: Critical Review with a Diverse Conclusion. Cardiovasc. Revascularization Med. 2019, 20, 907–914. [Google Scholar] [CrossRef]
- Kume, T.; Kawamoto, T.; Okura, H.; Toyota, E.; Neishi, Y.; Watanabe, N.; Hayashida, A.; Okahashi, N.; Yoshimura, Y.; Saito, K.; et al. Local Release of Catecholamines From the Hearts of Patients With Tako-Tsubo-Like Left Ventricular Dysfunction. Circ. J. 2008, 72, 106–108. [Google Scholar] [CrossRef]
- Abraham, J.; Mudd, J.O.; Kapur, N.; Klein, K.; Champion, H.C.; Wittstein, I.S. Stress Cardiomyopathy After Intravenous Administration of Catecholamines and Beta-Receptor Agonists. J. Am. Coll. Cardiol. 2009, 53, 1320–1325. [Google Scholar] [CrossRef]
- Ueyama, T.; Kasamatsu, K.; Hano, T.; Yamamoto, K.; Tsuruo, Y.; Nishio, I. Emotional stress induces transient left ventricular hypocontraction in the rat via activation of cardiac adrenoceptors: A possible animal model of ‘tako-tsubo’ cardiomyopathy. Circ. J. Off. J. Jpn. Circ. Soc. 2002, 66, 712–713. [Google Scholar] [CrossRef]
- Redfors, B.; Ali, A.; Shao, Y.; Lundgren, J.; Gan, L.-M.; Omerovic, E. Different catecholamines induce different patterns of takotsubo-like cardiac dysfunction in an apparently afterload dependent manner. Int. J. Cardiol. 2014, 174, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, A.; Despas, F.; Delmas, C.; Lairez, O.; Lambert, E.; Lambert, G.; Labrunee, M.; Guiraud, T.; Esler, M.; Galinier, M.; et al. Direct evidences for sympathetic hyperactivity and baroreflex impairment in Tako Tsubo cardiopathy. PLoS ONE 2014, 9, e93278. [Google Scholar] [CrossRef] [PubMed]
- Burgdorf, C. Regional alterations in myocardial sympathetic innervation in patients with transient left-ventricular apical ballooning (Tako-Tsubo cardiomyopathy). J. Nucl. Cardiol. Off. Publ. Am. Soc. Nucl. Cardiol. 2008, 15, 65–72. [Google Scholar] [CrossRef]
- Verberne, H.J.; van der Heijden, D.J.; van Eck-Smit, B.L.; Somsen, G.A. Persisting myocardial sympathetic dysfunction in takotsubo cardiomyopathy. J. Nucl. Cardiol. 2009, 16, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Haft, J.I. Cardiovascular injury induced by sympathetic catecholamines. Prog. Cardiovasc. Dis. 1974, 17, 73–86. [Google Scholar] [CrossRef]
- Shams, Y. Insights into the pathogenesis of takotsubo syndrome, which with persuasive reasons should be regarded as an acute cardiac sympathetic disease entity. ISRN Cardiol. 2012, 2012, 593735. [Google Scholar] [CrossRef][Green Version]
- Zaroff, J.G.; Rordorf, G.A.; Ogilvy, C.S.; Picard, M.H. Regional patterns of left ventricular systolic dysfunction after subarachnoid hemorrhage: Evidence for neurally mediated cardiac injury. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2000, 13, 774–779. [Google Scholar] [CrossRef]
- Spieker, L.E.; Hürlimann, D.; Ruschitzka, F.; Corti, R.; Enseleit, F.; Shaw, S.; Hayoz, D.; Deanfield, J.E.; Lüscher, T.F.; Noll, G. Mental Stress Induces Prolonged Endothelial Dysfunction via Endothelin-A Receptors. Circulation 2002, 105, 2817–2820. [Google Scholar] [CrossRef]
- Scantlebury, D.C.; Prasad, A.; Rabinstein, A.A.; Best, P.J.M. Prevalence of Migraine and Raynaud Phenomenon in Women With Apical Ballooning Syndrome (Takotsubo or Stress Cardiomyopathy). Am. J. Cardiol. 2013, 111, 1284–1288. [Google Scholar] [CrossRef]
- Vasilieva, E.; Vorobyeva, I.; Lebedeva, A.; Urazovskaya, I.; Kalinskaya, A.; Skrypnik, D.; Shpektor, A. Brachial artery flow-mediated dilation in patients with Tako-tsubo cardiomyopathy. Am. J. Med. 2011, 124, 1176–1179. [Google Scholar] [CrossRef] [PubMed]
- Naegele, M.; Flammer, A.J.; Enseleit, F.; Roas, S.; Frank, M.; Hirt, A.; Kaiser, P.; Cantatore, S.; Templin, C.; Fröhlich, G.; et al. Endothelial function and sympathetic nervous system activity in patients with Takotsubo syndrome. Int. J. Cardiol. 2016, 224, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Carbonara, R.; Giardinelli, F.; Pepe, M.; Luzzi, G.; Panettieri, I.; Vulpis, V.; Bortone, A.S.; Ciccone, M.M. Correlation between endothelial dysfunction and myocardial damage in acute phase of Tako-Tsubo cardiomyopathy: Brachial flow mediated dilation as a potential marker for assessment of patient with Tako-Tsubo. Heart Vessel. 2017, 33, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Tsuchihashi, K.; Ueshima, K.; Uchida, T.; Oh-mura, N.; Kimura, K.; Owa, M.; Yoshiyama, M.; Miyazaki, S.; Haze, K.; Ogawa, H.; et al. Transient left ventricular apical ballooning without coronary artery stenosis: A novel heart syndrome mimicking acute myocardial infarction. J. Am. Coll. Cardiol. 2001, 38, 11–18. [Google Scholar] [CrossRef]
- Migliore, F.; Maffei, E.; Perazzolo Marra, M.; Bilato, C.; Napodano, M.; Corbetti, F.; Zorzi, A.; Andres, A.L.; Sarais, C.; Cacciavillani, L.; et al. LAD coronary artery myocardial bridging and apical ballooning syndrome. JACC Cardiovasc. Imaging 2013, 6, 32–41. [Google Scholar] [CrossRef]
- Stiermaier, T.; Desch, S.; Blazek, S.; Schuler, G.; Thiele, H.; Eitel, I. Frequency and Significance of Myocardial Bridging and Recurrent Segment of the Left Anterior Descending Coronary Artery in Patients With Takotsubo Cardiomyopathy. Am. J. Cardiol. 2014, 114, 1204–1209. [Google Scholar] [CrossRef]
- Arcari, L.; Limite, L.R.; Cacciotti, L.; Alonzo, A.; Musumeci, M.B.; Passaseo, I.; Marazzi, G.; Berni, A.; Ansalone, G.; Volpe, M.; et al. Tortuosity, Recurrent Segments, and Bridging of the Epicardial Coronary Arteries in Patients With the Takotsubo Syndrome. Am. J. Cardiol. 2017, 119, 243–248. [Google Scholar] [CrossRef]
- Haghi, D.; Roehm, S.; Hamm, K.; Harder, N.; Suselbeck, T.; Borggrefe, M.; Papavassiliu, T. Takotsubo cardiomyopathy is not due to plaque rupture: An intravascular ultrasound study. Clin. Cardiol. 2010, 33, 307–310. [Google Scholar] [CrossRef]
- Vitale, C.; Rosano, G.M.; Kaski, J.C. Role of Coronary Microvascular Dysfunction in Takotsubo Cardiomyopathy. Circ. J. Off. J. Jpn. Circ. Soc. 2016, 80, 299–305. [Google Scholar] [CrossRef]
- Cohen, R.A.; Shepherd, J.T.; Vanhoutte, P.M. Prejunctional and postjunctional actions of endogenous norepinephrine at the sympathetic neuroeffector junction in canine coronary arteries. Circ. Res. 1983, 52, 16–25. [Google Scholar] [CrossRef]
- Uchida, Y.; Egami, H.; Uchida, Y.; Sakurai, T.; Kanai, M.; Shirai, S.; Nakagawa, O.; Oshima, T. Possible participation of endothelial cell apoptosis of coronary microvessels in the genesis of Takotsubo cardiomyopathy. Clin. Cardiol. 2010, 33, 371–377. [Google Scholar] [CrossRef]
- Kume, T.; Akasaka, T.; Kawamoto, T.; Yoshitani, H.; Watanabe, N.; Neishi, Y.; Wada, N.; Yoshida, K. Assessment of Coronary Microcirculation in Patients With Takotsubo-Like Left Ventricular Dysfunction. Circ. J. 2005, 69, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Rigo, F.; Sicari, R.; Citro, R.; Ossena, G.; Buja, P.; Picano, E. Diffuse, marked, reversible impairment in coronary microcirculation in stress cardiomyopathy: A Doppler transthoracic echo study. Ann. Med. 2009, 41, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Meimoun, P.; Malaquin, D.; Sayah, S.; Benali, T.; Luycx-Bore, A.; Levy, F.; Zemir, H.; Tribouilloy, C. The Coronary Flow Reserve Is Transiently Impaired in Tako-Tsubo Cardiomyopathy: A Prospective Study Using Serial Doppler Transthoracic Echocardiography. J. Am. Soc. Echocardiogr. 2008, 21, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Meimoun, P.; Clerc, J.; Vincent, C.; Flahaut, F.; Germain, A.L.; Elmkies, F.; Zemir, H.; Luycx-Bore, A. Non-invasive detection of tako-tsubo cardiomyopathy vs. acute anterior myocardial infarction by transthoracic Doppler echocardiography. Eur. Heart J. Cardiovasc. Imaging 2012, 14, 464–470. [Google Scholar] [CrossRef]
- Bybee, K.A.; Prasad, A.; Barsness, G.W.; Lerman, A.; Jaffe, A.S.; Murphy, J.G.; Wright, R.S.; Rihal, C.S. Clinical characteristics and Thrombolysis In Myocardial Infarction frame counts in women with transient left ventricular apical ballooning syndrome. Am. J. Cardiol. 2004, 94, 343–346. [Google Scholar] [CrossRef]
- Kurisu, S.; Inoue, I.; Kawagoe, T.; Ishihara, M.; Shimatani, Y.; Nishioka, K.; Umemura, T.; Nakamura, S.; Yoshida, M.; Sato, H. Myocardial perfusion and fatty acid metabolism in patients with tako-tsubo-like left ventricular dysfunction. J. Am. Coll. Cardiol. 2003, 41, 743–748. [Google Scholar] [CrossRef]
- Khalid, N.; Iqbal, I.; Coram, R.; Raza, T.; Fahsah, I.; Ikram, S. Thrombolysis In Myocardial Infarction Frame Count in Takotsubo Cardiomyopathy. Int. J. Cardiol. 2015, 191, 107–108. [Google Scholar] [CrossRef]
- Galiuto, L.; De Caterina, A.R.; Porfidia, A.; Paraggio, L.; Barchetta, S.; Locorotondo, G.; Rebuzzi, A.G.; Crea, F. Reversible coronary microvascular dysfunction: A common pathogenetic mechanism in Apical Ballooning or Tako-Tsubo Syndrome. Eur. Heart J. 2010, 31, 1319–1327. [Google Scholar] [CrossRef]
- Barletta, G.; Del Pace, S.; Boddi, M.; Del Bene, R.; Salvadori, C.; Bellandi, B.; Coppo, M.; Saletti, E.; Gensini, G.F. Abnormal coronary reserve and left ventricular wall motion during cold pressor test in patients with previous left ventricular ballooning syndrome. Eur. Heart J. 2009, 30, 3007–3014. [Google Scholar] [CrossRef][Green Version]
- Patel, S.M.; Lerman, A.; Lennon, R.J.; Prasad, A. Impaired coronary microvascular reactivity in women with apical ballooning syndrome (Takotsubo/stress cardiomyopathy). Eur. Heart J. Acute Cardiovasc. Care 2013, 2, 147–152. [Google Scholar] [CrossRef]
- Martin, E.A.; Prasad, A.; Rihal, C.S.; Lerman, L.O.; Lerman, A. Endothelial function and vascular response to mental stress are impaired in patients with apical ballooning syndrome. J. Am. Coll. Cardiol. 2010, 56, 1840–1846. [Google Scholar] [CrossRef] [PubMed]
- Nef, H.M.; Mollmann, H.; Kostin, S.; Troidl, C.; Voss, S.; Weber, M.; Dill, T.; Rolf, A.; Brandt, R.; Hamm, C.W.; et al. Tako-Tsubo cardiomyopathy: Intraindividual structural analysis in the acute phase and after functional recovery. Eur. Heart J. 2007, 28, 2456–2464. [Google Scholar] [CrossRef] [PubMed]
- Nef, H.M.; Mollmann, H.; Troidl, C.; Kostin, S.; Voss, S.; Hilpert, P.; Behrens, C.B.; Rolf, A.; Rixe, J.; Weber, M.; et al. Abnormalities in intracellular Ca2+ regulation contribute to the pathomechanism of Tako-Tsubo cardiomyopathy. Eur. Heart J. 2009, 30, 2155–2164. [Google Scholar] [CrossRef] [PubMed]
- Linck, B.; Bokník, P.; Baba, H.A.; Eschenhagen, T.; Haverkamp, U.; Jäckel, E.; Jones, L.R.; Kirchhefer, U.; Knapp, J.; Läer, S.; et al. Long-term beta adrenoceptor-mediated alteration in contractility and expression of phospholamban and sarcoplasmic reticulum Ca(++)-ATPase in mammalian ventricle. J. Pharmacol. Exp. Ther. 1998, 286, 531–538. [Google Scholar] [PubMed]
- Lipskaia, L.; Lompré, A.-M. Alteration in temporal kinetics of Ca2+ signaling and control of growth and proliferation. Biol. Cell 2004, 96, 55–68. [Google Scholar] [CrossRef]
- Paur, H.; Wright, P.T.; Sikkel, M.B.; Tranter, M.H.; Mansfield, C.; O’Gara, P.; Stuckey, D.J.; Nikolaev, V.O.; Diakonov, I.; Pannell, L.; et al. High Levels of Circulating Epinephrine Trigger Apical Cardiodepression in a β2-Adrenergic Receptor/Gi–Dependent Manner. Circulation 2012, 126, 697–706. [Google Scholar] [CrossRef]
- Lyon, A.R.; Rees, P.S.C.; Prasad, S.; Poole-Wilson, P.A.; Harding, S.E. Stress (Takotsubo) cardiomyopathy—A novel pathophysiological hypothesis to explain catecholamine-induced acute myocardial stunning. Nat. Clin. Pract. Cardiovasc. Med. 2008, 5, 22–29. [Google Scholar] [CrossRef]
- Heubach, J.F.; Ravens, U.; Kaumann, A.J. Epinephrine Activates Both Gs and Gi Pathways, but Norepinephrine Activates Only the Gs Pathway through Human β2-Adrenoceptors Overexpressed in Mouse Heart. Mol. Pharmacol. 2004, 65, 1313–1322. [Google Scholar] [CrossRef]
- Chesley, A.; Lundberg, M.S.; Asai, T.; Xiao, R.-P.; Ohtani, S.; Lakatta, E.G.; Crow, M.T. The β2-Adrenergic Receptor Delivers an Antiapoptotic Signal to Cardiac Myocytes Through Gi-Dependent Coupling to Phosphatidylinositol 3′-Kinase. Circ. Res. 2000, 87, 1172–1179. [Google Scholar] [CrossRef]
- Shao, Y.; Redfors, B.; Scharin Tang, M.; Mollmann, H.; Troidl, C.; Szardien, S.; Hamm, C.; Nef, H.; Boren, J.; Omerovic, E. Novel rat model reveals important roles of beta-adrenoreceptors in stress-induced cardiomyopathy. Int. J. Cardiol. 2013, 168, 1943–1950. [Google Scholar] [CrossRef]
- Nakano, T.; Onoue, K.; Nakada, Y.; Nakagawa, H.; Kumazawa, T.; Ueda, T.; Nishida, T.; Soeda, T.; Okayama, S.; Watanabe, M.; et al. Alteration of β-Adrenoceptor Signaling in Left Ventricle of Acute Phase Takotsubo Syndrome: A Human Study. Sci. Rep. 2018, 8, 12731. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Redfors, B.; Lundgren, J.; Alkhoury, J.; Oras, J.; Gan, L.-M.; Omerovic, E. Effects of pretreatment with cardiostimulants and beta-blockers on isoprenaline-induced takotsubo-like cardiac dysfunction in rats. Int. J. Cardiol. 2019, 281, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, L.; Trimarco, V.; Di Marino, S.; Marino, M.; Iaccarino, G.; Trimarco, B. L41Q polymorphism of the G protein coupled receptor kinase 5 is associated with left ventricular apical ballooning syndrome. Eur. J. Heart Fail. 2010, 12, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Figtree, G.A.; Bagnall, R.D.; Abdulla, I.; Buchholz, S.; Galougahi, K.K.; Yan, W.; Tan, T.; Neil, C.; Horowitz, J.D.; Semsarian, C.; et al. No association of G-protein-coupled receptor kinase 5 or β-adrenergic receptor polymorphisms with Takotsubo cardiomyopathy in a large Australian cohort. Eur. J. Heart Fail. 2013, 15, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Novo, G.; Giambanco, S.; Guglielmo, M.; Arvigo, L.; Sutera, M.R.; Giambanco, F.; Evola, S.; Vaccarino, L.; Bova, M.; Lio, D.; et al. G-protein-coupled receptor kinase 5 polymorphism and Takotsubo cardiomyopathy. J. Cardiovasc. Med. 2015, 16, 639–643. [Google Scholar] [CrossRef]
- Eitel, I.; Moeller, C.; Munz, M.; Stiermaier, T.; Meitinger, T.; Thiele, H.; Erdmann, J. Genome-wide association study in takotsubo syndrome - Preliminary results and future directions. Int J Cardiol 2017, 236, 335–339. [Google Scholar] [CrossRef]
- Vriz, O.; Minisini, R.; Citro, R.; Guerra, V.; Zito, C.; De Luca, G.; Pavan, D.; Pirisi, M.; Limongelli, G.; Bossone, E. Analysis of beta1 and beta2-adrenergic receptors polymorphism in patients with apical ballooning cardiomyopathy. Acta Cardiol. 2011, 66, 787–790. [Google Scholar] [CrossRef]
- Zaroff, J.G.; Pawlikowska, L.; Miss, J.C.; Yarlagadda, S.; Ha, C.; Achrol, A.; Kwok, P.Y.; McCulloch, C.E.; Lawton, M.T.; Ko, N.; et al. Adrenoceptor polymorphisms and the risk of cardiac injury and dysfunction after subarachnoid hemorrhage. Stroke 2006, 37, 1680–1685. [Google Scholar] [CrossRef]
- Sharkey, S.W.; Maron, B.J.; Nelson, P.; Parpart, M.; Maron, M.S.; Bristow, M.R. Adrenergic receptor polymorphisms in patients with stress (tako-tsubo) cardiomyopathy. J. Cardiol. 2009, 53, 53–57. [Google Scholar] [CrossRef]
- Ueyama, T.; Kasamatsu, K.; Hano, T.; Tsuruo, Y.; Ishikura, F. Catecholamines and estrogen are involved in the pathogenesis of emotional stress-induced acute heart attack. Ann. N. Y. Acad. Sci. 2008, 1148, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Komesaroff, P.A.; Esler, M.D.; Sudhir, K. Estrogen Supplementation Attenuates Glucocorticoid and Catecholamine Responses to Mental Stress in Perimenopausal Women1. J. Clin. Endocrinol. Metab. 1999, 84, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Sung, B.H.; Ching, M.; Izzo, J.L., Jr.; Dandona, P.; Wilson, M.F. Estrogen improves abnormal norepinephrine-induced vasoconstriction in postmenopausal women. J. Hypertens. 1999, 17, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Sader, M.A.; Celermajer, D.S. Endothelial function, vascular reactivity and gender differences in the cardiovascular system. Cardiovasc. Res. 2002, 53, 597–604. [Google Scholar] [CrossRef]
- Sato, T.; Hagiwara, K.; Nishikido, A.; Miyamoto, S.; Komiyama, K.; Matsuno, H.; Hashida, H.; Kobayakawa, N.; Akiyama, O. Takotsubo (Ampulla-shaped) Cardiomyopathy Associated with Microscopic Polyangiitis. Intern. Med. 2005, 44, 251–255. [Google Scholar] [CrossRef]
- Scally, C.; Abbas, H.; Ahearn, T.; Srinivasan, J.; Mezincescu, A.; Rudd, A.; Spath, N.; Yucel-Finn, A.; Yuecel, R.; Oldroyd, K.; et al. Myocardial and Systemic Inflammation in Acute Stress-Induced (Takotsubo) Cardiomyopathy. Circulation 2019, 139, 1581–1592. [Google Scholar] [CrossRef]
- Santoro, F.; Tarantino, N.; Ferraretti, A.; Ieva, R.; Musaico, F.; Guastafierro, F.; Di Martino, L.; Di Biase, M.; Brunetti, N.D. Serum interleukin 6 and 10 levels in Takotsubo cardiomyopathy: Increased admission levels may predict adverse events at follow-up. Atherosclerosis 2016, 254, 28–34. [Google Scholar] [CrossRef]
- Wilson, H.M.; Cheyne, L.; Brown, P.A.J.; Kerr, K.; Hannah, A.; Srinivasan, J.; Duniak, N.; Horgan, G.; Dawson, D.K. Characterization of the Myocardial Inflammatory Response in Acute Stress-Induced (Takotsubo) Cardiomyopathy. JACC Basic Transl. Sci. 2018, 3, 766–778. [Google Scholar] [CrossRef]
- Kołodzińska, A.; Czarzasta, K.; Szczepankiewicz, B.; Główczyńska, R.; Fojt, A.; Ilczuk, T.; Budnik, M.; Krasuski, K.; Folta, M.; Cudnoch-Jędrzejewska, A.; et al. Toll-like receptor expression and apoptosis morphological patterns in female rat hearts with takotsubo syndrome induced by isoprenaline. Life Sci. 2018, 199, 112–121. [Google Scholar] [CrossRef]
- Fitzgibbons, T.P.; Edwards, Y.J.K.; Shaw, P.; Iskandar, A.; Ahmed, M.; Bote, J.; et al. Activation of Inflammatory and Pro-Thrombotic Pathways in Acute Stress Cardiomyopathy. Front. Cardiovasc. Med. 2017, 4, 49. [Google Scholar] [CrossRef]
- Rawish, E.; Nording, H.; Munte, T.; Langer, H.F. Platelets as Mediators of Neuroinflammation and Thrombosis. Front. Immunol. 2020, 11, 548631. [Google Scholar] [CrossRef]
- Neubauer, S.; Horn, M.; Naumann, A.; Tian, R.; Hu, K.; Laser, M.; Friedrich, J.; Gaudron, P.; Schnackerz, K.; Ingwall, J.S. Impairment of energy metabolism in intact residual myocardium of rat hearts with chronic myocardial infarction. J. Clin. Investig. 1995, 95, 1092–1100. [Google Scholar] [CrossRef]
- Ciutac, A.M.; Dawson, D. The role of inflammation in stress cardiomyopathy. Trends Cardiovasc. Med. 2020. [Google Scholar] [CrossRef]
- Nef, H.M.; Möllmann, H.; Hilpert, P.; Troidl, C.; Voss, S.; Rolf, A.; Behrens, C.B.; Weber, M.; Hamm, C.W.; Elsässer, A. Activated cell survival cascade protects cardiomyocytes from cell death in Tako-Tsubo cardiomyopathy. Eur. J. Heart Fail. 2009, 11, 758–764. [Google Scholar] [CrossRef]
- Surikow, S.Y.; Raman, B.; Licari, J.; Singh, K.; Nguyen, T.H.; Horowitz, J.D. Evidence of nitrosative stress within hearts of patients dying of Tako-tsubo cardiomyopathy. Int. J. Cardiol. 2015, 189, 112–114. [Google Scholar] [CrossRef]
- Birenbaum, A.; Tesse, A.; Loyer, X.; Michelet, P.; Andriantsitohaina, R.; Heymes, C.; Riou, B.; Amour, J. Involvement of β3-Adrenoceptor in Altered β-Adrenergic Response in Senescent Heart. Anesthesiology 2008, 109, 1045–1053. [Google Scholar] [CrossRef]
- Surikow, S.Y.; Nguyen, T.H.; Stafford, I.; Chapman, M.; Chacko, S.; Singh, K.; Licari, G.; Raman, B.; Kelly, D.J.; Zhang, Y.; et al. Nitrosative Stress as a Modulator of Inflammatory Change in a Model of Takotsubo Syndrome. JACC Basic Transl. Sci. 2018, 3, 213–226. [Google Scholar] [CrossRef]
- Schulze, P.C.; Liu, H.; Choe, E.; Yoshioka, J.; Shalev, A.; Bloch, K.D.; Lee, R.T. Nitric Oxide–Dependent Suppression of Thioredoxin-Interacting Protein Expression Enhances Thioredoxin Activity. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2666–2672. [Google Scholar] [CrossRef]
- Chong, C.-R.; Chan, W.P.A.; Nguyen, T.H.; Liu, S.; Procter, N.E.K.; Ngo, D.T.; Sverdlov, A.L.; Chirkov, Y.Y.; Horowitz, J.D. Thioredoxin-Interacting Protein: Pathophysiology and Emerging Pharmacotherapeutics in Cardiovascular Disease and Diabetes. Cardiovasc. Drugs Ther. 2014, 28, 347–360. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Liu, S.; Ong, G.J.; Stafford, I.; Frenneaux, M.P.; Horowitz, J.D. Glycocalyx shedding is markedly increased during the acute phase of Takotsubo cardiomyopathy. Int. J. Cardiol. 2017, 243, 296–299. [Google Scholar] [CrossRef]
- Zhang, Z.; Jin, S.; Teng, X.; Duan, X.; Chen, Y.; Wu, Y. Hydrogen sulfide attenuates cardiac injury in takotsubo cardiomyopathy by alleviating oxidative stress. Nitric Oxide 2017, 67, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Shao, Y.; Liu, X.; Wang, D.; Li, X. The cardioprotective effects of icariin on the isoprenaline-induced takotsubo-like rat model: Involvement of reactive oxygen species and the TLR4/NF-κB signaling pathway. Int. Immunopharmacol. 2019, 74, 105733. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Redfors, B.; Ståhlman, M.; Täng, M.S.; Miljanovic, A.; Möllmann, H.; Troidl, C.; Szardien, S.; Hamm, C.; Nef, H.; et al. A mouse model reveals an important role for catecholamine-induced lipotoxicity in the pathogenesis of stress-induced cardiomyopathy. Eur. J. Heart Fail. 2013, 15, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Rawish, E.; Nickel, L.; Schuster, F.; Stolting, I.; Frydrychowicz, A.; Saar, K.; Hubner, N.; Othman, A.; Kuerschner, L.; Raasch, W. Telmisartan prevents development of obesity and normalizes hypothalamic lipid droplets. J. Endocrinol. 2020, 244, 95–110. [Google Scholar] [CrossRef]
- Borchert, T.; Hübscher, D.; Guessoum, C.I.; Lam, T.-D.D.; Ghadri, J.R.; Schellinger, I.N.; Tiburcy, M.; Liaw, N.Y.; Li, Y.; Haas, J.; et al. Catecholamine-Dependent β-Adrenergic Signaling in a Pluripotent Stem Cell Model of Takotsubo Cardiomyopathy. J. Am. Coll. Cardiol. 2017, 70, 975–991. [Google Scholar] [CrossRef]


| Diagnostic criteria | |
|---|---|
| 1 | Transient regional wall motion abnormalities of left ventricle (LV) or right ventricle (RV) myocardium which are frequently, but not always, preceded by a stressful trigger (emotional or physical). |
| 2 | The regional wall motion abnormalities usually a extend beyond a single epicardial vascular distribution and often result in circumferential dysfunction of the ventricular segments involved. |
| 3 | The absence of culprit atherosclerotic coronary artery disease including acute plaque rupture, thrombus formation, and coronary dissection or other pathological conditions to explain the observed pattern of temporary LV dysfunction (e.g., hypertrophic cardiomyopathy and viral myocarditis). |
| 4 | New and reversible electrocardiography (ECG) abnormalities (ST-segment elevation, ST depression, left bundle branch block (LBBB) b, T-wave inversion, and/or QTc prolongation) during the acute phase (3 months). |
| 5 | Significantly elevated level of serum natriuretic peptide (B-type natriuretic peptide (BNP) or N-terminal pro-B-type natriuretic peptide (NT-proBNP)) during the acute phase. |
| 6 | Positive but relatively small elevation in cardiac troponin measured with a conventional assay (i.e., disparity between the troponin level and the amount of dysfunctional myocardium present). c |
| 7 | Recovery of ventricular systolic function on cardiac imaging at follow-up (3–6 months) d. |
| Diagnostic Tool | Finding |
|---|---|
| ECG | ST-segment elevation (particularly in -aVR), ST depression, LBBB, T-wave inversion, QTc prolongation |
| Biomarkers | Elevated troponin T with higher NT-proBNP/troponin T ratio than in ST-segment elevation myocardial infarction (STEMI) |
| Angiography and ventriculography | Absence of culprit atherosclerotic coronary artery disease including acute plaque rupture, thrombus formation, and coronary dissection, as well as characteristic regional LV wall motion abnormality (RWMA). Apical nipple sign. |
| Echocardiography | RWMAs |
| CMR (cardiac magnetic resonance) | RWMAs, RV involvement, late gadolinium-enhancement signal intensity threshold < 5 SD, and edema using T2 weighted imaging in dysfunctional LV regions. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rawish, E.; Stiermaier, T.; Santoro, F.; Brunetti, N.D.; Eitel, I. Current Knowledge and Future Challenges in Takotsubo Syndrome: Part 1—Pathophysiology and Diagnosis. J. Clin. Med. 2021, 10, 479. https://doi.org/10.3390/jcm10030479
Rawish E, Stiermaier T, Santoro F, Brunetti ND, Eitel I. Current Knowledge and Future Challenges in Takotsubo Syndrome: Part 1—Pathophysiology and Diagnosis. Journal of Clinical Medicine. 2021; 10(3):479. https://doi.org/10.3390/jcm10030479
Chicago/Turabian StyleRawish, Elias, Thomas Stiermaier, Francesco Santoro, Natale D. Brunetti, and Ingo Eitel. 2021. "Current Knowledge and Future Challenges in Takotsubo Syndrome: Part 1—Pathophysiology and Diagnosis" Journal of Clinical Medicine 10, no. 3: 479. https://doi.org/10.3390/jcm10030479
APA StyleRawish, E., Stiermaier, T., Santoro, F., Brunetti, N. D., & Eitel, I. (2021). Current Knowledge and Future Challenges in Takotsubo Syndrome: Part 1—Pathophysiology and Diagnosis. Journal of Clinical Medicine, 10(3), 479. https://doi.org/10.3390/jcm10030479